Application of Catalytic Wet Peroxide Oxidation for Industrial and Urban Wastewater Treatment: A Review
Abstract
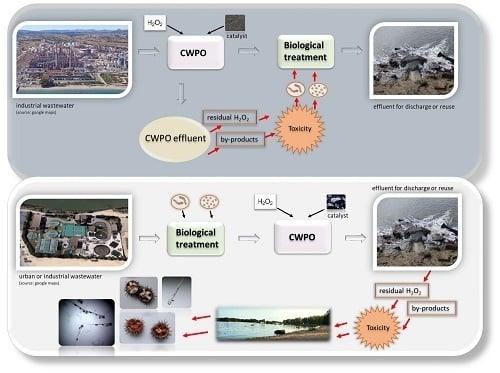
:1. Introduction
2. Main Principles and Mechanism of CWPO
- (1)
- Increasing the quality of the industrial or urban wastewater effluent. In the final step of the wastewater treatment process, CWPO is able to remove residual contaminants, such as persistent toxic endocrine-disruption or refractory compounds, and to increase the quality of the treated effluent for water reuse or safe discharge.
- (2)
- Increasing the biodegradability of industrial wastewater. In this case, CWPO can be applied before the biological process in order to increase the biodegradability of recalcitrant compounds and their suitability for biological treatment (conventional or not). It is important to mention that only non-biodegradable wastewaters are suitable for CWPO. The CWPO followed by biological processes can enhance the efficiency of the biological process and the viability of treatment from an economic point of view [35].
3. CWPO for the Enhancement of Industrial Wastewater Biodegradability
3.1. Catalysts
3.2. Temperature
3.3. Effect of Initial Concentration of Organic Pollutants in Wastewater
3.4. Effect of pH
3.5. Effect of H2O2 Concentration
3.6. Toxicity
3.7. Cost Estimation
4. CWPO as a Post-treatment Step for Urban and Industrial Wastewater Effluents
4.1. Catalysts
- High stability in wide temperature range;
- Stability under different pH conditions;
- High surface area;
- No leaching;
- Efficient for decomposition of H2O2; and,
- Low cost.
4.2. Temperature and pH
4.3. H2O2 Concentration and Toxicity
4.4. Cost Estimation
5. Conclusions, Knowledge Gaps, and Future Perspectives
- Metal leaching and deactivation (e.g. due to mechanical and thermal degradation, poisoning, fouling, etc.) are among the main drawbacks of iron-based catalysts for practical application of CWPO. Based on revised literature it can be suggested that carbon materials are among the most promising catalysts for the practical application of CWPO for wastewater treatment. Properties of carbon materials, such as stability in a wide range of pH and temperature, high surface area, absence of leaching, possibility to control some surface properties, and relatively low cost of catalysts [74], makes them especially attractive for application.
- It can be expected that the elimination of emerging pollutants and the decrease of toxicity of municipal wastewater effluents by CWPO can be very efficient. However, there is a lack of studies that are devoted to the application of CWPO as post-treatment for municipal wastewater effluents.
- To the best of our knowledge, there is a lack of data on the toxicity assessment of wastewater during the CWPO process. Moreover, in all studies dealing with CWPO for the treatment of wastewater, only acute toxicity bioassays were used.
- Cost estimation is very important to the evaluation of CWPO feasibility for wastewater treatment. Cost assessment was reported in only a few studies (some reviewed in this work). Interestingly, cost evaluation was reported only when CWPO was conducted at an ambient temperature and the natural pH of wastewater.
- Despite the fact that CWPO was shown to be a promising treatment method, majority of studies with industrial or urban wastewaters were conducted at the laboratory scale. Moreover, among the revised studies, mostly batch or semi-batch reactors were used, while continuous catalytic systems, such as fixed bed reactors, were less studied. Taking into account that fixed bed reactors are promising from the practical point of view (especially for recovery and reuse of catalyst) and the reaction mechanism in batch and fixed bed reactors may vary due to different ratio between catalyst and water [75], it can be expected that in the future these will be more studied. Catalysts with magnetic properties can also be of high interest for the practical application of CWPO for wastewater treatment, which is mainly due to the simplicity of catalyst separation after treatment. However, investigations that are focused on industrial wastewater treatment by CWPO catalysed by magnetic catalysts are lacking.
