Characterization of Highly Dispersed Rod- and Particle-Shaped CuFe19Ox Catalysts and Their Shape Effects on WGS
Abstract
:1. Introduction
2. Results and Discussion
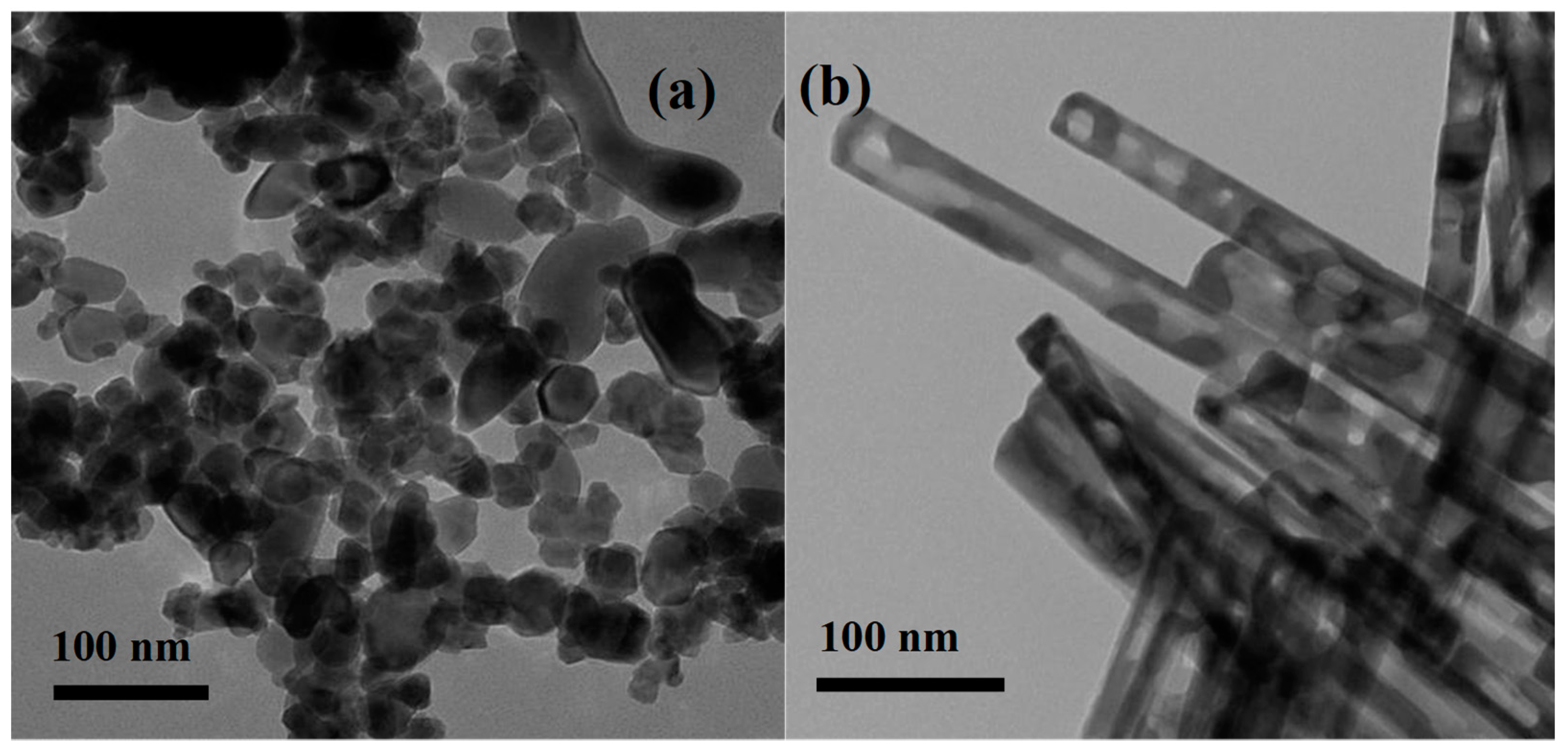
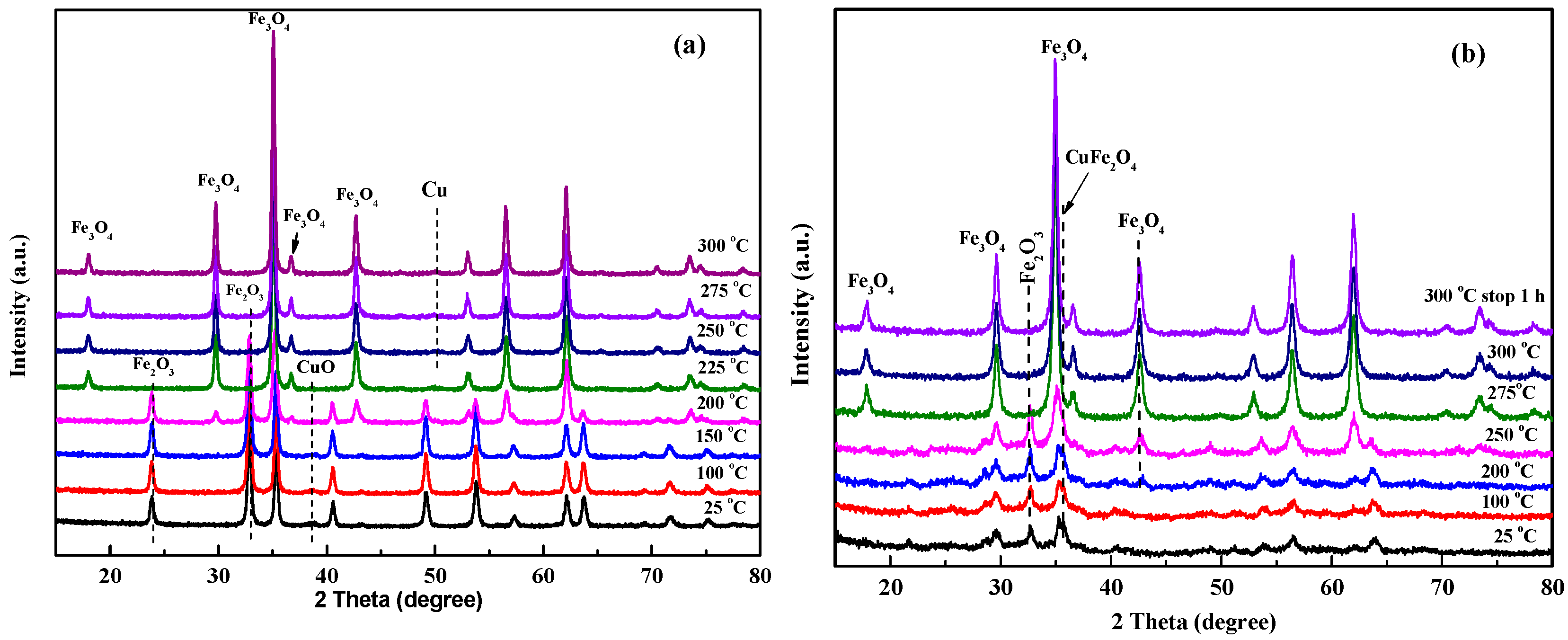
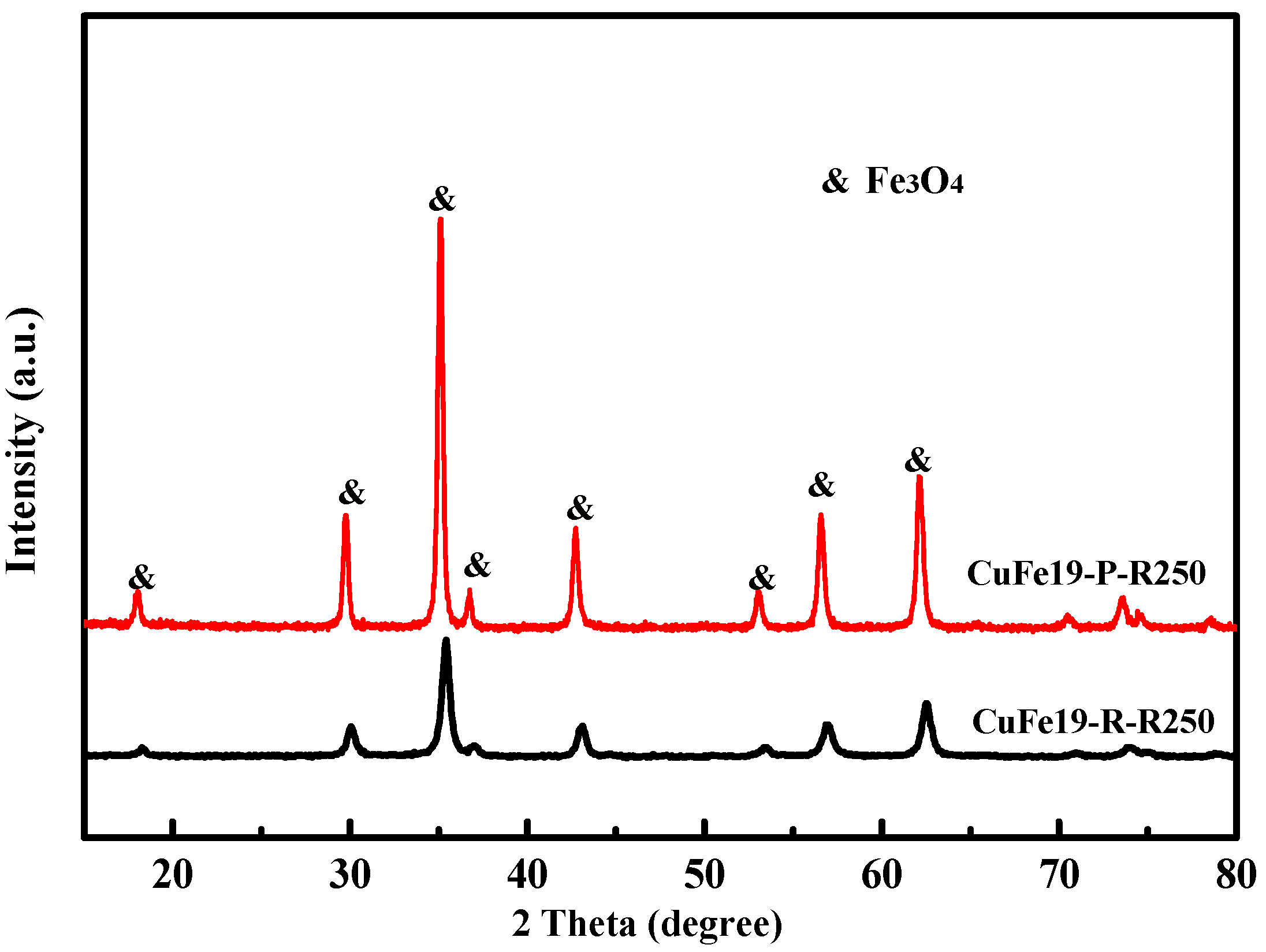
2.1. Characterization of Structures and Shapes of CuFe19Ox Catalysts
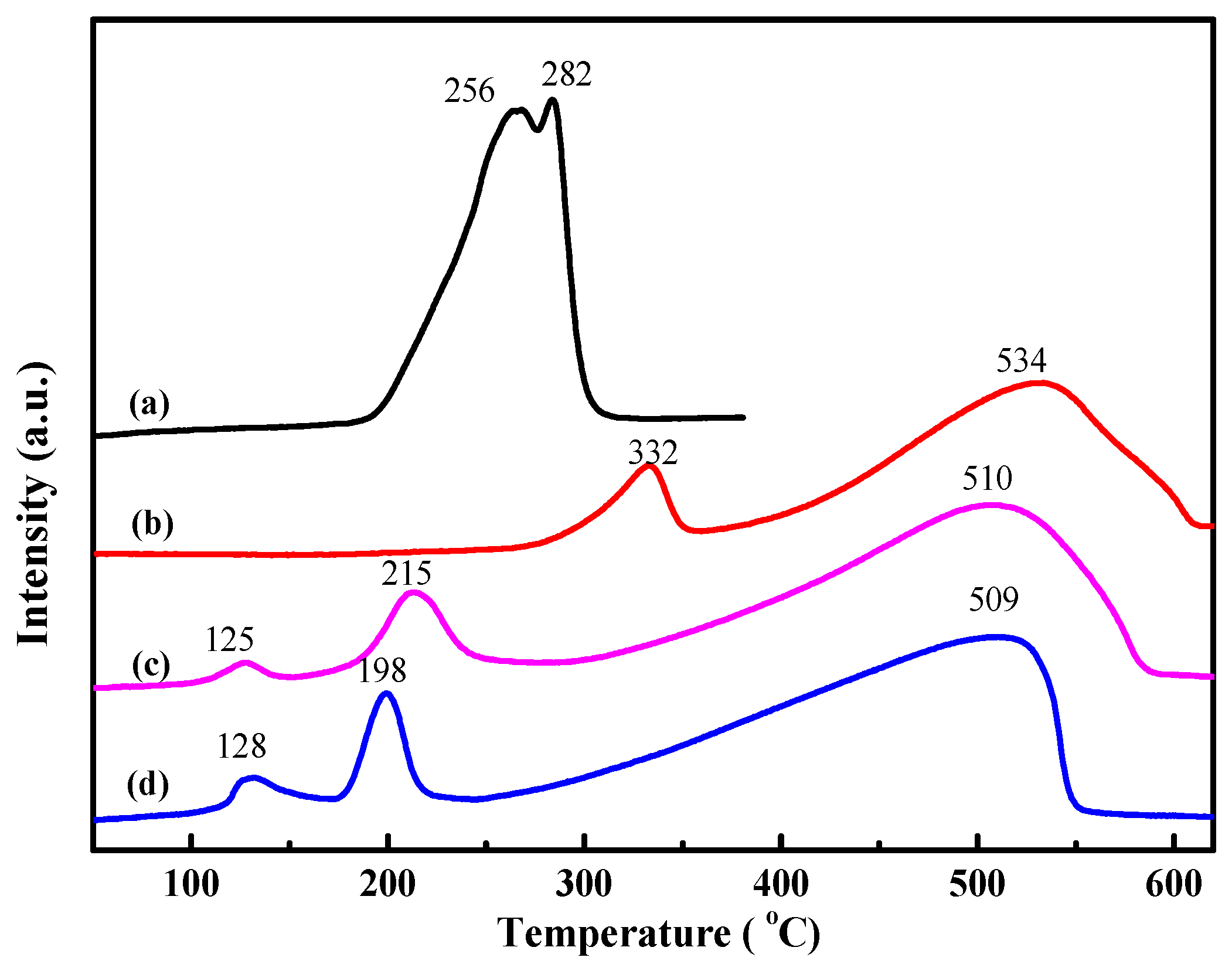
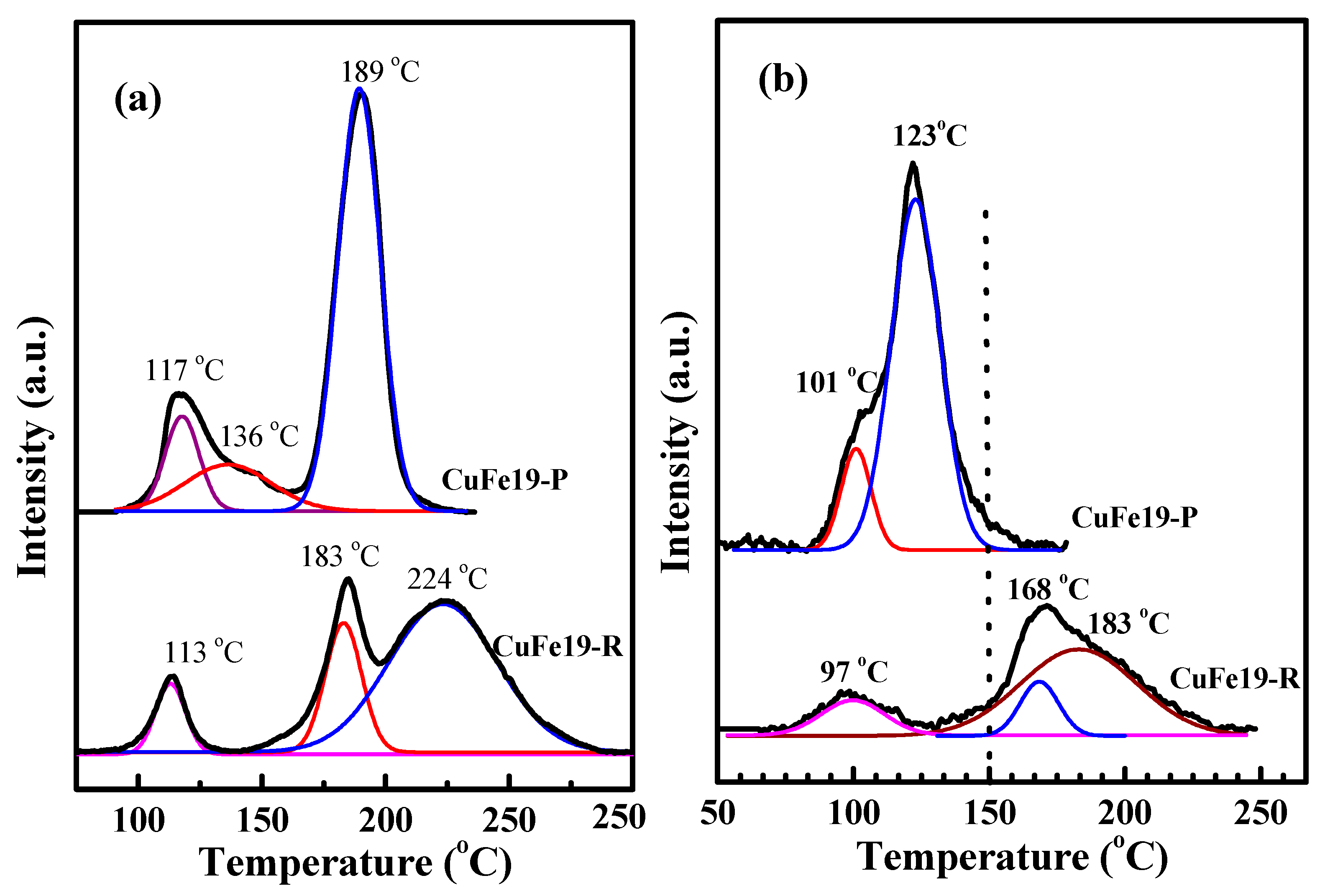
2.2. Reduction Behavior of CuFe19-P and CuFe19-R
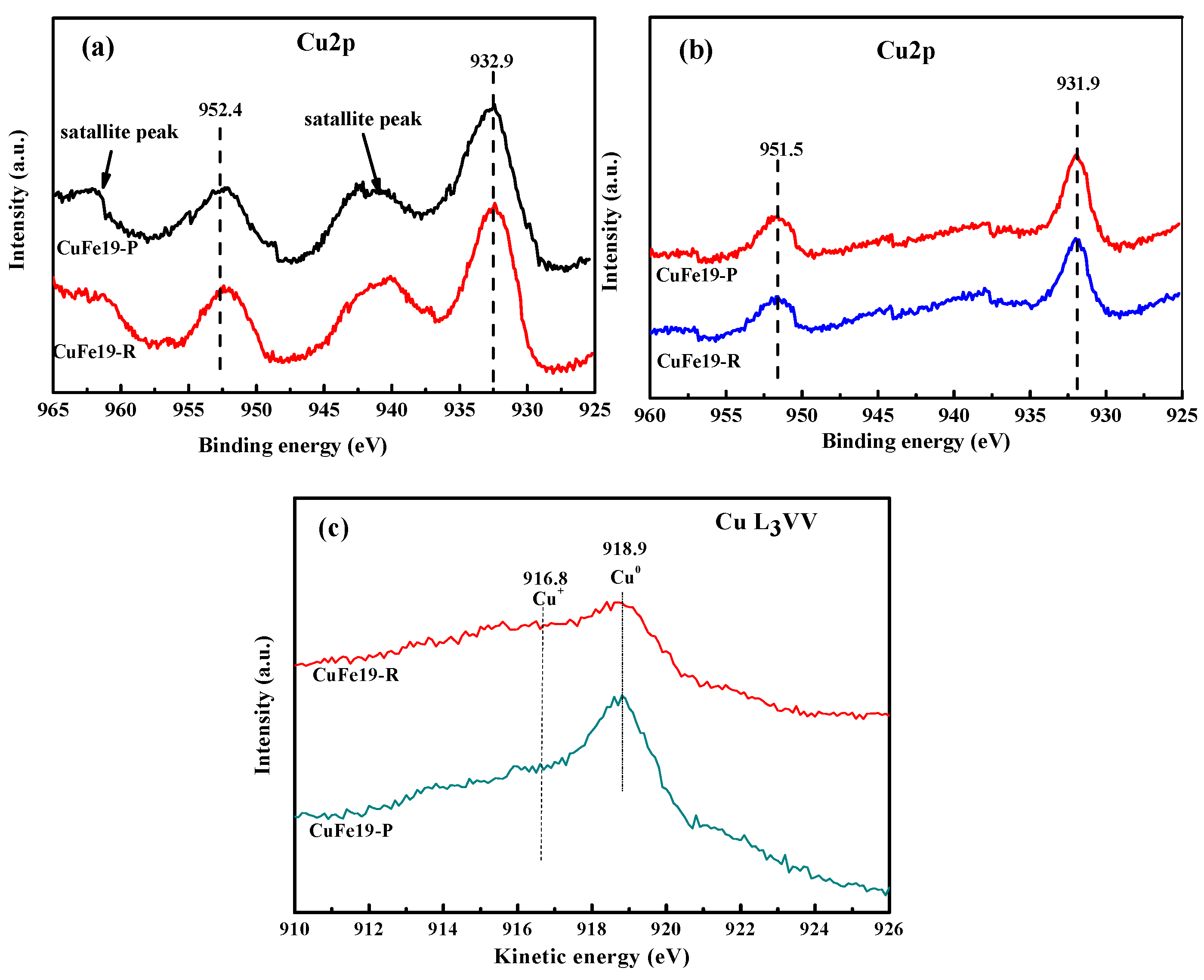
2.3. Dispersion and Surface Cu Species
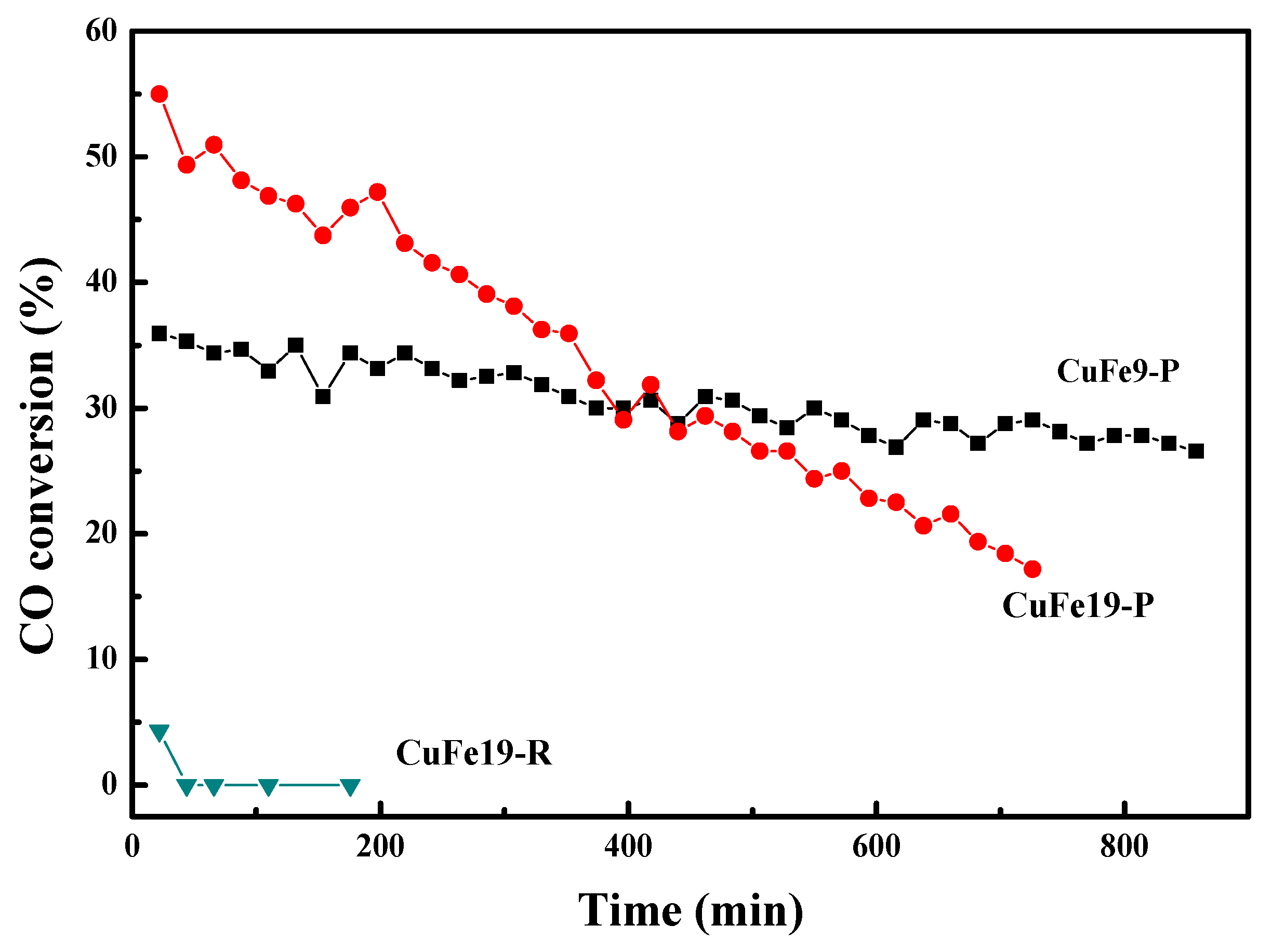
2.4. WGS Activity Test
3. Experiment
3.1. Synthesis of Catalysts
3.2. Characterization of Catalysts
3.3. Catalyst Pretreatment and Testing
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Li, Y.; Shen, W. Morphology-dependent nanocatalysis on metal oxides. Sci. Chi. Chem. 2012, 55, 2485–2496. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, W.; Qi, G.; Weng, D. Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J. Catal. 2012, 289, 21–29. [Google Scholar] [CrossRef]
