The Influence of the Washcoat Deposition Process on High Pore Density Open Cell Foams Activation for CO Catalytic Combustion
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization
2.2. Rheological Behavior
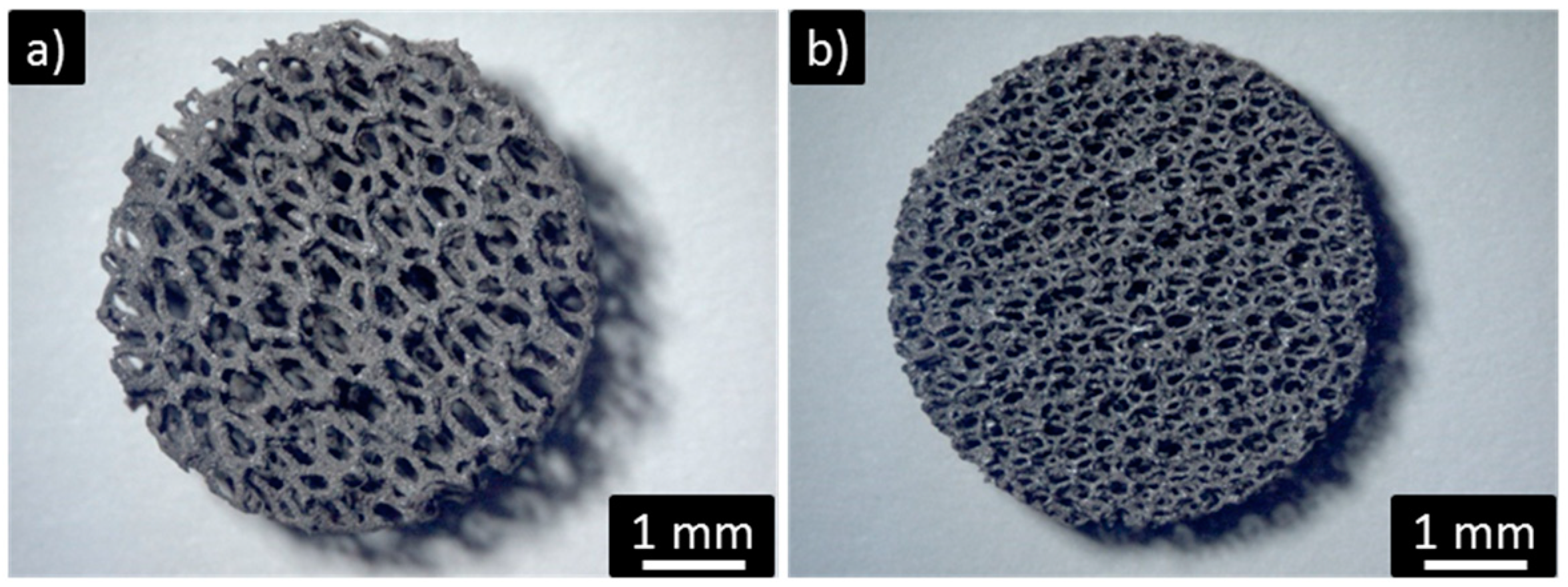
2.3. Washcoat Deposition
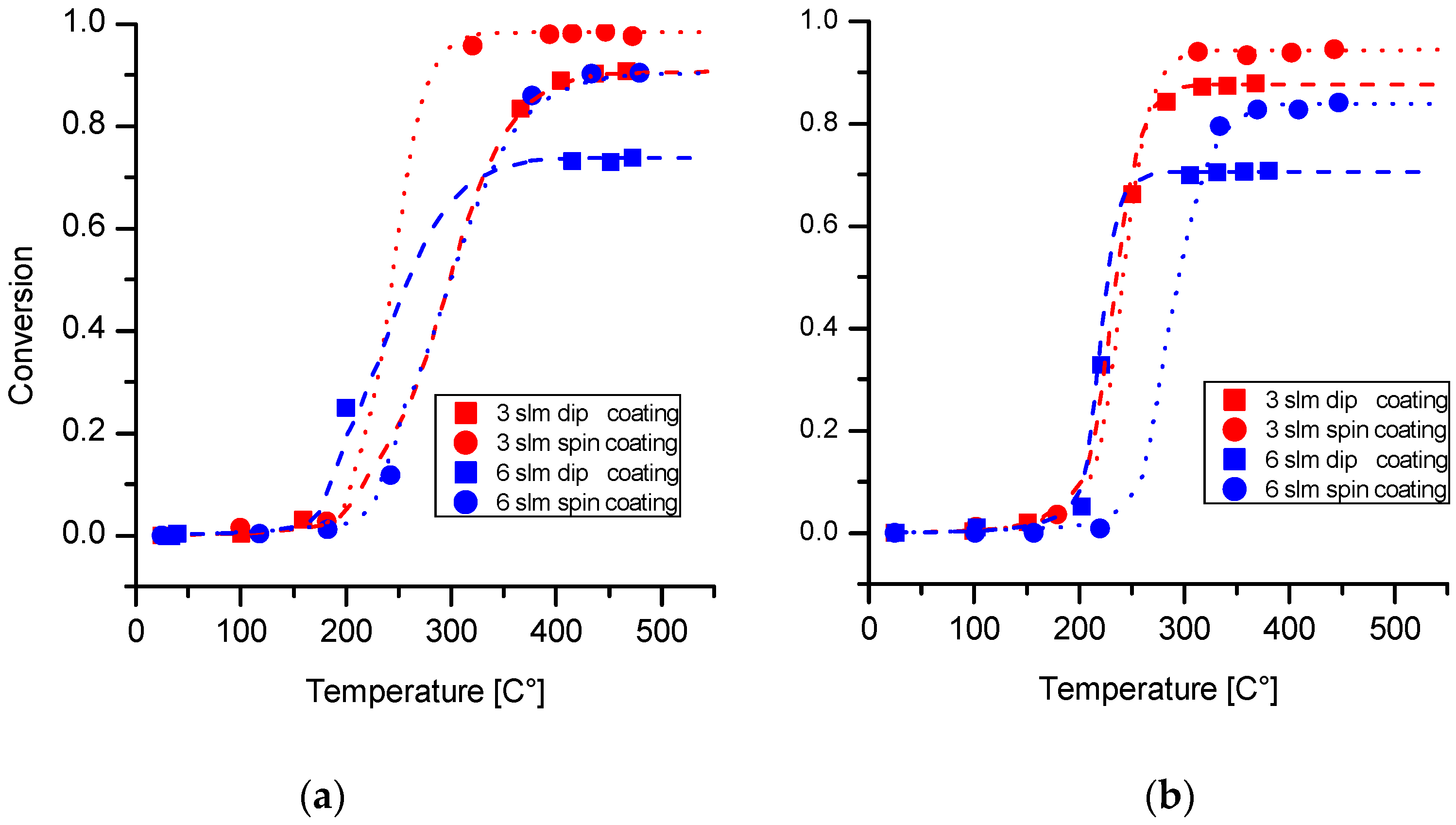
2.4. Catalytic Activity Test
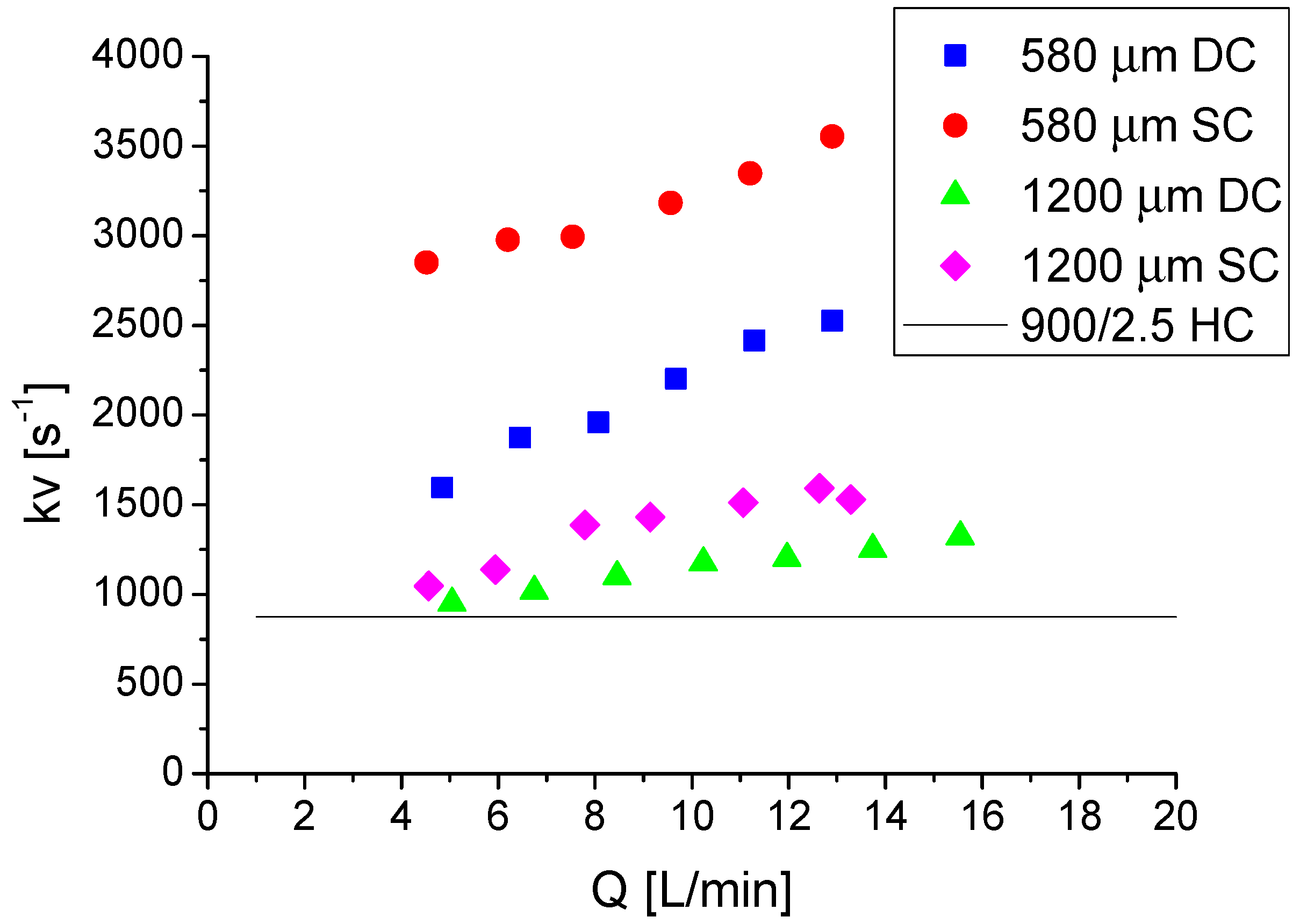
2.5. Analysis of Mass Transfer Performances