Author Contributions
Conflicts of Interest
References
- European Commission. Water Scarcity & Droughts in the European Union; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- WWAP. The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource; UNESCO: Paris, France, 2017. [Google Scholar]
- European Commission. Directive 2000/60/EC; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Ec-European Commission. A Blueprint to Safeguard Europe’s Water Resources, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; Ec-European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Jing, R.; Fusi, S.; Chan, A.; Capozzi, S.; Kjellerup, B.V. Distribution of polychlorinated biphenyls in effluent from a large municipal wastewater treatment plant: Potential for bioremediation? J. Environ. Sci. 2018. [CrossRef]
- Qiao, M.; Bai, Y.; Cao, W.; Huo, Y.; Zhao, X.; Liu, D.; Li, Z. Impact of secondary effluent from wastewater treatment plants on urban rivers: Polycyclic aromatic hydrocarbons and derivatives. Chemosphere 2018, 211, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lara-Martín, P.A.; González-Mazo, E.; Petrovic, M.; Barceló, D.; Brownawell, B.J. Occurrence, distribution and partitioning of nonionic surfactants and pharmaceuticals in the urbanised Long Island Sound Estuary (NY). Mar. Pollut. Bull. 2014, 85, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. Atmospheric pressure gas chromatography–time-of-flight-mass spectrometry (APGC–ToF-MS) for the determination of regulated and emerging contaminants in aqueous samples after stir bar sorptive extraction (SBSE). Anal. Chim. Acta 2014, 851, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Thai, P.K.; Ky, L.X.; Binh, V.N.; Nhung, P.H.; Nhan, P.T.; Hieu, N.Q.; Dang, N.T.T.; Tam, N.K.B.; Anh, N.T.K. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi. Vietnam. Sci. Total Environ. 2018, 645, 393–400. [Google Scholar] [CrossRef] [PubMed]
- François, G.; Mélanie, D.; Marlène, F.; Michel, F. Effects of a municipal effluent on the freshwater mussel Elliptio complanata following challenge with Vibrio anguillarum. J. Environ. Sci. 2015, 37, 91–99. [Google Scholar] [CrossRef]
- Deblonde, T.; Hartemann, P. Environmental impact of medical prescriptions: Assessing the risks and hazards of persistence, bioaccumulation and toxicity of pharmaceuticals. Public Health 2013, 127, 312–317. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Catalytic wet peroxide oxidation: A route towards the application of hybrid magnetic carbon nanocomposites for the degradation of organic pollutants. A review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering. Treatment and Reuse; McGraw-Hill Higher Education: Singapore, 2004. [Google Scholar]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment II: Hybrid methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Diaz de Tuesta, J.L.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. P-, B- and N-doped carbon black for the catalytic wet peroxide oxidation of phenol: Activity, stability and kinetic studies. Catal. Commun. 2017, 102, 131–135. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Combining efficiently catalytic hydrodechlorination and wet peroxide oxidation (HDC–CWPO) for the abatement of organochlorinated water pollutants. Appl. Catal. B Environ. 2014, 150, 197–203. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Improved wet peroxide oxidation strategies for the treatment of chlorophenols. Chem. Eng. J. 2013, 228, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Rashwan, W.E.; Fathy, N.A.; Elkhouly, S.M. A novel catalyst of ceria-nanorods loaded on carbon xerogel for catalytic wet oxidation of methyl green dye. J. Taiwan Inst. Chem. Eng. 2018, 88, 234–242. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Carabineiro, S.A.C.; Maldonado-Hódar, F.J.; Madeira, L.M. Wet peroxide oxidation of dye-containing wastewaters using nanosised Au supported on Al2O3. Catal. Today 2017, 280, 165–175. