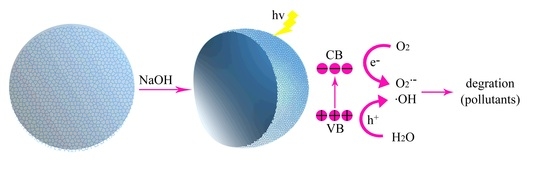
Low Temperature Synthesis of Nest-Like Microsphere with Exposed (001) Facets and Its Enhanced Photocatalytic Performance by NaOH Alkalization
Abstract
:1. Introduction
2. Results and Discussion
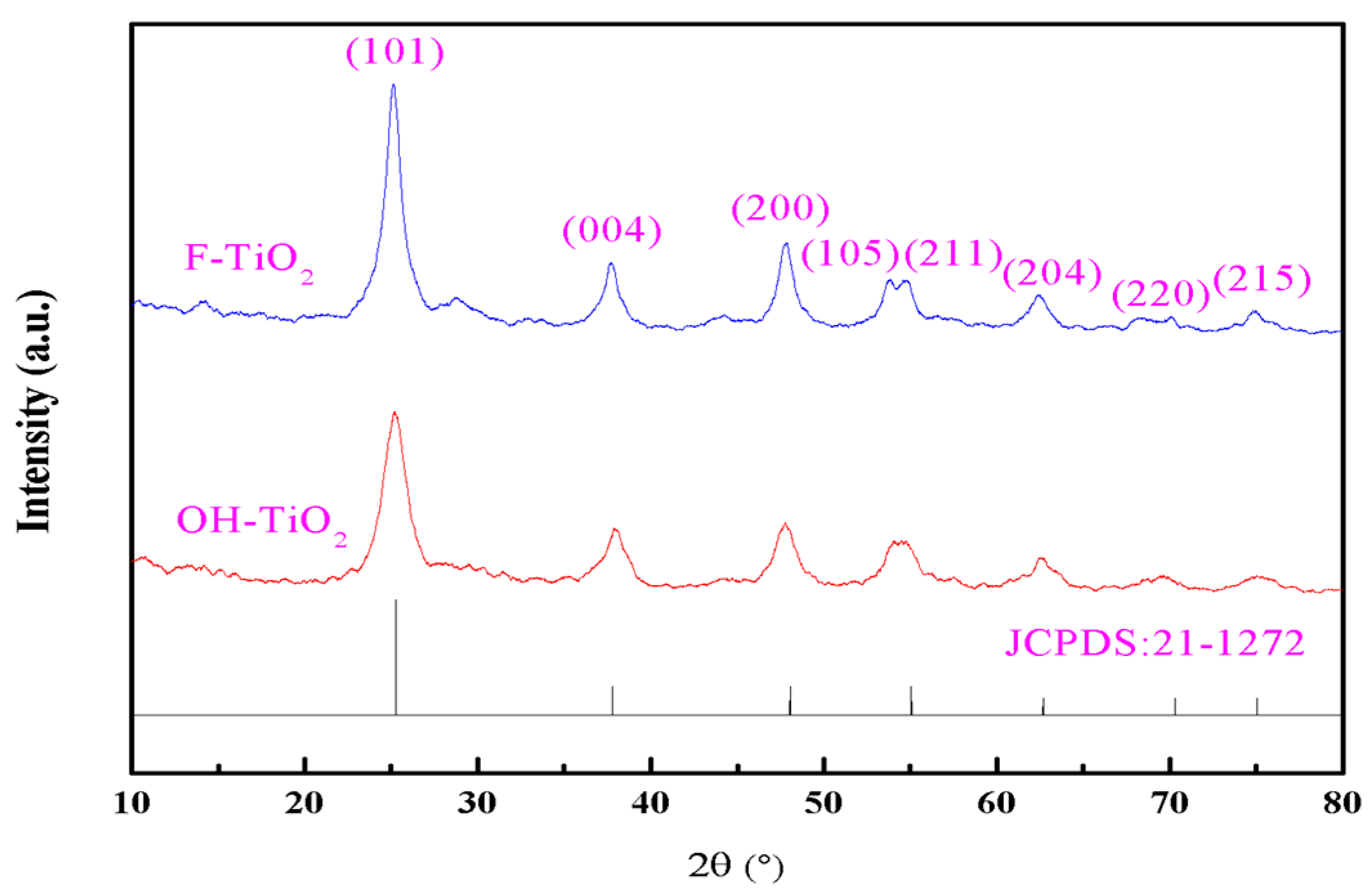
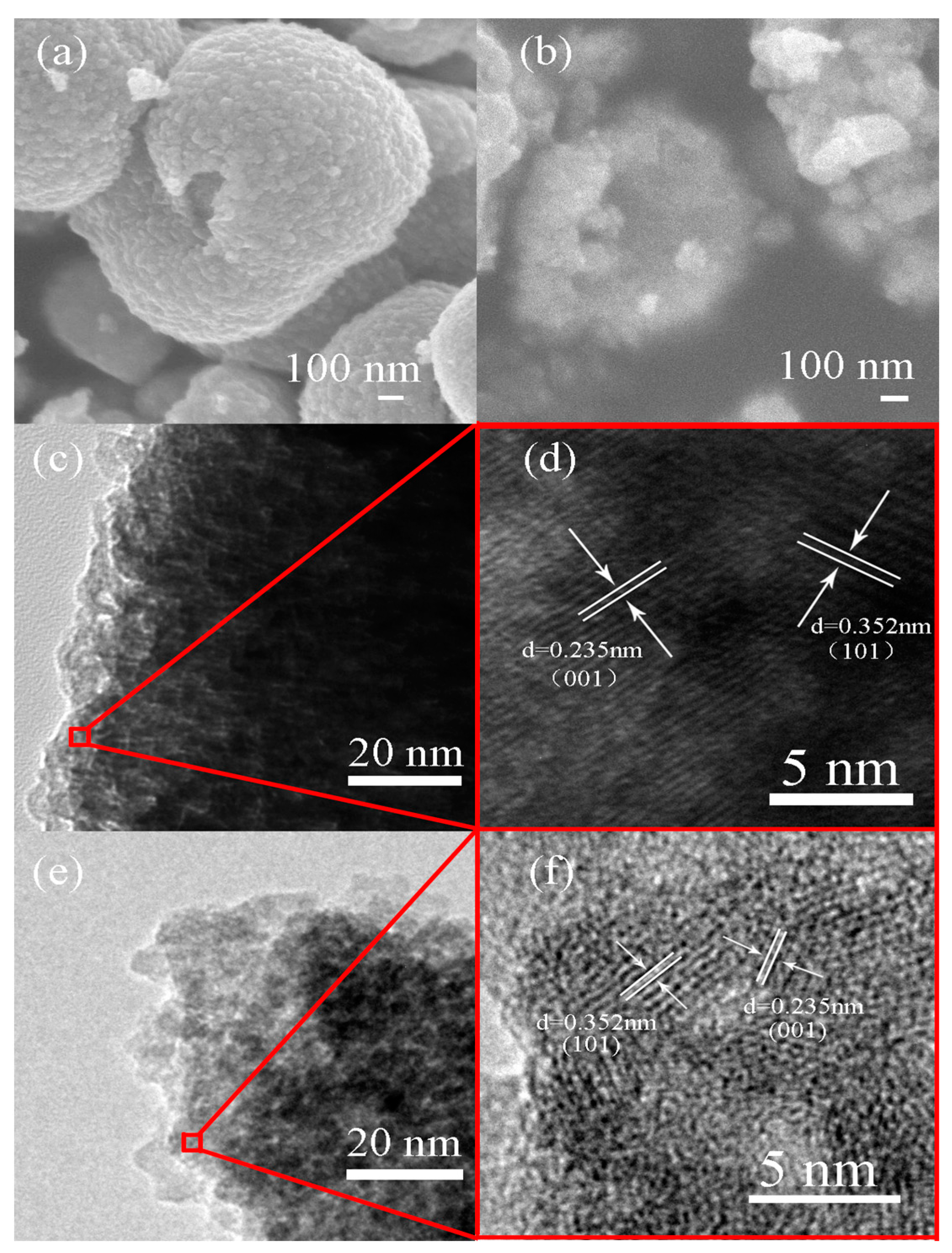
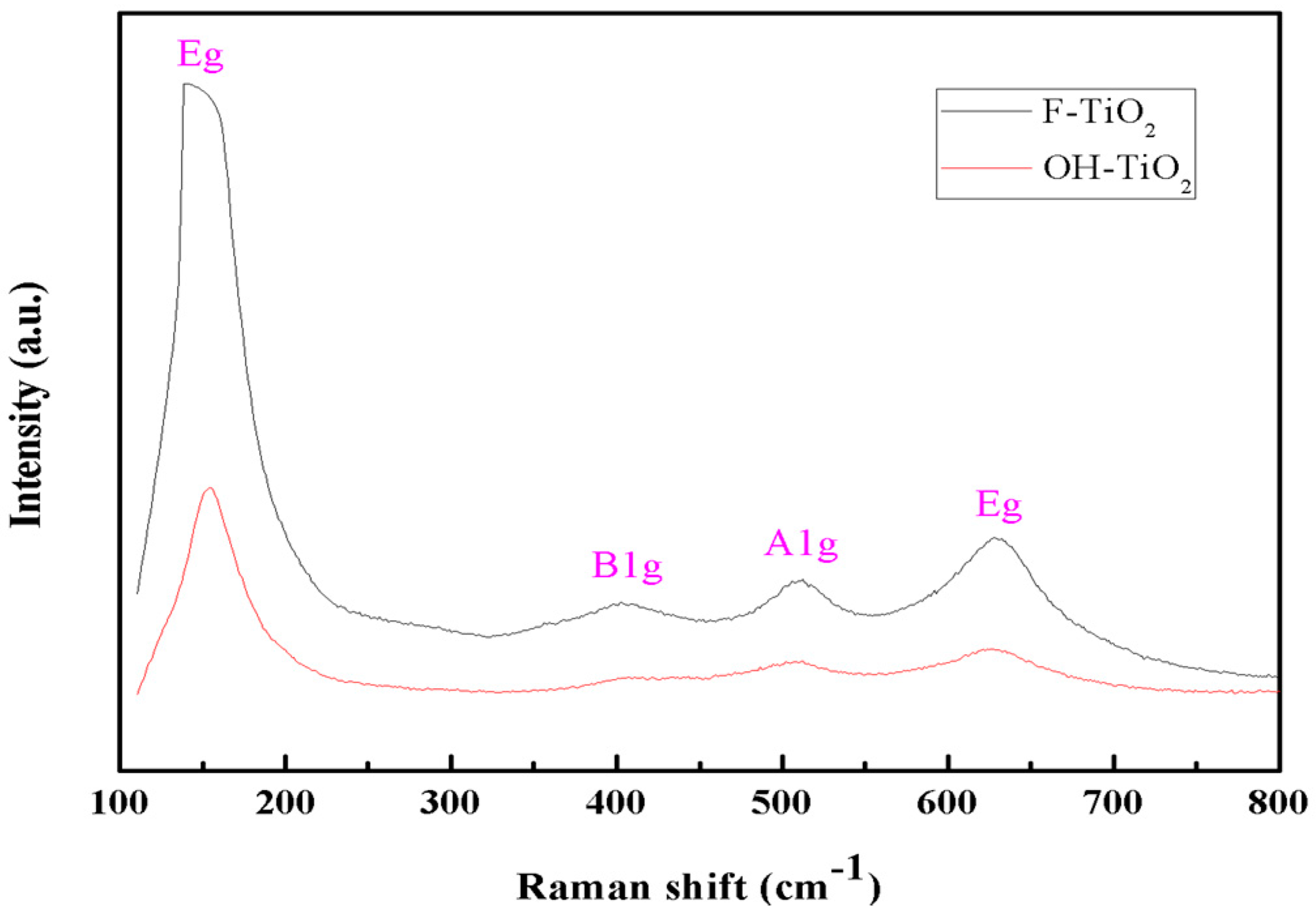
2.1. Structural Characterization
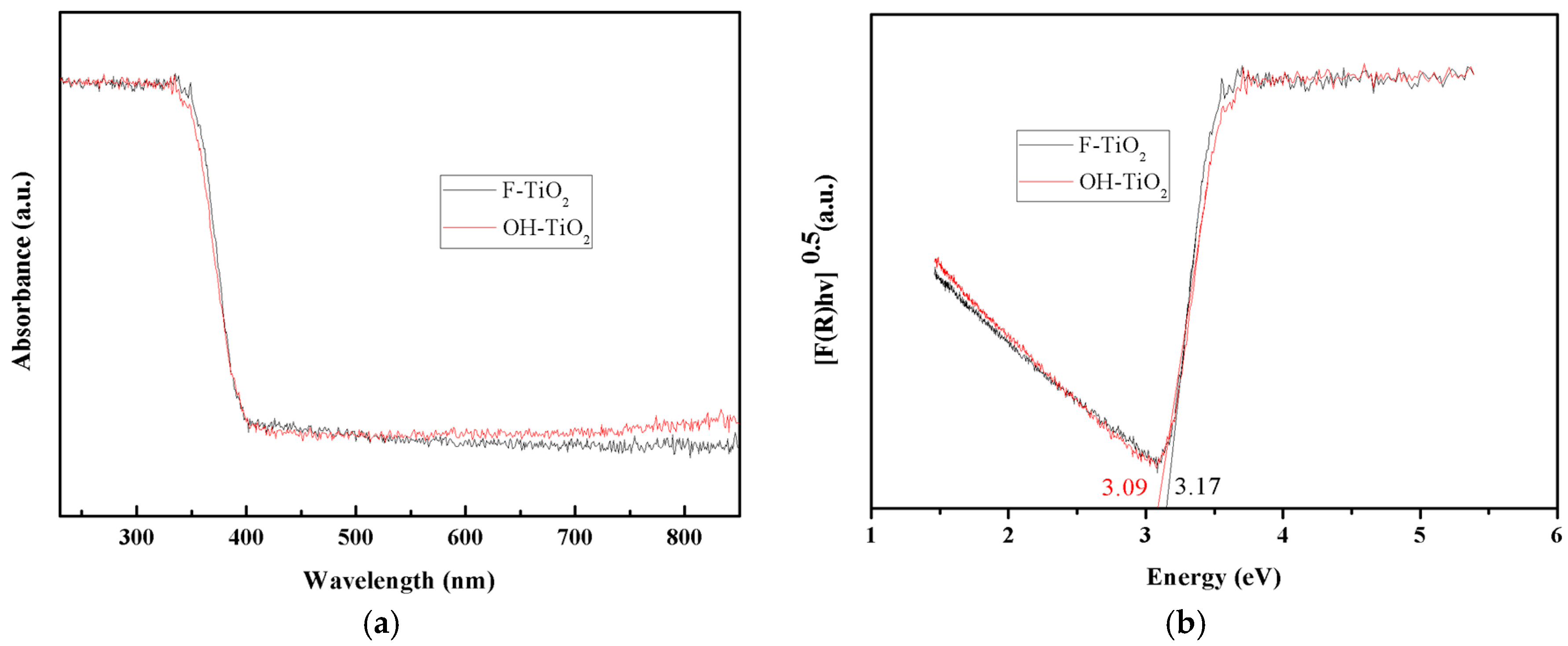
2.2. UV-Vis DRS Analysis
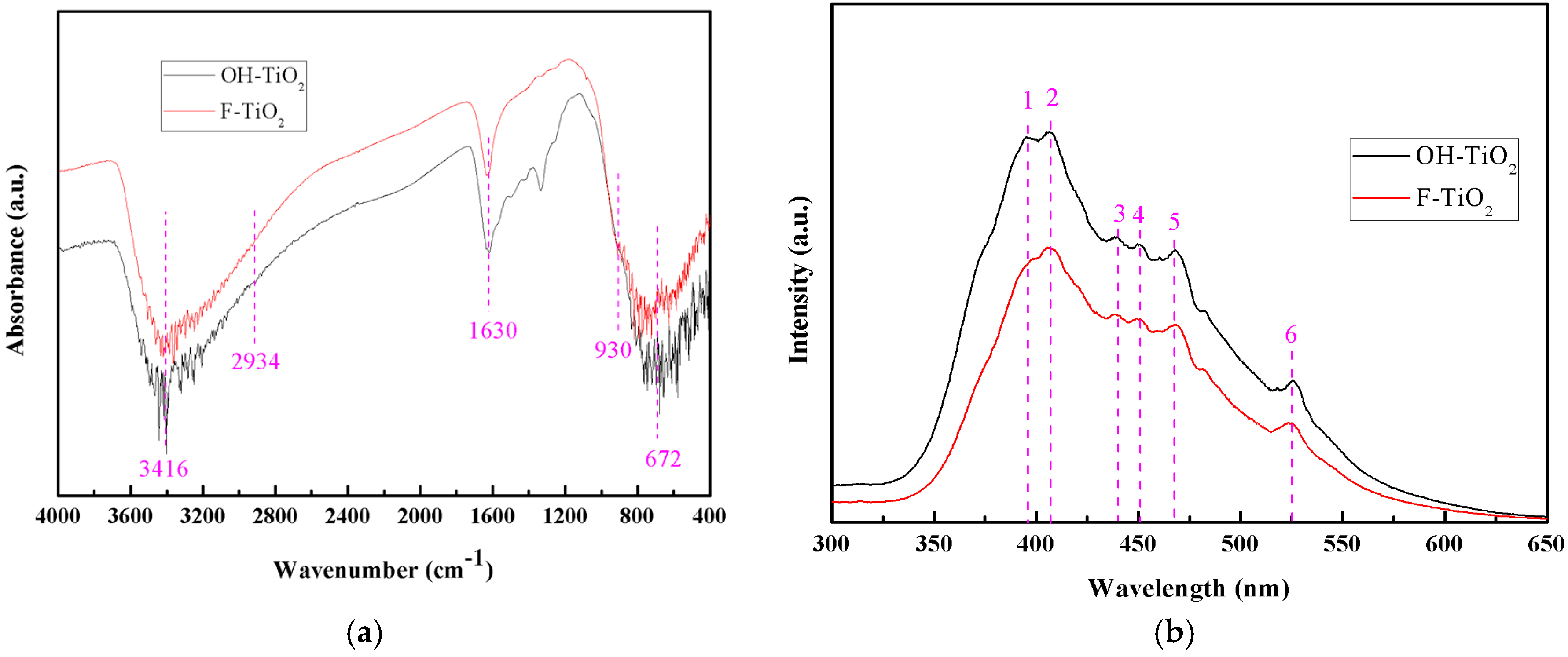
2.3. FT-IR Analysis
2.4. Photoluminescence Analysis
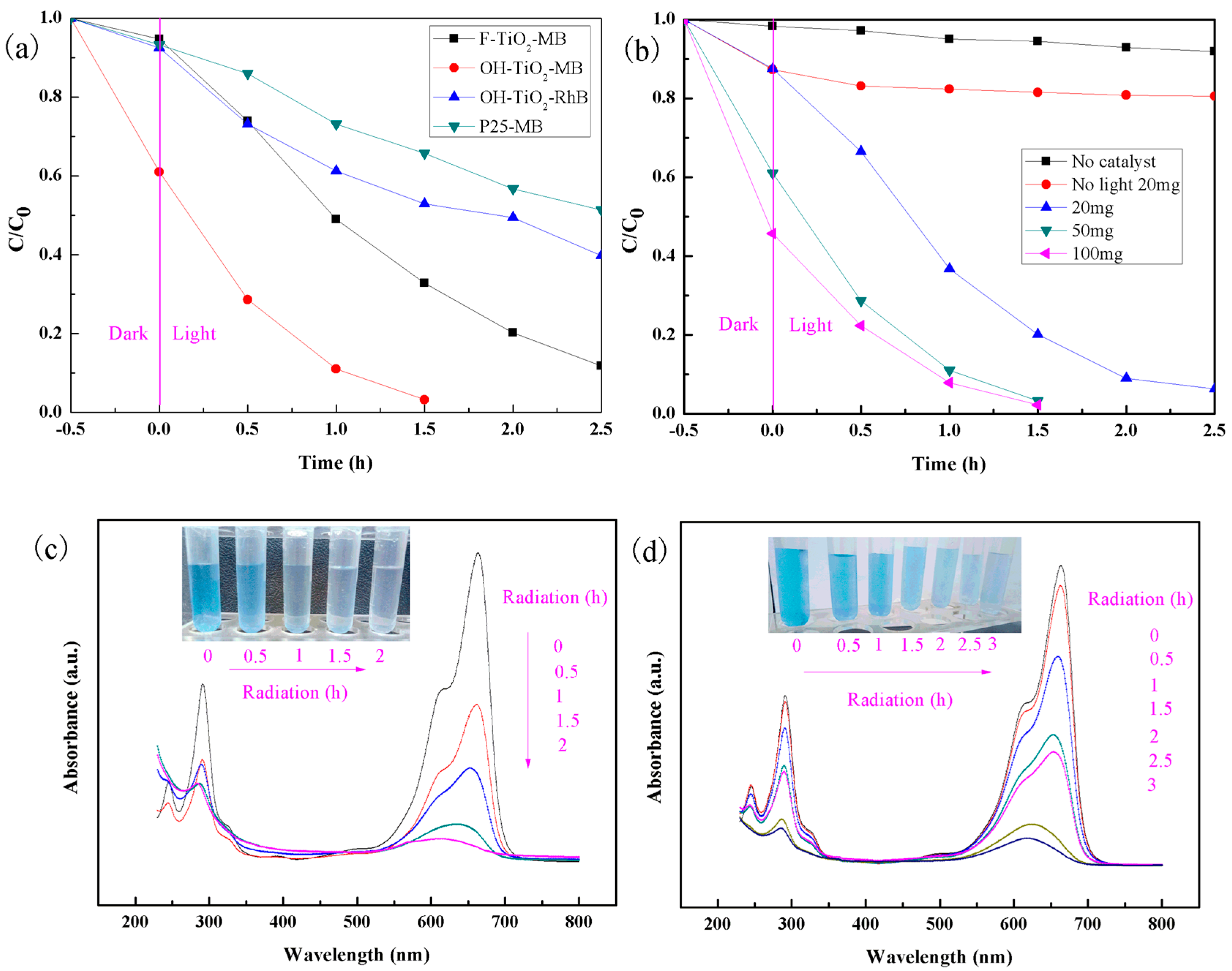
2.5. Photocatalytic Activity
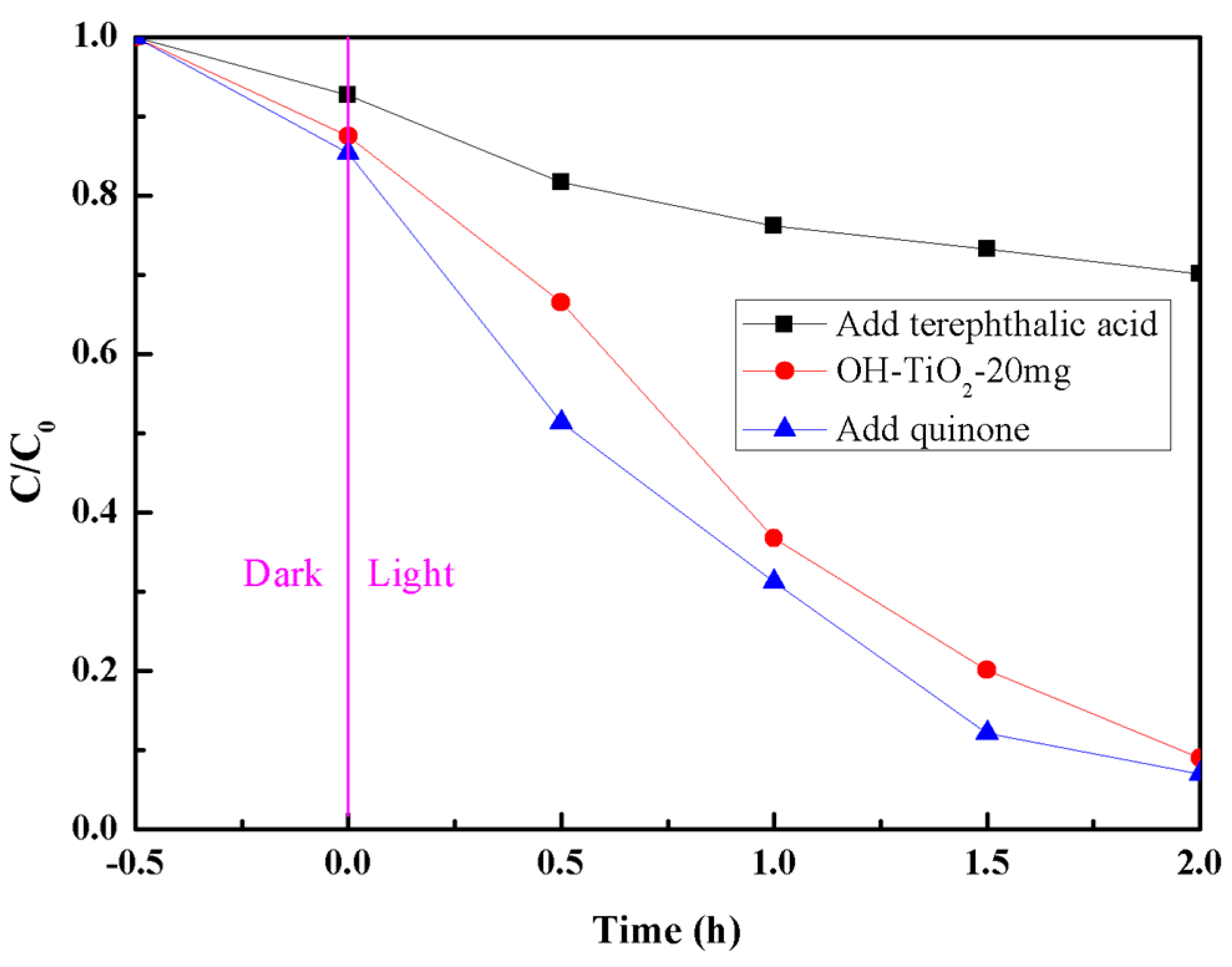
2.6. Radical-Scavenging Experiments
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalysts
3.3. Catalyst Characterization
3.4. Photocatalytic Activity Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wu, Z.; Chen, Q.; Yan, Y.; Cravotto, G.; Wu, Z. Microwave-induced crystallization of AC/TiO2 for improving the perfomnance of rhodamine B dye degradation. Appl. Surf. Sci. 2015, 351, 104–112. [Google Scholar] [CrossRef]
- Atout, H.; Álvarez, M.G.; Chebli, D.; Bouguettoucha, A.; Tichit, D.; Llorca, J.; Medina, F. Enhanced photocatalytic degradation of methylene blue: Preparation of TiO2/reduced graphene oxide nanocomposites by direct sol-gel and hydrothermal methods. Mater. Res. Bull. 2017, 50, 952–957. [Google Scholar] [CrossRef]