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Open Cell Foams Characterization
3.4. Washcoating
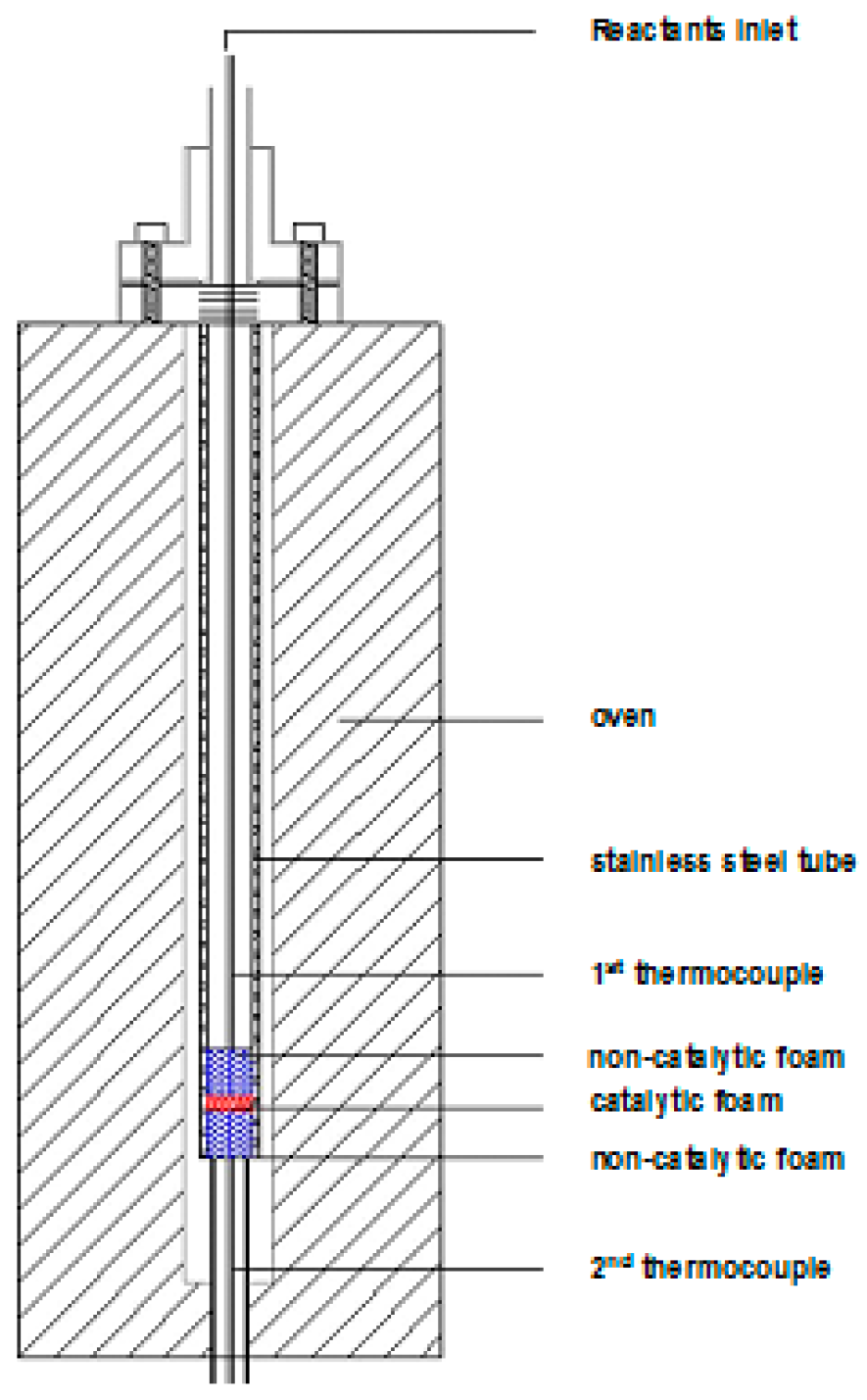
3.5. Catalyst Testing
4. Conclusions
- Spin coating allows effective control of wet coating formation onto metallic open cell foams with cell diameters down to 500 μm. Thanks to the higher shear stresses induced by rotation during wet film deposition, this technique is able to produce better results in terms of reduced clogging phenomena and higher film homogeneity with respect to dip coating.
- The use of the reported deposition methodologies allowed very thin catalytic layers to be obtained, without remarkably affecting the strut average thickness.
- The deposition method has a strong effect on the catalytic performances of the samples: foams prepared with spin coating outperformed samples activated via dip coating.