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Gharagozlou, M.; Emami, F. Catalytic wet peroxide oxidation of a reactive dye by magnetic copper ferrite nanoparticles. J. Environ. Chem. Eng. 2016, 4, 1530–1536. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. The role of cobalt in bimetallic iron-cobalt magnetic carbon xerogels developed for catalytic wet peroxide oxidation. Catal. Today 2017, 296, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Liu, Y.; Zhang, C.; Wan, J.; Hu, L.; Zhou, C.; Xiong, W. Efficient degradation of sulfamethazine in simulated and real wastewater at slightly basic pH values using Co-SAM-SCS/H2O2 Fenton-like system. Water Res. 2018, 138, 7–18. [Google Scholar] [CrossRef]
- Munoz, M.; Mora, F.J.; de Pedro, Z.M.; Alvarez-Torrellas, S.; Casas, J.A.; Rodriguez, J.J. Application of CWPO to the treatment of pharmaceutical emerging pollutants in different water matrices with a ferromagnetic catalyst. J. Hazard. Mater. 2017, 331, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Mena, I.F.; Diaz, E.; Moreno-Andrade, I.; Rodriguez, J.J.; Mohedano, A.F. Stability of carbon-supported iron catalysts for catalytic wet peroxide oxidation of ionic liquids. J. Environ. Chem. Eng. 2018, 6, 6444–6450. [Google Scholar] [CrossRef]
- Bedia, J.; Monsalvo, V.M.; Rodriguez, J.J.; Mohedano, A.F. Iron catalysts by chemical activation of sewage sludge with FeCl3 for CWPO. Chem. Eng. J. 2017, 318, 224–230. [Google Scholar] [CrossRef]
- Gosu, V.; Sikarwar, P.; Subbaramaiah, V. Mineralisation of pyridine by CWPO process using nFe0/GAC catalyst. J. Environ. Chem. Eng. 2018, 6, 1000–1007. [Google Scholar] [CrossRef]
- Mohedano, A.F.; Monsalvo, V.M.; Bedia, J.; Lopez, J.; Rodriguez, J.J. Highly stable iron catalysts from sewage sludge for CWPO. J. Environ. Chem. Eng. 2014, 2, 2359–2364. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Rizzo, L. Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 2011, 45, 4311–4340. [Google Scholar] [CrossRef]
- Blanco-Galvez, J.; Fernández-Ibáñez, P.; Malato-Rodríguez, S. Solar Photocatalytic Detoxification and Disinfection of Water: Recent Overview. J. Sol. Energy Eng. 2006, 129, 4–15. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, A.; Zazo, J.; Casas, J.; Bahamonde, A.; Rodriguez, J. Influence of the structural and surface characteristics of activated carbon on the catalytic decomposition of hydrogen peroxide. Appl. Catal. A Gen. 2011, 402, 146–155. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, Q.; Jérôme, F.; Duprez, D.; Zhang, H.; Royer, S. Shape-controlled nanostructured magnetite-type materials as highly efficient Fenton catalysts. Appl. Catal. B Environ. 2014, 144, 739–749. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.; Faria, P.; Órfão, J.J. Decolourisation of dye solutions by oxidation with H2O2 in the presence of modified activated carbons. J. Hazard. Mater. 2009, 162, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lu, M.; Chen, J.; Lee, C. Catalytic decomposition of hydrogen peroxide and 4-chlorophenol in the presence of modified activated carbons. Chemosphere 2003, 51, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Lücking, F.; Köser, H.; Jank, M.; Ritter, A. Iron powder, graphite and activated carbon as catalysts for the oxidation of 4-chlorophenol with hydrogen peroxide in aqueous solution. Water Res. 1998, 32, 2607–2614. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Pintado-Herrera, M.G.; Martín-Díaz, M.L.; Acevedo-Merino, A.; Manzano, M.A. Combined AOPs for potential wastewater reuse or safe discharge based on multi-barrier treatment (microfiltration-H2O2/UV-catalytic wet peroxide oxidation). Chem. Eng. J. 2015, 270, 80–90. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Sillanpää, M.; Pocostales, P.; Acevedo, A.; Manzano, M.A. Post-treatment of biologically treated wastewater containing organic contaminants using a sequence of H2O2 based advanced oxidation processes: Photolysis and catalytic wet oxidation. Water Res. 2015, 71, 85–96. [Google Scholar] [CrossRef]
