Gaseous Nitric Acid Activated Graphite Felts as Hierarchical Metal-Free Catalyst for Selective Oxidation of H2S
Abstract
:1. Introduction
2. Results and Discussion
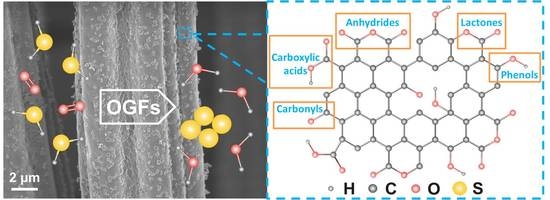
2.1. Characteristics of the Acid Treated Graphite Felts
2.2. OGFs as Metal-Free Catalyst for Selective Oxidation of H2S
3. Materials and Methods
3.1. Graphite Felts
3.2. Gaseous HNO3 Treatment of GFs
3.3. Characterization Techniques
3.4. Selective Oxidation Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.P.; Su, D.S. Recent progress on the growth mechanism of carbon nanotubes: A review. ChemSusChem 2011, 4, 824–847. [Google Scholar] [CrossRef] [PubMed]
- Duong-Viet, C.; Ba, H.; Truong-Phuoc, L.; Liu, Y.; Tessonnier, J.P.; Nhut, J.M.; Granger, P.; Pham-Huu, C. Nitrogen-Doped Carbon Composites as Metal-Free Catalysts; Elsevier Series Book; Parvulescu, V., Kemnitz, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 273–312. ISBN 978-0-444-63587-7. [Google Scholar]
- Liang, J.; Du, X.; Gibson, C.; Du, X.W.; Qiao, S.Z. N-doped graphene natively grown on hierarchical ordered porous carbon for enhanced oxygen reduction. Adv. Mater. 2013, 25, 6226–6231. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liang, H.; Parvez, K.; Zhuang, X.; Feng, X.; Mullen, K. Nitrogen-doped carbon nanosheets with size-defined mesopores as highly efficient metal-free catalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2014, 53, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Allen, B.L.; Kauffman, D.R.; Star, A. Electrocatalytic activity of nitrogen-doped carbon nanotube cups. J. Am. Chem. Soc. 2009, 131, 13200–13201. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, Z.; Wang, J.; Cui, R.; Sun, H.; Peng, F.; Wei, L.; Wang, Z.; Liang, X.; Peng, L.; et al. Nitrogen-doped single-walled carbon nanotubes grown on substrates: Evidence for framework doping and their enhanced properties. Adv. Funct. Mater. 2011, 21, 986–992. [Google Scholar] [CrossRef]
- Tuci, G.; Pilaski, M.; Ba, H.; Rossin, A.; Luconi, L.; Caporali, S.; Pham-Huu, C.; Palkovits, R.; Giambastiani, G. Unraveling surface basicity and bulk morphology relationship on covalent triazine frameworks with unique catalytic and gas adsorption properties. Adv. Funct. Mater. 2017, 27, 1605672. [Google Scholar] [CrossRef]
- Chizari, K.; Deneuve, A.; Ersen, O.; Florea, I.; Liu, Y.; Edouard, D.; Janowska, I.; Begin, D.; Pham-Huu, C. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for selective oxidation. ChemSusChem 2012, 5, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N, P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions. Angew. Chem. Int. Ed. 2016, 55, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Tuci, G.; Zafferoni, C.; Rossin, A.; Luconi, L.; Milella, A.; Ceppatelli, M.; Innocenti, M.; Liu, Y.; Pham-Huu, C.; Giambastiani, G. Chemical functionalization of N-doped carbon nanotubes: A powerful approach to cast light on the electrochemical role of specific N-functionalities in the oxygen reduction reaction. Catal. Sci. Technol. 2016, 6, 6226–6236. [Google Scholar] [CrossRef]