Author Contributions
Funding
Conflicts of Interest
References
- Ambrosetti, M.; Bracconi, M.; Groppi, G.; Tronconi, E. Analytical Geometrical Model of Open Cell Foams with Detailed Description of Strut-Node Intersection. Chem. Ing. Tech. 2017, 89, 915–925. [Google Scholar] [CrossRef]
- Bracconi, M.; Ambrosetti, M.; Maestri, M.; Groppi, G.; Tronconi, E. A fundamental investigation of gas/solid mass transfer in open-cell foams using a combined experimental and CFD approach. Chem. Eng. J. 2018, 352, 558–571. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E. Methods for the catalytic activation of metallic structured substrates. Catal. Sci. Technol. 2014, 4, 2846–2870. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego CA, USA, 1990; ISBN 9780080571034. [Google Scholar]
- Middleman, S. Fundamentals of Polymer Processing; McGraw-Hill Inc.: New York NY, USA, 1977; ISBN 978-0070418516. [Google Scholar]
- Cristiani, C.; Finocchio, E.; Latorrata, S.; Visconti, C.G.; Bianchi, E.; Tronconi, E.; Groppi, G.; Pollesel, P. Activation of metallic open-cell foams via washcoat deposition of Ni/MgAl2O4 catalysts for steam reforming reaction. Catal. Today 2012, 197, 256–264. [Google Scholar] [CrossRef]
- Valentini, M.; Groppi, G.; Cristiani, C.; Levi, M.; Tronconi, E.; Forzatti, P. The deposition of γ-Al2O3 layers on ceramic and metallic supports for the preparation of structured catalysts. Catal. Today 2001, 69, 307–314. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Kohler, S.; Johnsen, H.; Myrstad, R. Washcoating and chemical testing of a commercial Cu/ZnO/Al2O3 catalyst for the methanol synthesis over copper open-cell foams. Appl. Catal. A Gen. 2014, 481, 96–103. [Google Scholar] [CrossRef]
- Meille, V.; Pallier, S.; Santa Cruz Bustamante, G.V.; Marilyne Roumanie, J.-P.R.; Roumanie, M.; Reymond, J.-P. Deposition of gamma-Al2O3 layers on structured supports for the design of new catalytic reactors. Appl. Catal. A Gen. 2005, 286, 232–238. [Google Scholar] [CrossRef]
