Ir-Based Bimetallic Catalysts for Hydrogen Production through Glycerol Aqueous-Phase Reforming
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.1.1. Physical Properties
2.1.2. X-ray Diffraction
2.1.3. Energy Dispersive Spectroscopy
2.1.4. X-ray Photoelectron Spectroscopy
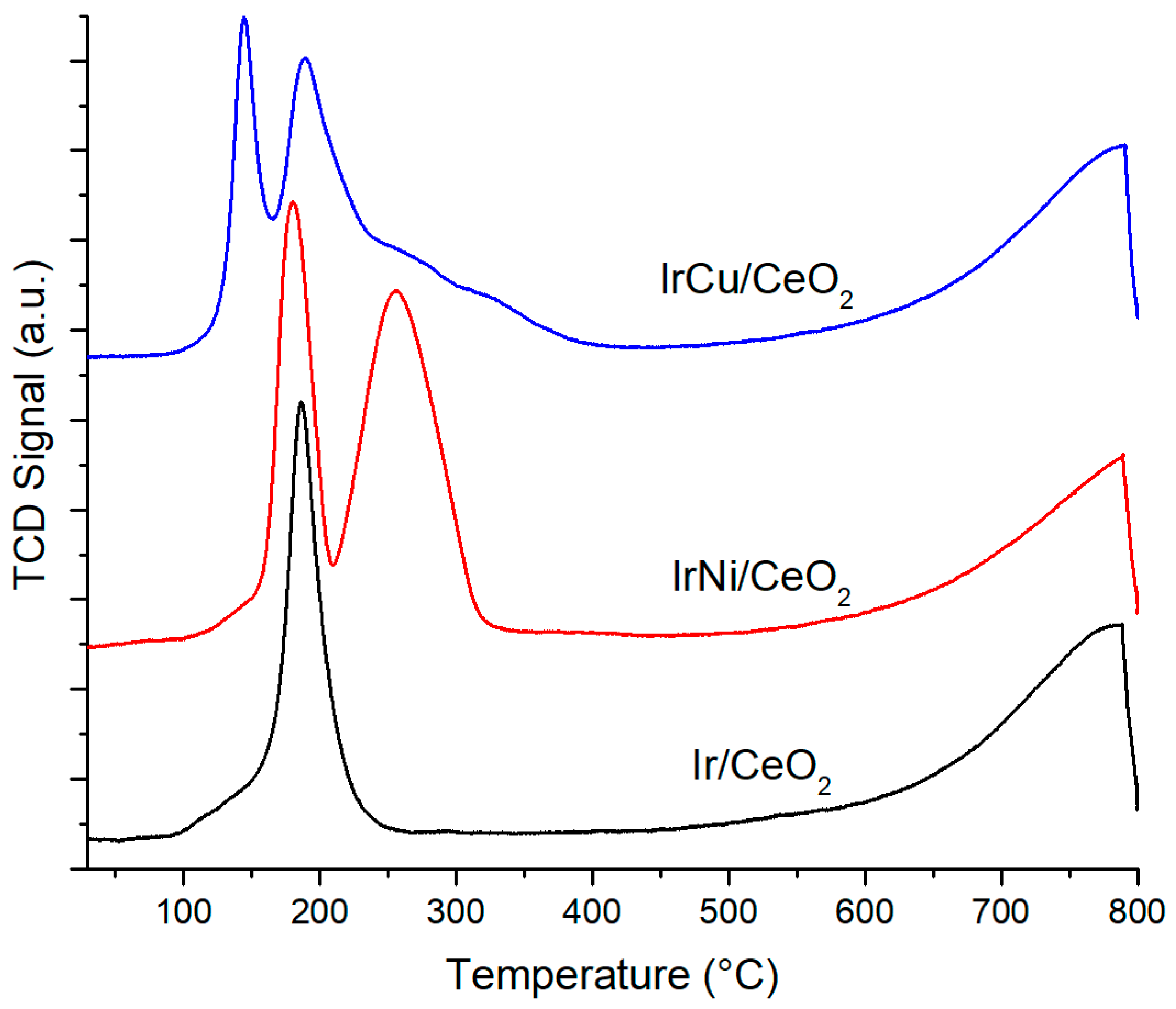
2.1.5. H2 Temperature Programmed Reduction
2.2. Catalytic Activity
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Catalysts Characterization
3.3. Activity Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newell, R.G.; Qian, Y.; Raimi, D. Global Energy Outlook 2015. 2016. Available online: http://www.nber.org/papers/w22075 (accessed on 29 March 2016).