- Georgi, A.; Kopinke, F. Interaction of adsorption and catalytic reactions in water decontamination processes: Part Oxidation of organic contaminants with hydrogen peroxide catalyzed by activated carbon. Appl. Catal. B Environ. 2005, 58, 9–18. [Google Scholar] [CrossRef]
- Anfruns, A.; Montes-Morán, M.A.; Gonzalez-Olmos, R.; Martin, M.J. H2O2-based oxidation processes for the regeneration of activated carbons saturated with volatile organic compounds of different polarity. Chemosphere 2013, 91, 48–54. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. The influence of structure and surface chemistry of carbon materials on the decomposition of hydrogen peroxide. Carbon 2013, 62, 97–108. [Google Scholar] [CrossRef]
- Azabou, S.; Najjar, W.; Bouaziz, M.; Ghorbel, A.; Sayadi, S. A compact process for the treatment of olive mill wastewater by combining wet hydrogen peroxide catalytic oxidation and biological techniques. J. Hazard. Mater. 2010, 183, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.B.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J. CWPO of 4-CP and industrial wastewater with Al-Fe pillared clays. Water Sci. Technol. 2010, 61, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Martínez, F.; Botas, J.A.; Molina, R.; Pariente, M.I. Heterogeneous catalytic wet peroxide oxidation systems for the treatment of an industrial pharmaceutical wastewater. Water Res. 2009, 43, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Giordano, G.; Granato, T.; Katovic, A.; Siciliano, A.; Tripicchio, F. Chemical pretreatment of olive oil mill wastewater using a metal-organic framework catalyst. J. Agric. Food Chem. 2005, 53, 8306–8309. [Google Scholar] [CrossRef] [PubMed]
- Pariente, M.; Melero, J.; Martínez, F.; Botas, J.; Gallego, A. Catalytic wet hydrogen peroxide oxidation of a petrochemical wastewater. Water Sci. Technol. 2010, 61, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Bautista, P.; Mohedano, A.; Casas, J.; Zazo, J.; Rodriguez, J. Oxidation of cosmetic wastewaters with H2O2 using a Fe/γ-Al2O3 catalyst. Water Sci. Technol. 2010, 61, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yin, W.; Jiang, Y.; Ge, H.; Li, P.; Wu, J. Depth treatment of coal-chemical engineering wastewater by a cost-effective sequential heterogeneous Fenton and biodegradation process. Environ. Sci. Pollut. Res. 2018, 25, 13118–13126. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, C.M.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Treatment of real winery wastewater by wet oxidation at mild temperature. Sep. Purif. Technol. 2014, 129, 121–128. [Google Scholar] [CrossRef]
- Martínez, F.; Melero, J.A.; Botas, J.Á.; Pariente, M.I.; Molina, R. Treatment of phenolic effluents by catalytic wet hydrogen peroxide oxidation over Fe2O3/SBA-15 extruded catalyst in a fixed-bed reactor. Ind. Eng. Chem. Res. 2007, 46, 4396–440557. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Rodríguez, J.J. Catalytic wet peroxide oxidation of phenol with a Fe/active carbon catalyst. Appl. Catal. B Environ. 2006, 65, 261–268. [Google Scholar] [CrossRef]
- Qin, H.; Xiao, R.; Shi, W.; Wang, Y.; Li, H.; Guo, L.; Cheng, H.; Chen, J. Magnetic core–shell-structured Fe3O4@ CeO2 as an efficient catalyst for catalytic wet peroxide oxidation of benzoic acid. RSC Adv. 2018, 8, 33972–33979. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Wang, Y. Photoinduced degradation of orange II on different iron (hydr) oxides in aqueous suspension: Rate enhancement on addition of hydrogen peroxide, silver nitrate, and sodium fluoride. Langmuir 2008, 24, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Pliego, G.; Zazo, J.A.; Blasco, S.; Casas, J.A.; Rodriguez, J.J. Treatment of highly polluted hazardous industrial wastewaters by combined coagulation–adsorption and high-temperature Fenton oxidation. Ind. Eng. Chem. Res. 2012, 51, 2888–2896. [Google Scholar] [CrossRef]
