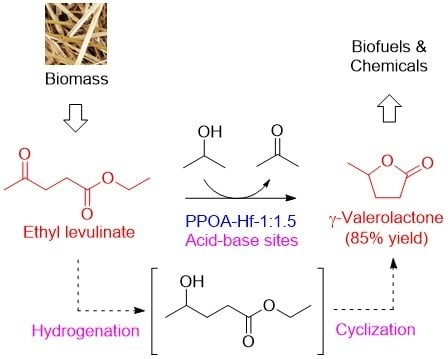
Acid–Base Bifunctional Hf Nanohybrids Enable High Selectivity in the Catalytic Conversion of Ethyl Levulinate to γ-Valerolactone
Abstract
:1. Introduction
2. Results and Discussions
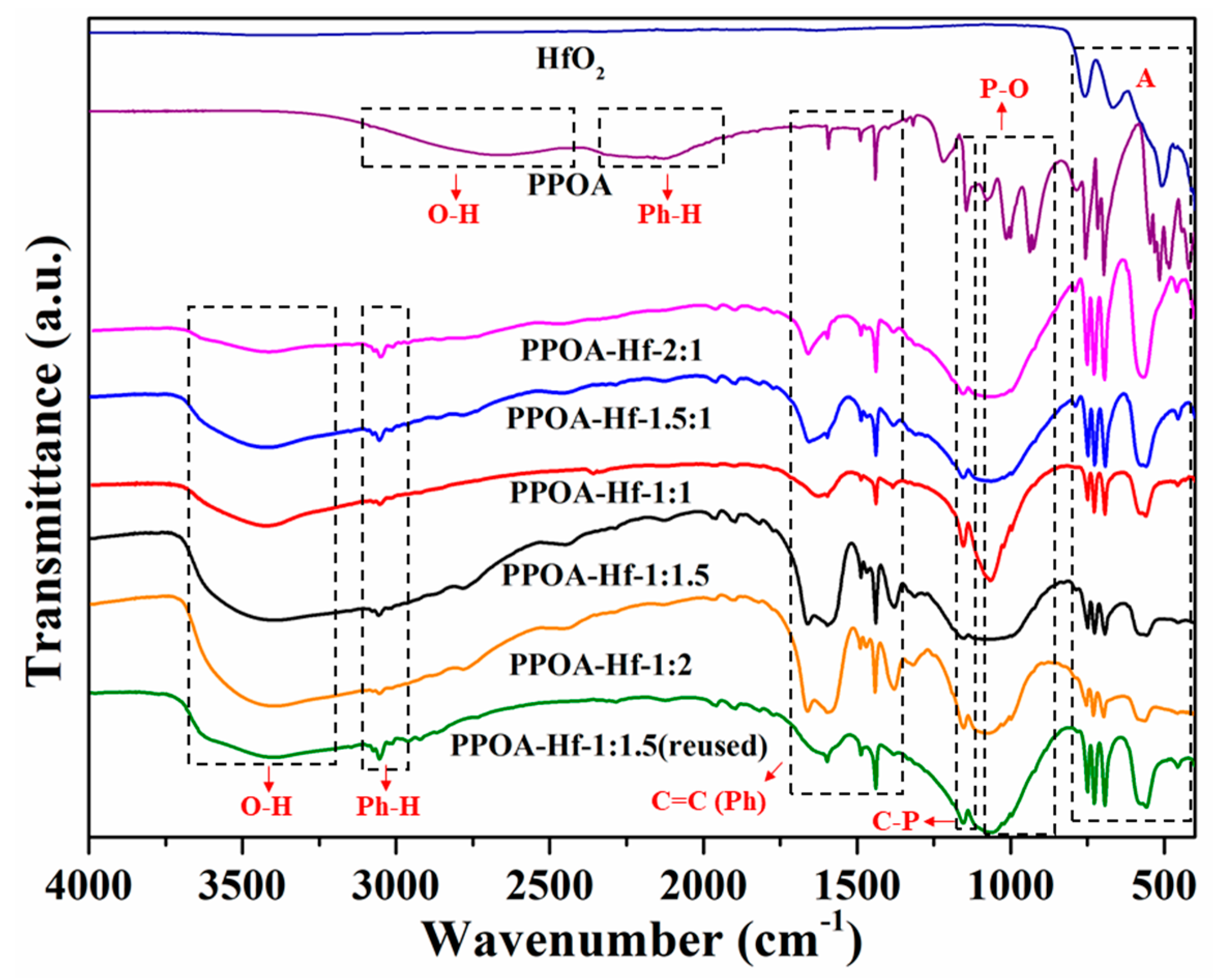