- Council, W.E. World Energy Resources 2016. Available online: https://www.worldenergy.org/wp-content/uploads/2016/10/World-Energy-Resources-Full-report-2016.10.03.pdf (accessed on 25 April 2017).
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Chiari, L.; Zecca, A. Constraints of fossil fuels depletion on global warming projections. Energy Policy 2011, 39, 5026–5034. [Google Scholar] [CrossRef]
- Hoel, M.; Kverndokk, S. Depletion of fossil fuels and the impacts of global warming. Resour. Energy Econ. 1996, 18, 115–136. [Google Scholar] [CrossRef]
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Da Silva Veras, T.; Mozer, T.S.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrog. Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Sherif, S.A.; Barbir, F.; Veziroglu, T.N. Towards a Hydrogen Economy. Electr. J. 2005, 18, 62–76. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Cifuentes, B.; Valero, M.; Conesa, J.; Cobo, M. Hydrogen Production by Steam Reforming of Ethanol on Rh-Pt Catalysts: Influence of CeO2, ZrO2, and La2O3 as Supports. Catalysts 2015, 5, 1872–1896. [Google Scholar] [CrossRef] [Green Version]
- Mhadmhan, S.; Natewong, P.; Prasongthum, N.; Samart, C.; Reubroycharoen, P. Investigation of Ni/SiO2 Fiber Catalysts Prepared by Different Methods on Hydrogen production from Ethanol Steam Reforming. Catalysts 2018, 8, 319. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrog. Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Shabaker, J.W.; Huber, G.W.; Davda, R.R.; Cortright, R.D.; Dumesic, J.A. Aqueous-Phase Reforming of Ethylene Glycol Over Supported Platinum Catalysts. Catal. Lett. 2003, 88, 1–8. [Google Scholar] [CrossRef]
- Shabaker, J.W.; Davda, R.R.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Aqueous-phase reforming of methanol and ethylene glycol over alumina-supported platinum catalysts. J. Catal. 2003, 215, 344–352. [Google Scholar] [CrossRef]
- Joensen, F.; Rostrup-Nielsen, J.R. Conversion of hydrocarbons and alcohols for fuel cells. J. Power Sources 2002, 105, 195–201. [Google Scholar] [CrossRef]
- Soares, R.R.; Simonetti, D.A.; Dumesic, J.A. Glycerol as a Source for Fuels and Chemicals by Low-Temperature Catalytic Processing. Angew. Chem. 2006, 45, 3982–3985. [Google Scholar] [CrossRef]
- Özgür, D.Ö.; Uysal, B.Z. Hydrogen production by aqueous phase catalytic reforming of glycerine. Biomass Bioenergy 2011, 35, 822–826. [Google Scholar] [CrossRef]
- Miyao, T.; Watanabe, Y.; Teramoto, M.; Naito, S. Catalytic activity of various supported Ir–Re catalysts for liquid phase methanol reforming with water. Catal. Commun. 2005, 6, 113–117. [Google Scholar] [CrossRef]
- Sakamoto, T.; Miyao, T.; Yoshida, A.; Naito, S. Effect of Re and Mo addition upon liquid phase methanol reforming with water over SiO2, ZrO2 and TiO2 supported Ir catalysts. Int. J. Hydrog. Energy 2010, 35, 6203–6209. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Aqueous-phase reforming of ethylene glycol on silica-supported metal catalysts. Appl. Catal. B Environ. 2003, 43, 13–26. [Google Scholar] [CrossRef]