- Zhang, S.T.; Ruan, Y.R.; Liu, C.; Wang, P.; Ma, Y.Q. The evolution of structure, chemical state and photocatalytic performance of α-Fe/FeTiO3/TiO2 with the nitridation at different temperatures. Mater. Res. Bull. 2017, 95, 503–508. [Google Scholar] [CrossRef]
- Bessegato, G.G.; Guaraldo, T.T.; Brito, J.F.; Brugnera, M.F. Achievements and trends in photoelectrocatalysis: From environmental to energy applications. Electrocatalysis 2015, 6, 415–441. [Google Scholar] [CrossRef]
- Michele, L.; Andrea, V.; Annabella, S. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 65, 155409. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Vione, D.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic ions Competitive reactions of phenol and alcohols on a titanium dioxide-fluoride system. Langmuir 2000, 16, 8964–8972. [Google Scholar] [CrossRef]
- Huang, W.C.; Ting, J.-M. Novel nitrogen-doped anatase TiO2 mesoporous bead photocatalysts for enhanced visible light response. Ceram. Int. 2017, 43, 9992–9997. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Gjipalaj, J.; Alessandri, I. Easy recovery; mechanical stability; enhanced adsorption capacity and recyclability of alginate-based TiO2 macrobead photocatalysts for water treatment. J. Environ. Chem. Eng. 2017, 5, 1763–1770. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Ghosh, S.; Basak, S.; Naskar, M.K. Mesoporous CuO-TiO2 microspheres for efficient catalytic oxidation of CO and photodegradation of methylene blue. J. Phys. Chem. Solids 2017, 104, 103–110. [Google Scholar] [CrossRef]