- Balzarotti, R.; Cristiani, C.; Latorrata, S.; Migliavacca, A. Washcoating of low surface area cerium oxide on complex geometry substrates. Part. Sci. Technol. 2016, 34, 184–193. [Google Scholar] [CrossRef]
- Balzarotti, R.; Italiano, C.; Pino, L.; Cristiani, C.; Vita, A. Ni/CeO2-thin ceramic layer depositions on ceramic monoliths for syngas production by oxy steam reforming of biogas. Fuel Process. Technol. 2016, 149, 40–48. [Google Scholar] [CrossRef]
- Italiano, C.; Balzarotti, R.; Vita, A.; Latorrata, S.; Fabiano, C.; Pino, L.; Cristiani, C. Preparation of structured catalysts with Ni and Ni-Rh/CeO2 catalytic layers for syngas production by biogas reforming processes. Catal. Today 2016, 273, 3–11. [Google Scholar] [CrossRef]
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating method for Pd/gamma-Al2O3 deposition on metallic foams. Appl. Catal. B Environ. 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Boettge, D.; Standke, G.; Fuessel, A.; Adler, J. Functionalization of open-celled foams by homogeneous slurry based coatings. J. Mater. Res. 2013, 28, 2220–2233. [Google Scholar] [CrossRef]
- Sanz, O.; Almeida, L.C.; Zamaro, J.M.; Montes, M.; Miro, E.E. Washcoating of Pt-ZSM5 onto aluminium foams. Appl. Catal. B Environ. 2008, 78, 166–175. [Google Scholar] [CrossRef]
- Gokon, N.; Kodama, T.; Imaizumi, N.; Umeda, J.; Seo, T. Ferrite/zirconia-coated foam device prepared by spin coating for solar demonstration of thermochemical water-splitting. Int. J. Hydrogen Energy 2011, 36, 2014–2028. [Google Scholar] [CrossRef]
- Zhang, H.; Suszynski, W.J.; Agrawal, K.V.; Tsapatsis, M.; Al Hashimi, S.; Francis, L.F. Coating of open cell foams. Ind. Eng. Chem. Res. 2012, 51, 9250–9259. [Google Scholar] [CrossRef]
- Zhao, C.Y. Review on thermal transport in high porosity cellular metal foams with open cells. Int. J. Heat Mass Transf. 2012, 55, 3618–3632. [Google Scholar] [CrossRef]