- Lv, R.; Cui, T.; Jun, M.-S.; Zhang, Q.; Cao, A.; Su, D.S.; Zhang, Z.; Yoon, S.-H.; Miyawaki, J.; Mochida, I.; et al. Open-ended, N-doped carbon nanotube-graphene hybrid nanostructures as high-performance catalyst support. Adv. Funct. Mater. 2011, 21, 999–1006. [Google Scholar] [CrossRef]
- Ba, H.; Liu, Y.; Truong-Phuoc, L.; Duong-Viet, C.; Nhut, J.-M.; Nguyen, D.L.; Ersen, O.; Tuci, G.; Giambastiani, G.; Pham-Huu, C. N-doped food-grade-derived 3D mesoporous foams as metal-free systems for catalysis. ACS Catal. 2016, 6, 1408–1419. [Google Scholar] [CrossRef]
- Tuci, G.; Luconi, L.; Rossin, A.; Berretti, E.; Ba, H.; Innocenti, M.; Yakhvarov, D.; Caporali, S.; Pham-Huu, C.; Giambastiani, G. Aziridine-functionalized multiwalled carbon nanotubes: Robust and versatile catalysts for the oxygen reduction reaction and knoevenagel condensation. ACS Appl. Mater. Interfaces 2016, 8, 30099–30106. [Google Scholar] [CrossRef] [PubMed]
- Duong-Viet, C.; Liu, Y.; Ba, H.; Truong-Phuoc, L.; Baaziz, W.; Nguyen-Dinh, L.; Nhut, J.-M.; Pham-Huu, C. Carbon nanotubes containing oxygenated decorating defects as metal-free catalyst for selective oxidation of H2S. Appl. Catal. B 2016, 191, 29–41. [Google Scholar] [CrossRef]
- Qi, W.; Liu, W.; Guo, X.; Schlogl, R.; Su, D. Oxidative dehydrogenation on nanocarbon: Intrinsic catalytic activity and structure-function relationships. Angew. Chem. Int. Ed. 2015, 54, 13682–13685. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Oxidative dehydrogenation of ethylbenzene on activated carbon catalysts. I. Influence of surface chemical groups. Appl. Catal. A 1999, 184, 153–160. [Google Scholar] [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.V.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for mwcnt functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Hiura, H.; Ebbesen, T.W.; Tanigaki, K. Opening and purification of carbon nanotubes in high yields. Adv. Mater. 1995, 7, 275–276. [Google Scholar] [CrossRef]
- Qui, N.V.; Scholz, P.; Krech, T.; Keller, T.F.; Pollok, K.; Ondruschka, B. Multiwalled carbon nanotubes oxidized by UV/H2O2 as catalyst for oxidative dehydrogenation of ethylbenzene. Catal. Commun. 2011, 12, 464–469. [Google Scholar] [CrossRef]