- Chou, S.; Huang, C. Application of a supported iron oxyhydroxide catalyst in oxidation of benzoic acid by hydrogen peroxide. Chemosphere 1999, 38, 2719–2731. [Google Scholar] [CrossRef] [Green Version]
- Prasad, J.; Tardio, J.; Jani, H.; Bhargava, S.K.; Akolekar, D.B.; Grocott, S.C. Wet peroxide oxidation and catalytic wet oxidation of stripped sour water produced during oil shale refining. J. Hazard. Mater. 2007, 146, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Pérez, J.A.; Román Sánchez, I.M.; Carra, I.; Cabrera Reina, A.; Casas López, J.L.; Malato, S. Economic evaluation of a combined photo-Fenton/MBR process using pesticides as model pollutant. Factors affecting costs. J. Hazard. Mater. 2013, 244, 195–203. [Google Scholar] [CrossRef]
- Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Sánchez Pérez, J.A.; Malato, S. Strategies for reducing cost by using solar photo-Fenton treatment combined with nanofiltration to remove microcontaminants in real municipal effluents: Toxicity and economic assessment. Chem. Eng. J. 2017, 318, 161–170. [Google Scholar] [CrossRef]
- García, E.B.; Rivas, G.; Arzate, S.; Sánchez Pérez, J.A. Wild bacteria inactivation in WWTP secondary effluents by solar photo-fenton at neutral pH in raceway pond reactors. Catal. Today 2017, 313, 72–78. [Google Scholar] [CrossRef]
- Sanabria, N.R.; Peralta, Y.M.; Montañez, M.K.; Rodríguez-Valencia, N.; Molina, R.; Moreno, S. Catalytic oxidation with Al–Ce–Fe–PILC as a post-treatment system for coffee wet processing wastewater. Water Sci. Technol. 2012, 66, 1663–1668. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Levchuk, I.; Uski, J.; Sillanpää, M.; Acevedo, A.; Manzano, M.A. Post-treatment of plywood mill effluent by Multi-Barrier Treatment: A pilot-scale study. Chem. Eng. J. 2016, 283, 21–28. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Levchuk, I.; Salcedo, I.; Acevedo-Merino, A.; Manzano, M.A. Post-treatment of refinery wastewater effluent using a combination of AOPs (H2O2 photolysis and catalytic wet peroxide oxidation) for possible water reuse. Comparison of low and medium pressure lamp performance. Water Res. 2016, 91, 86–96. [Google Scholar]
- Drábková, M.; Matthijs, H.; Admiraal, W.; Maršálek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 2007, 45, 363–369. [Google Scholar] [CrossRef]
- St. Laurent, J.B.; de Buzzaccarini, F.; De Clerck, K.; Demeyere, H.; Labeque, R.; Lodewick, R.; van Langenhove, L. Laundry Cleaning of Textiles; Elsevier: Amsterdam, The Netherlands, 2007; pp. 57–102. [Google Scholar]
- U.S. Department of the Interior Bureau of Reclamation. Reverse Osmosis Treatment of Central Arizona Project Water for the City of Tucson. Desalination Research and Development Program Report No. 36, Costs 20 Appendix C. 2004. Available online: https://www.usbr.gov/research/dwpr/reportpdfs/report036.pdf (accessed on 2 October 2018).
- Nguyen, T.V.; Jeong, S.; Pham, T.T.N.; Kandasamy, J.; Vigneswaran, S. Effect of granular activated carbon filter on the subsequent flocculation in seawater treatment. Desalination 2014, 354, 9–16. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Ocón, P.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Highly efficient application of activated carbon as catalyst for wet peroxide oxidation. Appl. Catal. B Environ. 2013, 140, 663–670. [Google Scholar] [CrossRef]
- Munoz, M.; Garcia-Muñoz, P.; Pliego, G.; Pedro, Z.M.D.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J. Application of intensified Fenton oxidation to the treatment of hospital wastewater: Kinetics, ecotoxicity and disinfection. J. Environ. Chem. Eng. 2016, 4, 4107–4112. [Google Scholar] [CrossRef]
- Pliego, G.; Zazo, J.A.; Garcia-Muñoz, P.; Munoz, M.; Casas, J.A.; Rodriguez, J.J. Trends in the intensification of the Fenton process for wastewater treatment: An overview. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2611–2692. [Google Scholar] [CrossRef]