- Cao, X.; Luo, S.; Liu, C.; Chen, J. Synthesis of Bentonite-Supported Fe2O3-Doped TiO2 superstructures for highly promoted photocatalytic activity and recyclability. Adv. Powder Technol. 2017, 28, 993–999. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Tunable Photocatalytic Selectivity of Hollow TiO2 Microspheres Composed of Anatase Polyhedra with Exposed {001} Facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, W.; Li, X.; Zhang, X. Low temperature synthesis of water dispersible F-doped TiO2 nanorods with enhanced photocatalytic activity. RSC Adv. 2017, 7, 21547–21555. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Zhou, J.Q.; He, Z.L.; Li, J.Q.; Liu, H. Preparation, characterisation and activity of TiO2−xFx spherical photocatalyst: Influence of sodium fluoride on methyl orange degradation. Mater. Res. Innov. 2011, 15, 78–82. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, C.; Zhao, D.; Ma, W.; Zhao, J. Change of Adsorption Modes of Dyes on Fluorinated TiO2 and Its Effect on Photocatalytic Degradation of Dyes under Visible Irradiation. Langmuir 2008, 24, 7338–7345. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Ho, W.K.; Yu, J.G.; Hark, S.K.; Iu, K. Effects of trifluoroacetic acid modification on the surface microstructures and photocatalytic activity of mesoporous TiO2 thin films. Langmuir 2003, 19, 3889–3896. [Google Scholar] [CrossRef]