- Konsolakis, M. The role of copper–ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Reina, T.R.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Boosting the activity of a Au/CeO2/Al2O3 catalyst for the WGS reaction. Catal. Today 2015, 253, 149–154. [Google Scholar] [CrossRef]
- Santosa, J.L.; Reinaa, T.R.; Ivanovaa, S.; Centenoa, M.A.; Odriozolaa, J.A. Gold promoted Cu/ZnO/Al2O3 catalysts prepared from hydrotalcite precursors: Advanced materials for the WGS reaction. Appl. Catal. B Environ. 2017, 201, 310–317. [Google Scholar] [CrossRef]
- Estrella, M.; Barrio, L.; Zhou, G.; Wang, X.; Wang, Q.; Wen, W.; Hanson, J.C.; Frenkel, A.I.; Rodriguez, J.A. In Situ Characterization of CuFe2O4 and Cu/Fe3O4 water−gas shift catalysts. J. Phys. Chem. C 2009, 113, 14411–14417. [Google Scholar] [CrossRef]
- Yahiro, H.; Sagata, K.; Yamamoto, T.; Saiki, K.; Asamoto, M.; Yamaura, H. Promotion effect of FeOx addition on the catalytic activity of supported Cu catalysts for the water-gas shift reaction. Catal. Lett. 2008, 124, 233–237. [Google Scholar] [CrossRef]
- Lin, X.; Li, R.; Zhang, Y.; Zhan, Y.; Chen, C.; Zheng, Q.; Ma, J. The role of surface copper species in CueFe composite oxide catalysts for the water gas shift reaction. Int. J. Hydrog. Energy 2015, 40, 1735–1741. [Google Scholar] [CrossRef]
- Lin, X.; Li, R.; Zhang, Y.; Zhan, Y.; Chen, C.; Zheng, Q.; Ma, J. Characterization and catalytic performance of copper-based WGS catalysts derived from copper ferrite. Int. J. Hydrog. Energy 2014, 39, 6424–6432. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 oxides. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Yan, H.; Qin, X.; Yin, Y.; Teng, Y.; Jin, Z.; Jia, C. Promoted Cu-Fe3O4 catalysts for low-temperature water gas shift reaction: Optimization of Cu content. Appl. Catal. B 2018, 226, 182–193. [Google Scholar] [CrossRef]