- Bergault, I.; Rajashekharam, M.; Chaudhari, R.; Schweich, D.; Delmas, H. Modeling and comparison of acetophenone hydrogenation in trickle-bed and slurry airlift reactors. Chem. Eng. Sci. 1997, 52, 4033–4043. [Google Scholar] [CrossRef]





| Reference | Type of Catalyst | Type of the Wastewater | Experimental Conditions | Main Outcomes |
|---|---|---|---|---|
| [52] | Fe/γ-Al2O3 (in form of powder) | Wastewater from cosmetic industry (TOC 691 mg/L and COD 2 376 mg/L) | Operating conditions: pH 3, 50–85 °C, concentration of catalyst 2,500–5,000 mg/L, concentration of H2O2 2,272–9,088 mg/L | About 80% of COD was eliminated at 85 °C, H2O2 2272 mg/L and space-time of 9.4 kgcath/kgCOD. The H2O2 was fully consumed. Stability of catalyst during 100h was demonstrated. Leaching of Fe from catalyst was lower than 3%. |
| [51] | Fe2O3/SBA-15 (silica supported) | Diluted wastewater from petrochemical industry (TOC 0.22–2.2 g/L) | Operating conditions: 5 g of catalyst was used in fixed-bed reactor, 120–160 °C, 7, 14 and 21 g of H2O2/g of TOC (at 160 °C) | Removal of TOC was not affected by increase in temperature. As the temperature increased, the leaching of iron decreased. An increase of H2O2 concentration enhanced TOC removal (at 160 °C). Optimal conditions were 160 °C and 14 g of H2O2/g of TOC. |
| [53] | Fe0 (powder) | Coal-chemical engineering wastewater effluent (COD 341 ± 6 mg/L) | Operating conditions: Fe0 0.1–4 g/L, pH 2–8, H2O2 5–50 mmol/L, 25 °C | In optimal operational conditions (pH 6.8, Fe0 2g/L, H2O2 25 mmol/L) 66% of COD removal was achieved. |
| [47] | Al-Fe-PILC | Olive mill wastewater (COD 12.5 g/L) | Operating conditions: 25, 50 and 70 °C, atmospheric pressure, Al-Fe-PILC 0.5 g/L, H2O2 2·10−2 M, pH 5.2 (natural for WW) | In optimal operational conditions (50 °C, 8 h) about 50% of initial COD was eliminated. Moreover, toxicity of water (bioluminescent test with Vibrio Vischeri) decreased by 70%. |
| [48] | Al-Fe-PILC | Wastewater from cosmetic factory (COD 4200 mg/L, for the majority of experiments it was diluted 10 times) | Operating conditions: 90 °C, Fe load (Fe/(Fe+Al) molar ratio) 0.05–0.15, catalyst 1250–3750 mg/L, H2O2/COD ratios 0.5–2 stoichiometric doses (2.12 g H2O2/g COD) | Highest levels of COD removal (about 70%) from wastewater were achieved at highest Fe loading and catalyst dose. With increase of H2O2/COD ratio, the elimination of COD increased. |
| [49] | Fe2O3/SBA-15 nanocomposite (fixed bed) | Pharmaceutical wastewater (COD 1901 mg O2/L, TOC 860 mg/L) | Operating conditions: 60, 80 and 100 °C, pH 3 and 5.6, H2O2/C mass ratio 7 (5400 mg/L of H2O2) and 14 (10800 mg/L of H2O2), 2.9 g of catalyst | Optimal operating conditions at continuous up-flow fixed bed reactor were pH 3, initial H2O2 concentration 10,800 mg/L, feed flow rate 0.25 mL/min, 80 °C, amount of catalyst 2.9 g. Decrease of COD and TOC at optimal conditions was 81% and 59%, respectively. |
| [54] | graphite, activated carbon, carbon black | Winery wastewater (COD 35 ± 2.5 g/L, TOC 11.3 ± 0.9 g/L) | Operating conditions: 80, 100, 125 °C, pH 2.2–7, H2O2 doses 0–1.6 stoichiometric amount related to COD. | About 80% of COD elimination and a significant decrease in wastewater toxicity (Photobacterium phosphoreum) was obtained using 5g/L of graphite at natural pH of the wastewater (3.8), 125 °C and stoichiometric amount of H2O2 (added stepwise). |
| [50] | Cu3(BTC)2(H2O)3 BTC–benzene 1,3,5-tricarboxylic acid | Olive oil mill wastewater (COD 57.7 g/L) | Operating conditions: catalyst dose 0.97 g/L, H2O2 113.2 mg/L, max temperature 32.85 °C | About 96% of polyphenol present in wastewater was removed after CWPO. Biodegradability of wastewater significantly increased after treatment. |
| Reference | Type of catalyst | Type of the Wastewater | Experimental Conditions | Main Outcomes |
|---|---|---|---|---|