- Lewandowski, M.; Ollis, D.F. Halide acid pretreatments of photocatalysts for oxidation of aromatic air contaminants: rate enhancement, rate inhibition and a thermodynamic rationale. Catalysis 2003, 217, 38–46. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-D.; Lin, H.-H.; Lee, C.-T.; Tseng, C.-M.; Suryanarayanan, V.; Vittala, R.; Ho, K.-C. Hierarchically assembled microspheres consisting of nanosheets of highly exposed (001)-facets TiO2 for dye-sensitized solar cells. RSC Adv. 2016, 6, 14178–14191. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- He, Z.; Wen, L.; Wang, D.; Xue, Y.; Lu, Q.; Wu, C.; Chen, J.; Song, S. Photocatalytic Reduction of CO2 in Aqueous Solution on Surface-Fluorinated Anatase TiO2 Nanosheets with Exposed {001} Facets. Energy Fuels 2014, 28, 3982–3993. [Google Scholar] [CrossRef]
- He, Z.; Cai, Q.; Hong, F.; Jiang, Z.; Chen, J.; Song, S. Effective Enhancement of the Degradation of Oxalic Acid by Catalytic Ozonation with TiO2 by Exposure of {001} Facets and Surface Fluorination. Ind. Eng. Chem. Res. 2012, 51, 5662–5668. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Lasek, J.; Jeffrey, C.S.; Yu, J.C.-C. Titania nanosheet photocatalysts with dominantly exposed (001) reactive facets for photocatalytic NOx abatement. Appl. Catal. B Environ. 