- Kameoka, S.; Tanabe, T.; Tsai, A.P. Spinel CuFe2O4: A precursor for copper catalyst with high thermal stability and activity. Catal. Lett. 2005, 100, 89–93. [Google Scholar] [CrossRef]
- Yang, S.C.; Su, W.N.; Lin, S.D.; Rick, J.; Cheng, J.H.; Liu, J.Y.; Pan, C.J.; Liu, D.G.; Lee, J.F.; Chan, T.S.; et al. Preparation of nano-sized Cu from a rod-like CuFe2O4: Suitable for high performance catalytic applications. Appl. Catal. B 2011, 106, 650–656. [Google Scholar] [CrossRef]
- Hou, M.; Ma, L.; Ma, H.; Yue, M. In situ characterization of Cu-Fe-Ox catalyst for water–gas shift reaction. J. Mater. Sci. 2018, 53, 1065–1075. [Google Scholar] [CrossRef]
- Ma, H.; Ma, L.; Hou, M.; Yue, M. Rod-like CuFe2O4 composite: Controllable synthesis and catalytic performance in isoamylic alcohol dehydrogenation. Chin. J. Inorg. Chem. 2017, 33, 2193–2200. [Google Scholar]
- Hoang, D.L.; Dang, T.T.H.; Engeldinger, J.; Schneider, M.; Radnik, J.; Richter, M.; Martin, A. TPR investigations on the reducibility of Cu supported on Al2O3, zeolite Y and SAPO-5. J. Solid State Chem. 2011, 184, 1915–1923. [Google Scholar] [CrossRef]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.; Shen, W. Rod-shaped Fe2O3 as an efficient catalyst for the selective reduction of nitrogen oxide by ammonia. Angew. Chem. Int. Ed. 2012, 51, 2989–2993. [Google Scholar] [CrossRef]
- Lohitharn, N.; James, G.; Goodwin, J. Impact of Cr, Mn and Zr addition on Fe Fischer–Tropsch synthesis catalysis: Investigation at the active site level using SSITKA. J. Catal. 2008, 257, 142–151. [Google Scholar] [CrossRef]
- Mo, L.; Saw, E.T.; Du, Y.; Borgna, A.; Ang, M.; Kathiraser, Y.; Li, Z.; Thitsartarn, W.; Lin, M.; Kawi, S. Highly dispersed supported metal catalysts prepared via in-situ self-assembled core-shell precursor route. Int. J. Hydrog. Energy 2015, 40, 13388–13398. [Google Scholar] [CrossRef]