- Mahata, N.; Pereira, M.F.; Suarez-Garcia, F.; Martinez-Alonso, A.; Tascon, J.M.; Figueiredo, J.L. Tuning of texture and surface chemistry of carbon xerogels. J. Colloid Interface Sci. 2008, 324, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.M.; Nichols, B.M.; Baker, S.E.; Marcus, M.S.; Castellini, O.M.; Lee, C.-S.; Hamers, R.J.; Eriksson, M.A. Effect of ozone oxidation on single-walled carbon nanotubes. J. Phys. Chem. B 2006, 110, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xu, J.; Wang, J.; Sun, X.; Qi, W.; Liang, C.; Su, D.S. Oxygen breaks into carbon nanotubes and abstracts hydrogen from propane. Carbon 2016, 96, 631–640. [Google Scholar] [CrossRef]
- Luo, J.; Liu, Y.; Wei, H.; Wang, B.; Wu, K.-H.; Zhang, B.; Su, D.S. A green and economical vapor-assisted ozone treatment process for surface functionalization of carbon nanotubes. Green Chem. 2017, 19, 1052–1062. [Google Scholar] [CrossRef]
- Hu, J.; Guo, Z.; Chu, W.; Li, L.; Lin, T. Carbon dioxide catalytic conversion to nano carbon material on the iron–nickel catalysts using CVD-IP method. J. Energy Chem. 2015, 24, 620–625. [Google Scholar] [CrossRef]
- Liu, Y.; Dintzer, T.; Ersen, O.; Pham-Huu, C. Carbon nanotubes decorated α-Al2O3 containing cobalt nanoparticles for fischer-tropsch reaction. J. Energy Chem. 2013, 22, 279–289. [Google Scholar] [CrossRef]
- Więckowska, J. Catalytic and adsorptive desulphurization of gases. Catal. Today 1995, 24, 405–465. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Qu, S.; Da, J.; Hao, Z. H2S-selective catalytic oxidation: Catalysts and processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar] [CrossRef]
- Bashkova, S.; Baker, F.S.; Wu, X.; Armstrong, T.R.; Schwartz, V. Activated carbon catalyst for selective oxidation of hydrogen sulphide: On the influence of pore structure, surface characteristics, and catalytically-active nitrogen. Carbon 2007, 45, 1354–1363. [Google Scholar] [CrossRef]
- Shinkarev, V.V.; Glushenkov, A.M.; Kuvshinov, D.G.; Kuvshinov, G.G. Nanofibrous carbon with herringbone structure as an effective catalyst of the H2S selective oxidation. Carbon 2010, 48, 2004–2012. [Google Scholar] [CrossRef]
- PiéPlu, A.; Saur, O.; Lavalley, J.-C.; Legendre, O.; NéDez, C. Claus catalysis and H2S selective oxidation. Catal. Rev. Sci. Eng. 1998, 40, 409–450. [Google Scholar] [CrossRef]
- Liu, Y.; Duong-Viet, C.; Luo, J.; Hébraud, A.; Schlatter, G.; Ersen, O.; Nhut, J.-M.; Pham-Huu, C. One-pot synthesis of a nitrogen-doped carbon composite by electrospinning as a metal-free catalyst for oxidation of H2S to sulfur. ChemCatChem 2015, 7, 2957–2964. [Google Scholar] [CrossRef]
- Keller, N.; Pham-Huu, C.; Ledoux, M.J. Continuous process for selective oxidation of H2S over SiC-supported iron catalysts into elemental sulfur above its dewpoint. Appl. Catal. A 2001, 217, 205–217. [Google Scholar] [CrossRef]
- Lee, E.-K.; Jung, K.-D.; Joo, O.-S.; Shul, Y.-G. Catalytic wet oxidation of H2S to sulfur on V/MgO catalyst. Catal. Lett. 2004, 98, 259–263. [Google Scholar] [CrossRef]
- Claus Process. Available online: https://en.wikipedia.org/wiki/Claus_process (accessed on 3 April 2018).