2017, 219, 391–400. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, S.; Wang, L.; Liu, R.; Zeng, Y.; Xia, X.; Liu, Y.; Luo, S. Facile synthesis of bird’s nest-like TiO2 microstructure with exposed (001) facets for photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 391, 228–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, T.; Shang, M.; Wallenmeyer, P.; Katelyn, D.; Peterson, A.; Murowchick, J.; Dong, L.; Chen, X. Structural evolution from TiO2 nanoparticles to nanosheets and their photocatalytic performance in hydrogen generation and environmental pollution removal. RSC Adv. 2014, 4, 16146–16152. [Google Scholar] [CrossRef]
- Chu, L.; Qin, Z.; Yang, J.; Li, X. Anatase TiO2 Nanoparticles with Exposed {001} Facets for Efficient Dye-Sensitized Solar Cells. Sci. Rep. 2015, 5, 12143. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhou, C.; Yin, H.; Zhang, D.; Xu, X. Reactivity of the alkaline pretreated nanoporous gold for the CO oxidation. Catal. Lett. 2011, 141, 1026–1031. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Zampieri, A.; Schwieger, W. Mesoporous ZSM-5 zeolites via alkali treatment for the direct hydroxylation of benzene to phenol with N2O. J. Catal. 2008, 260, 193–197. [Google Scholar] [CrossRef]