- Kameoka, S.; Tanabe, T.; Tsai, A.P. Self-assembled porous nano-composite with high catalytic performance by reduction of tetragonal spinel CuFe2O4. Appl. Catal. A Gen. 2010, 375, 163–171. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Li, Y.; Xu, Y.; Xin, Q.; Shen, W. CuO/CeO2 catalysts: Redox features and catalytic behaviors. Appl. Catal. A Gen. 2005, 288, 116–125. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, K.; Zhang, L.; Qin, B.; Chen, T.; Yin, Y.; Su, H. Deactivation analyses of CeO2/CuO catalysts in the preferential oxidation of carbon monoxide. J. Power. Sources 2014, 261, 46–54. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.J.; Liu, L.J.; Zhang, H.L.; Gao, F.; Dong, L.; Chen, Y. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl. Catal. B 2012, 119–120, 308–320. [Google Scholar] [CrossRef]
- Ma, L.; Ma, H.; Han, D.; Qiu, M.; Guan, Y.; Hu, Y. Evolution of copper supported on Fe3O4 nanorods for WGS reaction. Catalysts 2018, 8, 415. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Yuma, K.; Obata, Y. Distinction between surface and bulk oxidation of Cu through N2O decomposition. J. Catal. 2000, 196, 195–199. [Google Scholar] [CrossRef]
- Gervasini, A.; Bennici, S. Dispersion and surface states of copper catalysts by temperature-programmed-reduction of oxidized surfaces (s-TPR). Appl. Catal. A Gen. 2005, 281, 199–205. [Google Scholar] [CrossRef]
- Grift, C.J.G.V.D.; Wielers, A.F.H.; Jogh, B.P.J.; Beunum, J.V.; Boer, M.D.; Versluijs-Helder, M.; Geus, J.W. Effect of the reduction treatment on the structure and reactivity of silica-support copper particles. J. Catal. 1991, 131, 178–189. [Google Scholar]
- Yuan, Z.L.; Wang, L.N.; Wang, J.H.; Xia, S.X.; Chen, P.; Hou, Z.Y.; Zheng, X.M. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts. Appl. Catal. B 2011, 101, 431–440. [Google Scholar] [CrossRef]


| Samples | Shape | Cu/(Cu+Fe) (ICP) | BET Surface Area (m2/g) |
|---|---|---|---|
| CuFe19-P | nanoparticle | 5.8% | 25 |