- Zhou, W.; Ke, Y.; Wang, Q.; Wan, S.; Lin, J.; Zhang, J.; Hui, K.S. Development of cylindrical laminated methanol steam reforming microreactor with cascading metal foams as catalyst support. Fuel 2017, 191, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ping, D.; Dong, X.; Dong, Y.; Zang, Y. Construction of Ru/Ni-Al-oxide/Ni-foam monolithic catalyst for deep-removing CO in hydrogen-rich gas via selective methanation. Fuel Process. Technol. 2016, 148, 367–371. [Google Scholar] [CrossRef]
- Yu, H.; Chen, H.; Pan, M.; Tang, Y.; Zeng, K.; Peng, F.; Wang, H. Effect of the metal foam materials on the performance of methanol steam micro-reformer for fuel cells. Appl. Catal. A Gen. 2007, 327, 106–113. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Yu, H.; Peng, F.; Wang, H. Metal-foam-supported Pd/Al2O3 catalysts for catalytic combustion of methane: Effect of interaction between support and catalyst. Int. J. Chem. React. Eng. 2015, 13, 83–93. [Google Scholar] [CrossRef]
- Ho, P.H.; Ambrosetti, M.; Groppi, G.; Tronconi, E.; Jaroszewicz, J.; Ospitali, F.; Rodriguez-Castellon, E.; Fornasari, G.; Vaccari, A.; Benito, P. One-step electrodeposition of Pd-CeO2 on high pore density foams for environmental catalytic processes. Catal. Sci. Technol. 2018, 8, 4678–4689. [Google Scholar] [CrossRef]
- Ho, P.; Scavetta, E.; Tonelli, D.; Fornasari, G.; Vaccari, A.; Benito, P. Hydrotalcite-type materials electrodeposited on open-cell metallic foams as structured catalysts. Inorganics 2018, 6, 74. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, X.; Li, Y.; Chai, R.; Zhao, G.; Wang, C.; Gong, X.; Liu, Y.; Lu, Y. High-Performance PdNi Nanoalloy Catalyst in Situ Structured on Ni Foam for Catalytic Deoxygenation of Coalbed Methane: Experimental and DFT Studies. ACS Catal. 2016, 6, 6236–6245. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Chai, R.; Zhao, G.; Liu, Y.; Lu, Y. Low-temperature active oscillation-free PdNi (alloy)/Ni-foam catalyst with enhanced heat transfer for coal bed methane deoxygenation via catalytic combustion. Appl. Catal. B Environ. 2016, 187, 238–248. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Chai, R.; Zhao, G.; Cao, F.; Liu, Y.; Lu, Y. Metal-foam-structured Ni-Al2O3 catalysts: Wet chemical etching preparation and syngas methanation performance. Appl. Catal. A Gen. 2016, 510, 216–226. [Google Scholar] [CrossRef]
- Chai, R.; Li, Y.; Zhang, Q.; Zhao, G.; Liu, Y.; Lu, Y. Monolithic Ni-MOx/Ni-foam (M = Al, Zr or Y) catalysts with enhanced heat/mass transfer for energy-efficient catalytic oxy-methane reforming. Catal. Commun. 2015, 70, 1–5. [Google Scholar] [CrossRef]
- Roberts, M.; Huang, A.F.; Johns, P.; Owen, J. Dip-spin coating of reticulated vitreous carbon with composite materials to act as an electrode for 3D microstructured lithium ion batteries. J. Power Sources 2013, 224, 250–259. [Google Scholar] [CrossRef]
- Groppi, G.; Cristiani, C.; Lietti, L.; Ramella, C.; Valentini, M.; Forzatti, P. Effect of ceria on palladium supported catalysts for high temperature combustion of CH4 under lean conditions. Catal. Today 1999, 50, 399–412. [Google Scholar] [CrossRef]
- Balzarotti, R.; Ciurlia, M.; Cristiani, C.; Paparella, F. Washcoat deposition of Ni- and Co-ZrO2 low surface area powders onto ceramic open-cell foams: Influence of slurry formulation and rheology. Catalysts 2015, 5, 2271–2286. [Google Scholar] [CrossRef] [Green Version]
- Olhero, S.M.; Ferreira, J.M.F. Influence of particle size distribution on rheology and particle packing of silica-based suspensions. Powder Technol. 2004, 139, 69–75. [Google Scholar] [CrossRef]
- Zupancic, A.; Kristo, A. Influence of particle concentration on rheological properties of aqueous Al2O3 suspensions. J. Eur. Ceram. Soc. 1998, 18, 467–477. [Google Scholar] [CrossRef]