- Su, L.L.; Zhang, J.Q.; Wang, H.X.; Li, Y.G.; Shen, W.J.; Xu, Y.D.; Bao, X.H. Creating mesopores in ZSM-5 zeolite by alkali treatment: A new way to enhance the catalytic performance of methane dehydroaromatization on Mo/HZSM-5 catalysts. Catal. Lett. 2003, 91, 155–167. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Lv, K.L.; Yu, J.G. Pivotal role of fluorine in enhanced photocatalytic activity of anatase TiO2 nanosheets with dominant (001) facets for the photocatalytic degradation of acetone in air. Appl. Catal. B Environ. 2010, 96, 557–564. [Google Scholar] [CrossRef]
- Hou, C.; Liu, W.; Zhu, J. Synthesis of NaOH-Modified TiOF2 and Its Enhanced Visible Light Photocatalytic Performance on RhB. Catalysts 2017, 7, 243. [Google Scholar] [CrossRef]
- Yang, H.G.; Zeng, H.C. Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening. Phys. Chem. B 2004, 108, 3492–3495. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, N.; Wang, H.; Mao, L. Sacrificial template synthesis of coreshell SrTiO3/TiO2heterostructured microspheres photocatalyst. Ceram. Int. 2017, 43, 4807–4813. [Google Scholar] [CrossRef]
- Zo, M.; Liu, H.; Feng, L.; Xiong, F.; Thoma, T.; Yang, M. Effect of nitridation on visible light photocatalytic behavior of microporous (Ag, Ag2O) co-loaded TiO2. Microporous Mesoporous Mater. 2017, 240, 137–144. [Google Scholar] [CrossRef]
- Guo, S.-Y.; Dai, J.-G.; Zhao, T.-J.; Hou, S.-D.; Zhang, P.; Wang, P.; Sun, G.-X. A novel microporous morphous-ZnO@TiO2/graphene ternary nanocomposite with enhanced photocatalytic activity. RSC Adv. 2017, 7, 36787–36792. [Google Scholar] [CrossRef]