| [67] | GAC (supported in column) | Refinery wastewater effluent after H2O2/UVC. Two different influents: 1) TOC: 17 mg/L, COD 20 mg/L and 2) TOC: 19 mg/L, COD 15 mg/L. | Two experiments in ambient conditions (20 ± 2 °C ) were passing through GAC (141.1 g/L). Initial concentrations of H2O2 during CWPO were 1) 160 mg/L and 2) 96 mg/L. | The H2O2 concentration after CWPO treatments was not detected (in either experiment). The concentrations of TOC and COD were 1) 1.75 and 9 mg/L and 2) 3.5 and 6.4 mg/L, respectively. The contact time for CWPO was 6 and 3.5 min for experiments 1) and 2). Toxicity evaluation of influent and effluent of CWPO was evaluated using P. lividus embryo larvae and fertilisation tests. The toxicity of the water after treatment decreased more than 220 times and reduced the Toxic Units from IV to 0. |
| [43] | GAC (supported in column) | Simulated industrial wastewater effluent in urban wastewater matrix after H2O2/UVC (TOC 15 mg/L and COD 35.4 mg/L) | The effluent (0.5 L) of photo-Fenton in ambient conditions (20 °C) was passing through AC column (141.1 g/L). Initial concentration of H2O2 was 79.3 mg/L. | TOC, COD and H2O2 were sufficiently removed by 57, 76.6 and 100%, respectively after 2.3 min of contact time. The final effluent was recommended for safe discharge in marine water bodies after toxicity evaluation using Sparus aurata larvae and Vibrio fischeri. |
| [66] | GAC (supported in column) | Plywood mill effluent (diluted 10 times) after H2O2/UVC treatment (TOC 27 mg/L and COD 59.6 mg/L). | The effluent of H2O2/UVC in ambient conditions (20 ± 2 °C) was passing through GAC (141.1 g/L). Initial concentration of H2O2 was 100 mg/L and pH 6.0. | TOC, COD and H2O2 were sufficiently removed by 56, 39 and 100%, respectively after 5 min of contact time. The pH of the water after treatment was 8.0. |
| [42] | GAC (supported in column) | Simulated industrial wastewater effluent in urban wastewater matrix after H2O2/UVC (TOC 21 mg/L and COD 39 mg/L) | The effluent of H2O2/UVC int ambient conditions was passing through GAC column (141.1 g/L). Initial concentration of H2O2 was 161 mg/L. The pH of the water was 7.4. | Concentration of TOC, COD and H2O2 after 3.5 min of contact time were 4.2, 16.4 and < D.L, respectively. The pH of the water after the experiment was 7.9. The toxicity of the final effluent was evaluated using V. fischeri and P. lividus (embryo larvae development and fertilisation test). The most sensitive test, embryo larvae development, demonstrated that the water decreased in toxicity after CWPO by around 350 times (based on EC50). |
| [65] | Al-Ce-Fe-PILC (pillared inter-layered clays) | Coffee wet processing wastewater after biological treatment (COD 551 mg O2/L) | Operational conditions: 25 °C, Al-Ce-Fe-PILC 5 g/L, H2O2 0.1M, pH adjusted to 3.7 | After CWPO of wastewater (5h) 50% of mineralisation, 70% of phenolic compound conversion. |
| [26] | Fe3O4/γ-Al2O3 | Hospital wastewater (COD 365 mg/L, TOC 110 mg/L [73] and MWW effluent (TOC 2.6 mg/L) spiked with six pharmaceuticals | Operational conditions: 75 °C, catalyst dose 2 g/L, pH 3, H2O2 730 mg/L or 100 mg/L (when concentration of spiked pharmaceuticals was 10 µg/L of each) | Complete elimination of spiked pharmaceuticals (at high concentrations) from hospital wastewater and urban wastewater effluent was achieved after 90 min (H2O2 730 mg/L). When pharmaceuticals were spiked at lower concentrations, complete degradation was reached after 30 min (H2O2 100 mg/L). |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueda Márquez, J.J.; Levchuk, I.; Sillanpää, M. Application of Catalytic Wet Peroxide Oxidation for Industrial and Urban Wastewater Treatment: A Review. Catalysts 2018, 8, 673. https://doi.org/10.3390/catal8120673
Rueda Márquez JJ, Levchuk I, Sillanpää M. Application of Catalytic Wet Peroxide Oxidation for Industrial and Urban Wastewater Treatment: A Review. Catalysts. 2018; 8(12):673. https://doi.org/10.3390/catal8120673
Chicago/Turabian StyleRueda Márquez, Juan José, Irina Levchuk, and Mika Sillanpää. 2018. "Application of Catalytic Wet Peroxide Oxidation for Industrial and Urban Wastewater Treatment: A Review" Catalysts 8, no. 12: 673. https://doi.org/10.3390/catal8120673