- Tronconi, E.; Forzatti, P. Adequacy of lumped parameter models for SCR reactors with monolith structure. AIChE J. 1992, 38, 201–210. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 3rd ed.; Pearson: Upper Saddle River, NJ, USA, 2001; ISBN 978-0201610918. [Google Scholar]
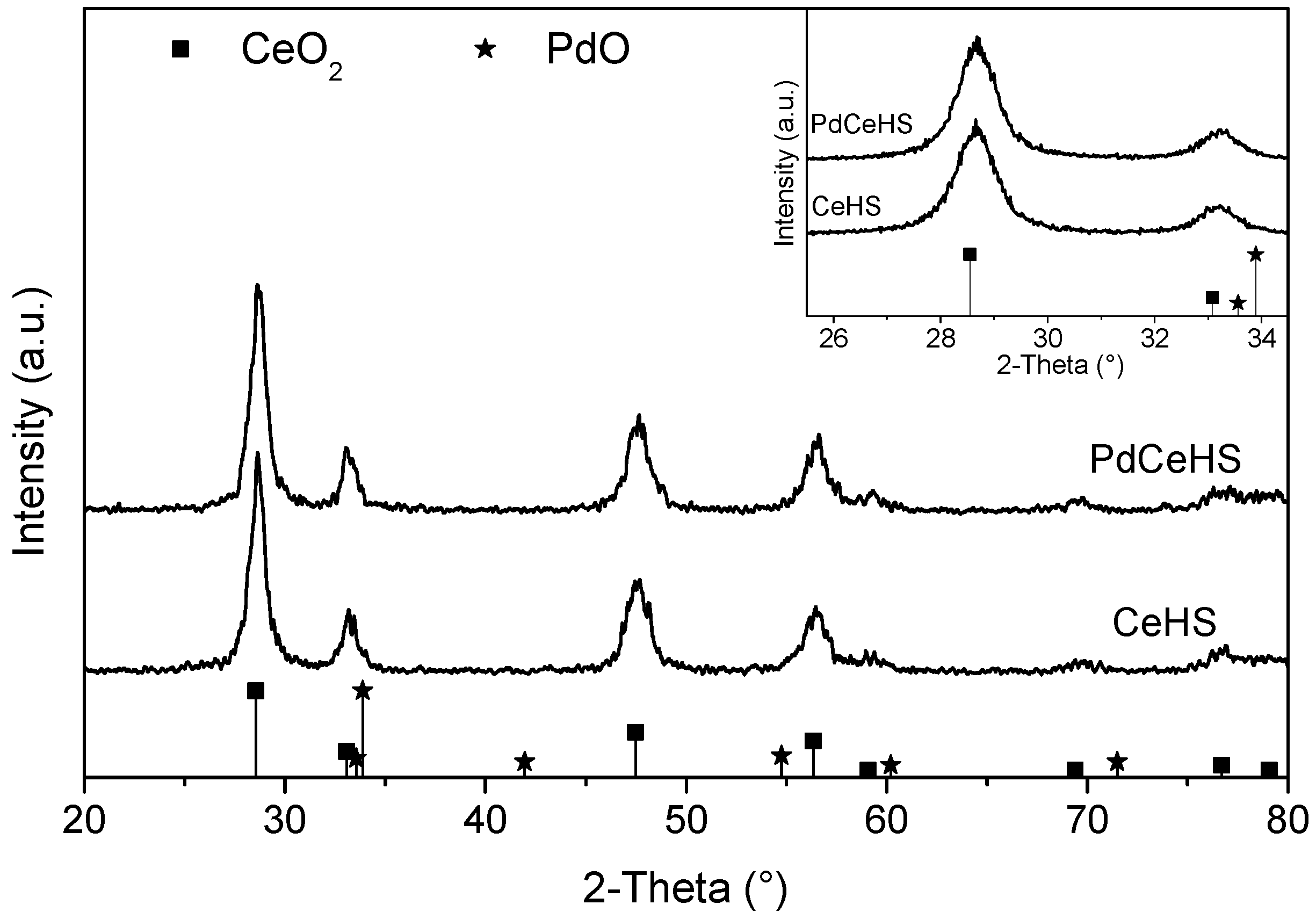
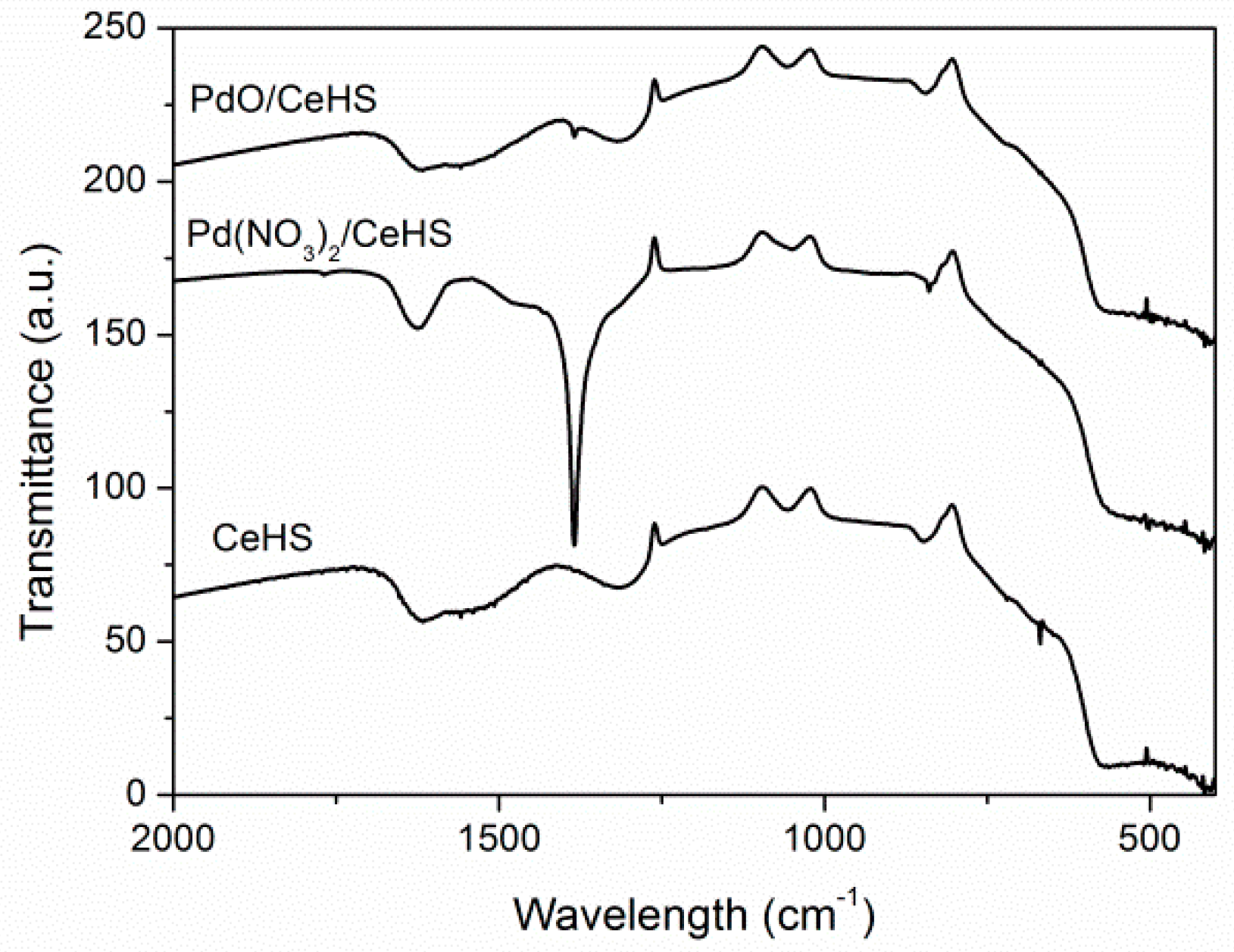



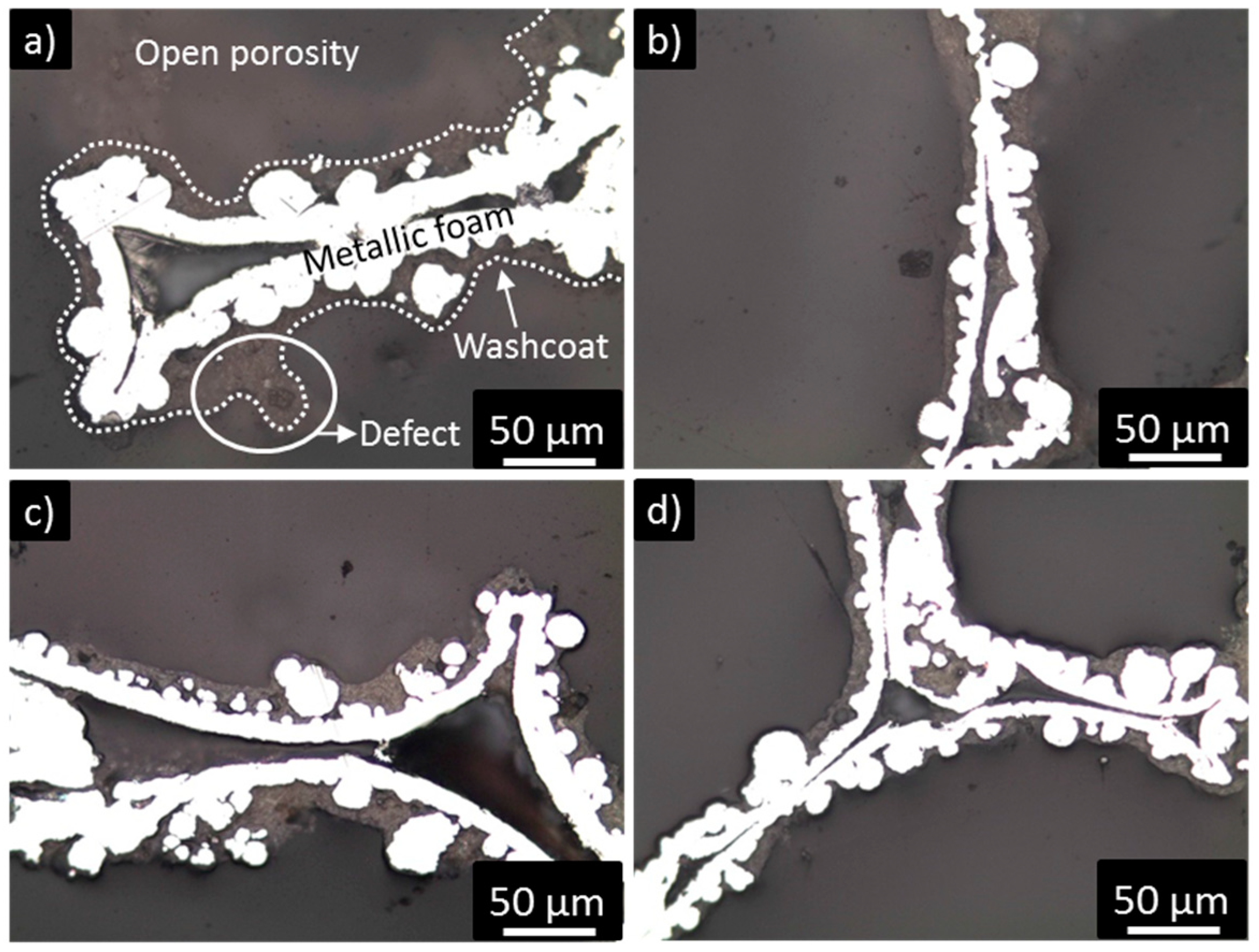
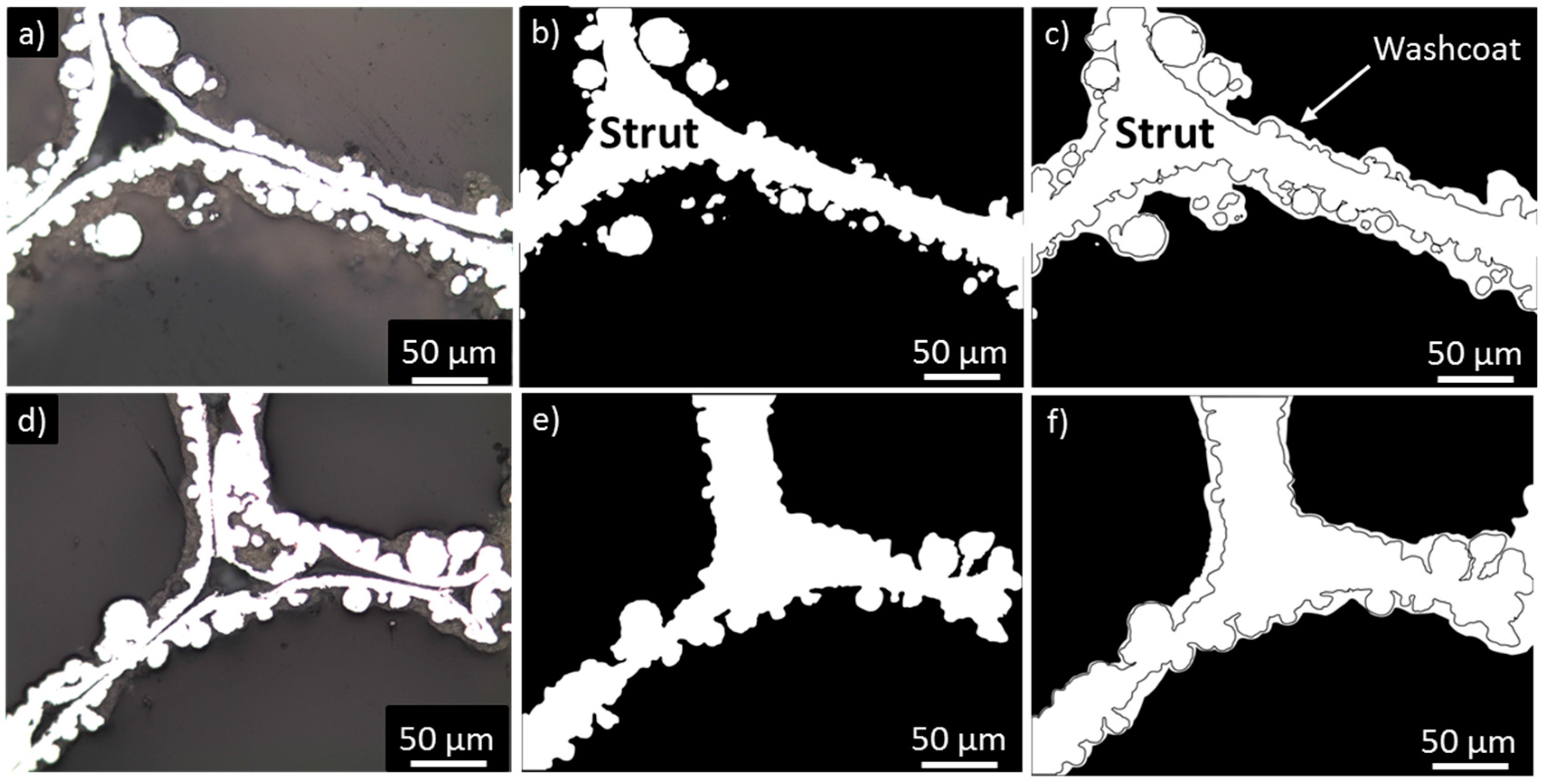

| Nominal Content (% wt.) | TG (% wt.) | SEM-EDS (% wt.) |
|---|---|---|
| 3 | 2.9 | 2.5 ± 0.3 |
| Sample | Load (% wt.%) | Dcell (mm) | ε (-) | Sv (m−1) | Lc (mm) |
|---|---|---|---|---|---|
| 580 µm DC | 24.2 | 0.551 (0.643) | 0.813 (0.915) | 6523 (4185) | 0.141(0.099) |
| 580 µm SC | 7.9 | 0.625 (0.643) | 0.880 (0.915) | 4973 (4185) | 0.117 (0.099) |
| 1200 µm DC | 23.4 | 1.131 (1.278) | 0.881 (0.9345) | 2906 (1860) | 0.236 (0.157) |
| 1200 µm SC | 17.2 | 1.171 (1.278) | 0.892 (0.9345) | 2627 (1860) | 0.217 (0.157) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosetti, M.; Balzarotti, R.; Cristiani, C.; Groppi, G.; Tronconi, E. The Influence of the Washcoat Deposition Process on High Pore Density Open Cell Foams Activation for CO Catalytic Combustion. Catalysts 2018, 8, 510. https://doi.org/10.3390/catal8110510
Ambrosetti M, Balzarotti R, Cristiani C, Groppi G, Tronconi E. The Influence of the Washcoat Deposition Process on High Pore Density Open Cell Foams Activation for CO Catalytic Combustion. Catalysts. 2018; 8(11):510. https://doi.org/10.3390/catal8110510
Chicago/Turabian StyleAmbrosetti, Matteo, Riccardo Balzarotti, Cinzia Cristiani, Gianpiero Groppi, and Enrico Tronconi. 2018. "The Influence of the Washcoat Deposition Process on High Pore Density Open Cell Foams Activation for CO Catalytic Combustion" Catalysts 8, no. 11: 510. https://doi.org/10.3390/catal8110510