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462. [Google Scholar] [CrossRef]
- Xu, S.H.; Shangguan, W.F.; Yuan, J. Synthesis and performance of novel magnetically separable nanospheres of titanium dioxide photocatalyst with egg-like structure. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Watanabe, A.; Iseki, M.; Watanabe, M.; Kandor, H. Strong Donation of the Hydrogen Bond of Tyrosine during Photoactivation of the BLUF Domain. Phys. Chem. Lett. 2011, 2, 1015–1019. [Google Scholar] [CrossRef]
- Ning, Y. Structural Identification of Organic Compounds and Organic Spectroscopy; Science Press: Beijing, China, 2000; ISBN 978-7-03-0074-126. [Google Scholar]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-Light-Driven N-F-Codoped TiO2 Photocatalysts. 2. Optical Characterization, Photocatalysis, and Potential Application to Air Purification. Chem. Mater. 2005, 17, 2596–2602. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, X.; Pang, Z.; Cai, Y.; Zhou, H.; Lv, P.; Wei, Q. Fabrication of hierarchical TiO2 nanofibers by microemulsion electrospinning for photocatalysis applications. Ceram. Int. 2017, 43, 15911–15917. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Zheng, Y.; Chen, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 nanocrystalline heterostructure: A wide spectrum responsive photocatalyst towards the highly efficient decomposition of gaseous benzene. Appl. Catal. B Environ. 