- Nozawa, T.; Yoshida, A.; Hikichi, S.; Naito, S. Effects of Re addition upon aqueous phase reforming of ethanol over TiO2 supported Rh and Ir catalysts. Int. J. Hydrog. Energy 2015, 40, 4129–4140. [Google Scholar] [CrossRef]
- Yang, G.; Yu, H.; Huang, X.; Peng, F.; Wang, H. Effect of calcium dopant on catalysis of Ir/La2O3 for hydrogen production by oxidative steam reforming of glycerol. Appl. Catal. B Environ. 2012, 127, 89–98. [Google Scholar] [CrossRef]
- Wang, F.; Cai, W.; Tana; Provendier, H.; Schuurman, Y.; Descorme, C.; Mirodatos, C.; Shen, W. Ageing analysis of a model Ir/CeO2 catalyst in ethanol steam reforming. Appl. Catal. B Environ. 2012, 125, 546–555. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, B.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production by oxidative steam reforming of ethanol over an Ir/CeO2 catalyst. Catal. Commun. 2007, 8, 1588–1594. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, W.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production by steam reforming of ethanol over an Ir/CeO2 catalyst: Reaction mechanism and stability of the catalyst. Int. J. Hydrog. Energy 2008, 33, 4377–4386. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; Zhan, E.; van Veen, A.C.; Mirodatos, C.; Shen, W. Hydrogen production from ethanol over Ir/CeO2 catalysts: A comparative study of steam reforming, partial oxidation and oxidative steam reforming. J. Catal. 2008, 257, 96–107. [Google Scholar] [CrossRef]
- Wang, F.; Cai, W.; Provendier, H.; Schuurman, Y.; Descorme, C.; Mirodatos, C.; Shen, W. Hydrogen production from ethanol steam reforming over Ir/CeO2 catalysts: Enhanced stability by PrOx promotion. Int. J. Hydrog. Energy 2011, 36, 13566–13574. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production from steam reforming of ethanol and glycerol over ceria-supported metal catalysts. Int. J. Hydrog. Energy 2007, 32, 2367–2373. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; Daniel, C.; van Veen, A.C.; Schuurman, Y.; Descorme, C.; Provendier, H.; Shen, W.; Mirodatos, C. Oxidative steam reforming of ethanol over Ir/CeO2 catalysts: A structure sensitivity analysis. J. Catal. 2012, 286, 137–152. [Google Scholar] [CrossRef]
- Fleming, P.; Farrell, R.A.; Holmes, J.D.; Morris, M.A. The Rapid Formation of La(OH)3 from La2O3 Powders on Exposureto Water Vapor. J. Am. Ceram. Soc. 2010, 93, 1187–1194. [Google Scholar] [CrossRef]
- Said, A.E.-A.A.; El-Wahab, M.M.M.A.; El-Aal, M.A. Catalytic dehydration of methanol to dimethyl ether over nanosized WO3/Al2O3 system under inert and oxidative atmosphere. Chem. Mon. 2016, 147, 1507–1516. [Google Scholar] [CrossRef]
- Thyssen, V.V.; Maia, T.A.; Assaf, E.M. Ni supported on La2O3–SiO2 used to catalyze glycerol steam reforming. Fuel 2013, 105, 358–363. [Google Scholar] [CrossRef]
- Atanasoska, L.; Gupta, P.; Deng, C.; Warner, R.; Larson, S.; Thompson, J. XPS, AES, and Electrochemical Study of Iridium Oxide Coating Materials for Cardiovascular Stent Application. ECS Trans. 2009, 16, 37–48. [Google Scholar] [CrossRef]
- XPS Simplified. Available online: http://xpssimplified.com/elements/nickel.php (accessed on 5 April 2018).