- Xia, W.; Jin, C.; Kundu, S.; Muhler, M. A highly efficient gas-phase route for the oxygen functionalization of carbon nanotubes based on nitric acid vapor. Carbon 2009, 47, 919–922. [Google Scholar] [CrossRef]
- Cancado, L.G.; Jorio, A.; Ferreira, E.H.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Thanh, T.T.; Ba, H.; Truong-Phuoc, L.; Nhut, J.-M.; Ersen, O.; Begin, D.; Janowska, I.; Nguyen, D.L.; Granger, P.; Pham-Huu, C. A few-layer graphene–graphene oxide composite containing nanodiamonds as metal-free catalysts. J. Mater. Chem. A 2014, 2, 11349–11357. [Google Scholar] [CrossRef]
- Luo, J.; Wei, H.; Liu, Y.; Zhang, D.; Zhang, B.; Chu, W.; Pham-Huu, C.; Su, D.S. Oxygenated group and structural defect enriched carbon nanotubes for immobilizing gold nanoparticles. Chem. Commun. 2017, 53, 12750–12753. [Google Scholar] [CrossRef] [PubMed]
- Ersen, O.; Hirlimann, C.; Drillon, M.; Werckmann, J.; Tihay, F.; Pham-Huu, C.; Crucifix, C.; Schultz, P. 3D-TEM characterization of nanometric objects. Solid State Sci. 2007, 9, 1088–1098. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351–9364. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, B.; Lin, Y.; Wang, Q.; Zhang, Q.; Su, D.S. Enhanced chemoselective hydrogenation through tuning the interaction between pt nanoparticles and carbon supports: Insights from identical location transmission electron microscopy and X-ray photoelectron spectroscopy. ACS Catal. 2016, 6, 7844–7854. [Google Scholar] [CrossRef]
- Scheibe, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Oxidation and reduction of multiwalled carbon nanotubes—Preparation and characterization. Mater. Charact. 2010, 61, 185–191. [Google Scholar] [CrossRef]
- Chu, W.; Ran, M.; Zhang, X.; Wang, N.; Wang, Y.; Xie, H.; Zhao, X. Remarkable carbon dioxide catalytic capture (CDCC) leading to solid-form carbon material via a new cvd integrated process (CVD-IP): An alternative route for CO2 sequestration. J. Energy Chem. 2013, 22, 136–144. [Google Scholar] [CrossRef]
- Bom, D.; Andrews, R.; Jacques, D.; Anthony, J.; Chen, B.; Meier, M.S.; Selegue, J.P. Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: Evidence for the role of defect sites in carbon nanotube chemistry. Nano Lett. 2002, 2, 615–619. [Google Scholar] [CrossRef]
- Ran, M.; Sun, W.; Liu, Y.; Chu, W.; Jiang, C. Functionalization of multi-walled carbon nanotubes using water-assisted chemical vapor deposition. J. Solid State Chem. 2013, 197, 517–522. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. Significant contribution of intrinsic carbon defects to oxygen reduction activity. ACS Catal. 2015, 5, 6707–6712. [Google Scholar] [CrossRef]
- Shinkarev, V.V.; Glushenkov, A.M.; Kuvshinov, D.G.; Kuvshinov, G.G. New effective catalysts based on mesoporous nanofibrous carbon for selective oxidation of hydrogen sulfide. Appl. Catal. B 2009, 85, 180–191. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Truong-Phuoc, L.; Tran-Thanh, T.; Nhut, J.-M.; Nguyen-Dinh, L.; Janowska, I.; Begin, D.; Pham-Huu, C. Nitrogen-doped carbon nanotubes decorated silicon carbide as a metal-free catalyst for partial oxidation of H2S. Appl. Catal. A 2014, 482, 397–406. [Google Scholar]
- Liu, Y.; Ersen, O.; Meny, C.; Luck, F.; Pham-Huu, C. Fischer–tropsch reaction on a thermally conductive and reusable silicon carbide support. ChemSusChem 2014, 7, 1218–1239. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Dreibine, L.; de Tymowski, B.; Vigneron, F.; Edouard, D.; Bégin, D.; Nguyen, P.; Pham, C.; Savin-Poncet, S.; Luck, F.; et al. Silicon carbide foam composite containing cobalt as a highly selective and re-usable Fischer–Tropsch synthesis catalyst. Appl. Catal. A 2011, 397, 62–72. [Google Scholar] [CrossRef]
- Elamin, M.M.; Muraza, O.; Malaibari, Z.; Ba, H.; Nhut, J.-M.; Pham-Huu, C. Microwave assisted growth of SAPO-34 on β-SiC foams for methanol dehydration to dimethyl ether. Chem. Eng. J. 2015, 274, 113–122. [Google Scholar] [CrossRef]
- Liu, Y.; Podila, S.; Nguyen, D.L.; Edouard, D.; Nguyen, P.; Pham, C.; Ledoux, M.J.; Pham-Huu, C. Methanol dehydration to dimethyl ether in a platelet milli-reactor filled with H-ZSM5/SiC foam catalyst. Appl. Catal. A 2011, 409–410, 113–121. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, X.; Jiang, C.; Tian, C.; Yang, Z.; Zhang, J. Hierarchical ZSM-5/SiC nano-whisker/SiC foam composites: Preparation and application in mtp reactions. J. Catal. 2015, 332, 70–76. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Ba, H.; El-Berrichi, Z.; Nhut, J.-M.; Ledoux, M.J.; Liu, Y.; Pham-Huu, C. Silicon carbide foam as a porous support platform for catalytic applications. New J. Chem. 2016, 40, 4285–4299. [Google Scholar] [CrossRef]
- Mares, B.; Prosernat Com. Personal communication, 2018.