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Park, H.; Choi, W. Photocatalytic Reactivities of Nafion-Coated TiO2 for the Degradation of Charged Organic Compounds under UV or Visible Light. J. Phys. Chem. B 2005, 109, 11667–11674. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.; Zhu, J.; Song, Q. Low Temperature Synthesis of Nest-Like Microsphere with Exposed (001) Facets and Its Enhanced Photocatalytic Performance by NaOH Alkalization. Catalysts 2018, 8, 70. https://doi.org/10.3390/catal8020070
Hou C, Zhu J, Song Q. Low Temperature Synthesis of Nest-Like Microsphere with Exposed (001) Facets and Its Enhanced Photocatalytic Performance by NaOH Alkalization. Catalysts. 2018; 8(2):70. https://doi.org/10.3390/catal8020070
Chicago/Turabian StyleHou, Chentao, Jiaming Zhu, and Qiaoqiao Song. 2018. "Low Temperature Synthesis of Nest-Like Microsphere with Exposed (001) Facets and Its Enhanced Photocatalytic Performance by NaOH Alkalization" Catalysts 8, no. 2: 70. https://doi.org/10.3390/catal8020070
APA StyleHou, C., Zhu, J., & Song, Q. (2018). Low Temperature Synthesis of Nest-Like Microsphere with Exposed (001) Facets and Its Enhanced Photocatalytic Performance by NaOH Alkalization. Catalysts, 8(2), 70. https://doi.org/10.3390/catal8020070