- Sunding, M.F.; Hadidi, K.; Diplas, S.; Løvvik, O.M.; Norby, T.E.; Gunnæs, A.E. XPS characterisation of in situ treated lanthanum oxide and hydroxide using tailored charge referencing and peak fitting procedures. J. Electron Spectrosc. Relat. Phenom. 2011, 184, 399–409. [Google Scholar] [CrossRef]
- Lebugle, A.; Axelsson, U.; Nyholm, R.; Mårtensson, N. Experimental L and M Core Level Binding Energies for the Metals 22Ti to 30Zn. Phys. Scr. 1981, 23, 825. [Google Scholar] [CrossRef]
- Miller, A.C.; Simmons, G.W. Nickel by XPS. Surf. Sci. Spectra 1992, 1, 312–317. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Yao, H.C.; Yao, Y.F.Y. Ceria in automotive exhaust catalysts: I. Oxygen storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Harrison, B.; Diwell, A.F.; Hallett, C. Promoting Platinum Metals by Ceria. Platin. Met. Rev. 1988, 32, 73–83. [Google Scholar]
- Jha, A.; Jeong, D.-W.; Jang, W.-J.; Rode, C.V.; Roh, H.-S. Mesoporous NiCu–CeO2 oxide catalysts for high-temperature water–gas shift reaction. RSC Adv. 2015, 5, 1430–1437. [Google Scholar] [CrossRef]
- Chen, H.; Yu, H.; Tang, Y.; Pan, M.; Yang, G.; Peng, F.; Wang, H.; Yang, J. Hydrogen production via autothermal reforming of ethanol over noble metal catalysts supported on oxides. J. Nat. Gas Chem. 2009, 18, 191–198. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Orozco, J.; Moronta, D.; Fontal, B.; Imbert, F.E. Methane Conversion Over Sr2+/La2O3 Catalyst Modified with Nickel and Copper. React. Kinet. Catal. Lett. 2000, 69, 145–152. [Google Scholar] [CrossRef]
- Dokjampa, S.; Rirksomboon, T.; Phuong, D.T.M.; Resasco, D.E. Ring opening of 1,3-dimethylcyclohexane on Ir catalysts: Modification of product distribution by addition of Ni and K to improve fuel properties. J. Mol. Catal. A Chem. 2007, 274, 231–240. [Google Scholar] [CrossRef]
- Guo, Y.; Wan, T.; Zhu, A.; Shi, T.; Zhang, G.; Wang, C.; Yu, H.; Shao, Z. Performance and durability of a layered proton conducting solid oxide fuel cell fueled by the dry reforming of methane. RSC Adv. 2017, 7, 44319–44325. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Wang, F.; Tana; Li, Y.; Huang, X.; Shen, W. A highly efficient Cu/La2O3 catalyst for transfer dehydrogenation of primary aliphatic alcohols. Green Chem. 2010, 12, 108–113. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Ma, H.; Xu, Z.; Tian, Z. Production of hydrogen by aqueous-phase reforming of glycerol. Int. J. Hydrog. Energy 2008, 33, 6657–6666. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Azmat, M.U.; Xu, W.; Ren, J.; Wang, Y.; Lu, G. Hydrogen production by aqueous-phase reforming of glycerol over Ni-B catalysts. Int. J. Hydrog. Energy 2012, 37, 227–234. [Google Scholar] [CrossRef]
- Ni, J.; Chen, L.; Lin, J.; Schreyer, M.K.; Wang, Z.; Kawi, S. High performance of Mg–La mixed oxides supported Ni catalysts for dry reforming of methane: The effect of crystal structure. Int. J. Hydrog. Energy 2013, 38, 13631–13642. [Google Scholar] [CrossRef]