| Treatment Duration | SSA a m2/g | Mass Loss b % | ID/IGc | ID’/IGc | O at % d | TWLe °C |
|---|---|---|---|---|---|---|
| 0 h | 10 | 0 | 0.77 | 0.26 | 7.3 | 802 |
| 4 h | 112 | 7 | 1.64 | 0.55 | - | - |
| 8 h | 196 | 12 | - | - | - | - |
| 16 h | 268 | 31 | 1.83 | 0.66 | - | 685 |
| 24 h | 298 | 44 | 1.86 | 0.80 | 9.4 | 612 |
| 30 h | 329 | 48 | - | - | - | - |
| Treatment Duration | CO2 Desorption µmol/g | CO Desorption µmol/g | Total O µmol/g | ||||
|---|---|---|---|---|---|---|---|
| Carboxylic Acids | Anhydrides | Lactones | Anhydrides | Phenols | Carbonyls | ||
| 0 h | - | - | - | - | - | - | 165 |
| 4 h | 53 | 187 | 128 | 141 | 951 | 230 | 1691 |
| 8 h | 134 | 486 | 148 | 332 | 2199 | 498 | 3797 |
| 16 h | 304 | 626 | 412 | 527 | 3865 | 660 | 6393 |
| 30 h | 422 | 1186 | 424 | 735 | 5462 | 887 | 9116 |
| Catalysts | T °C | [H2S] vol. % | [O2] vol. % | [H2O] vol. % | WHSV h−1 | XH2Sa % | SSb % | YSc % | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| OGFs-16 | 250 | 1 | 2.5 | 10 | 0.1 | 98 | 86 | 84 | This work |
| O-CNT-250-24 | 230 | 1 | 2.5 | 30 | 0.6 | 95 | 76 | 72 | 16 |
| N-CNT/SiC-750 | 190 | 1 | 2.5 | 30 | 0.6 | 97 | 75 | 73 | 52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Duong-Viet, C.; Ba, H.; Li, B.; Truong-Huu, T.; Nguyen-Dinh, L.; Pham-Huu, C. Gaseous Nitric Acid Activated Graphite Felts as Hierarchical Metal-Free Catalyst for Selective Oxidation of H2S. Catalysts 2018, 8, 145. https://doi.org/10.3390/catal8040145
Xu Z, Duong-Viet C, Ba H, Li B, Truong-Huu T, Nguyen-Dinh L, Pham-Huu C. Gaseous Nitric Acid Activated Graphite Felts as Hierarchical Metal-Free Catalyst for Selective Oxidation of H2S. Catalysts. 2018; 8(4):145. https://doi.org/10.3390/catal8040145
Chicago/Turabian StyleXu, Zhenxin, Cuong Duong-Viet, Housseinou Ba, Bing Li, Tri Truong-Huu, Lam Nguyen-Dinh, and Cuong Pham-Huu. 2018. "Gaseous Nitric Acid Activated Graphite Felts as Hierarchical Metal-Free Catalyst for Selective Oxidation of H2S" Catalysts 8, no. 4: 145. https://doi.org/10.3390/catal8040145