| CuFe19-R | nanorod | 6.1% | 45 |
| Samples | Peak Position (°C) | Peak Area | Peak Sum |
|---|---|---|---|
| CuFe19-P | 101 | 0.021 | 0.191 |
| 123 | 0.17 | ||
| CuFe19-R | 100 | 0.0226 | 0.147 |
| 168 | 0.0213 | ||
| 183 | 0.103 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Han, D.; Ma, H.; Liu, L.; Guo, H. Characterization of Highly Dispersed Rod- and Particle-Shaped CuFe19Ox Catalysts and Their Shape Effects on WGS. Catalysts 2018, 8, 635. https://doi.org/10.3390/catal8120635
Ma L, Han D, Ma H, Liu L, Guo H. Characterization of Highly Dispersed Rod- and Particle-Shaped CuFe19Ox Catalysts and Their Shape Effects on WGS. Catalysts. 2018; 8(12):635. https://doi.org/10.3390/catal8120635
Chicago/Turabian StyleMa, Lingjuan, Dawei Han, Hongbin Ma, Longgang Liu, and Huichao Guo. 2018. "Characterization of Highly Dispersed Rod- and Particle-Shaped CuFe19Ox Catalysts and Their Shape Effects on WGS" Catalysts 8, no. 12: 635. https://doi.org/10.3390/catal8120635