- Han, X.; Chu, W.; Ni, P.; Luo, S.-Z.; Zhang, T. Promoting effects of iridium on nickel based catalyst in ammonia decomposition. J. Fuel Chem. Technol. 2007, 35, 691–695. [Google Scholar] [CrossRef]
- Manfro, R.L.; da Costa, A.F.; Ribeiro, N.F.P.; Souza, M.M.V.M. Hydrogen production by aqueous-phase reforming of glycerol over nickel catalysts supported on CeO2. Fuel Process. Technol. 2011, 92, 330–335. [Google Scholar] [CrossRef]
- Luo, N.; Fu, X.; Cao, F.; Xiao, T.; Edwards, P.P. Glycerol aqueous phase reforming for hydrogen generation over Pt catalyst—Effect of catalyst composition and reaction conditions. Fuel 2008, 87, 3483–3489. [Google Scholar] [CrossRef]














| Catalyst | SBET/m2 g−1 | Vp/cm3 g–1 | Pore Size (Average Radius/Å) |
|---|---|---|---|
| La2O3 | 9.1 ± 0.5 | (2.5 ± 0.3) × 10−2 | 55.4 ± 0.1 |
| Ir/La2O3 | 10.2 ± 0.6 | (2.2 ± 0.2) × 10−2 | 42.5 ± 0.1 |
| IrCu/La2O3 | 6.7 ± 0.4 | (1.3 ± 0.1) × 10−2 | 39.2 ± 0.1 |
| IrNi/La2O3 | 10.1 ± 0.6 | (2.4 ± 0.3) × 10−2 | 47.0 ± 0.1 |
| CeO2 | 7.7 ± 0.5 | (2.8 ± 0.3) × 10−2 | 67.8 ± 0.1 |
| Ir/CeO2 | 4.2 ± 0.2 | (6.7 ± 0.7) × 10−3 | 32.1 ± 0.2 |
| IrCu/CeO2 | 5.3 ± 0.3 | (7.9 ± 0.8) × 10−3 | 29.8 ± 0.2 |
| IrNi/CeO2 | 4.8 ± 0.3 | (8.5 ± 0.9) × 10−3 | 35.7 ± 0.2 |
| Catalyst | Ir | Ni | Cu |
|---|---|---|---|
| Ir/La2O3 | 3.3 | - | - |
| IrNi/La2O3 | 2.8 | 2.2 | - |
| IrCu/La2O3 | 2.8 | - | 2.5 |
| Ir/CeO2 | 3.4 | - | - |
| IrNi/CeO2 | 3.4 | 2 | - |
| IrCu/CeO2 | 2.4 | - | 2.7 |
| Catalyst | Yield Rate of H2 Production (µmol min−1 gcat−1) | Glycerol Conversion (%) | S-H2 (%) | S-CH4 (%) | S-CO (%) | S-CO2 (%) | C to GAS Conv. (%) |
|---|---|---|---|---|---|---|---|
| Ir/La2O3 | 21.2 | 58.2 | 95.4 | 48.8 | 8.8 | 42.4 | 0.7 |
| IrCu/La2O3 | 16.9 | 76.5 | 54.5 | 37.1 | 7.3 | 55.6 | 0.9 |
| IrNi/La2O3 | 317.7 | 92.0 | 23.1 | 40.8 | 11.0 | 48.2 | 43.6 |
| Ir/CeO2 | 16.0 | 52.6 | 39.3 | 40.6 | 9.0 | 50.4 | 1.3 |
| IrCu/CeO2 | 4.7 | 35.5 | 23.7 | 43.3 | 9.4 | 473. | 0.6 |
| IrNi/CeO2 | 162.9 | 55.7 | 34.8 | 27.8 | 14.7 | 57.5 | 14.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Moreno, F.; Balla, P.; Shen, W.; Chavarria-Hernandez, J.C.; Ruiz-Gómez, M.; Tlecuitl-Beristain, S. Ir-Based Bimetallic Catalysts for Hydrogen Production through Glycerol Aqueous-Phase Reforming. Catalysts 2018, 8, 613. https://doi.org/10.3390/catal8120613
Espinosa-Moreno F, Balla P, Shen W, Chavarria-Hernandez JC, Ruiz-Gómez M, Tlecuitl-Beristain S. Ir-Based Bimetallic Catalysts for Hydrogen Production through Glycerol Aqueous-Phase Reforming. Catalysts. 2018; 8(12):613. https://doi.org/10.3390/catal8120613
Chicago/Turabian StyleEspinosa-Moreno, Francisco, Putrakumar Balla, Wenjie Shen, Juan C. Chavarria-Hernandez, Miguel Ruiz-Gómez, and Saúl Tlecuitl-Beristain. 2018. "Ir-Based Bimetallic Catalysts for Hydrogen Production through Glycerol Aqueous-Phase Reforming" Catalysts 8, no. 12: 613. https://doi.org/10.3390/catal8120613