2.1. Catalyst Characterization
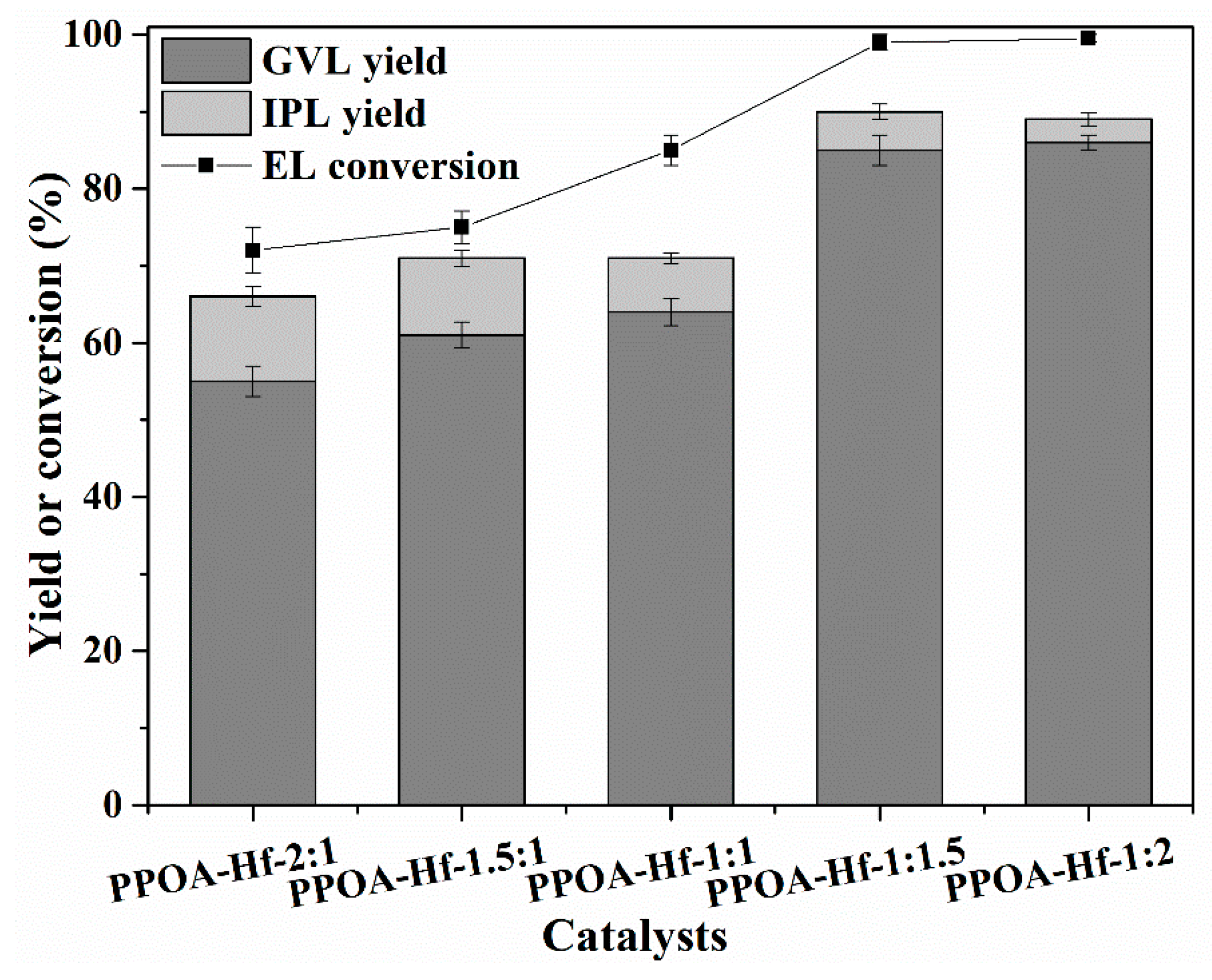
2.2. Catalytic Performance of PPOA–Hf-x
2.3. Effect of Reaction Time and Temperature
2.4. Effect of Catalyst Dosage and Reactivity Comparison with Various Catalysts
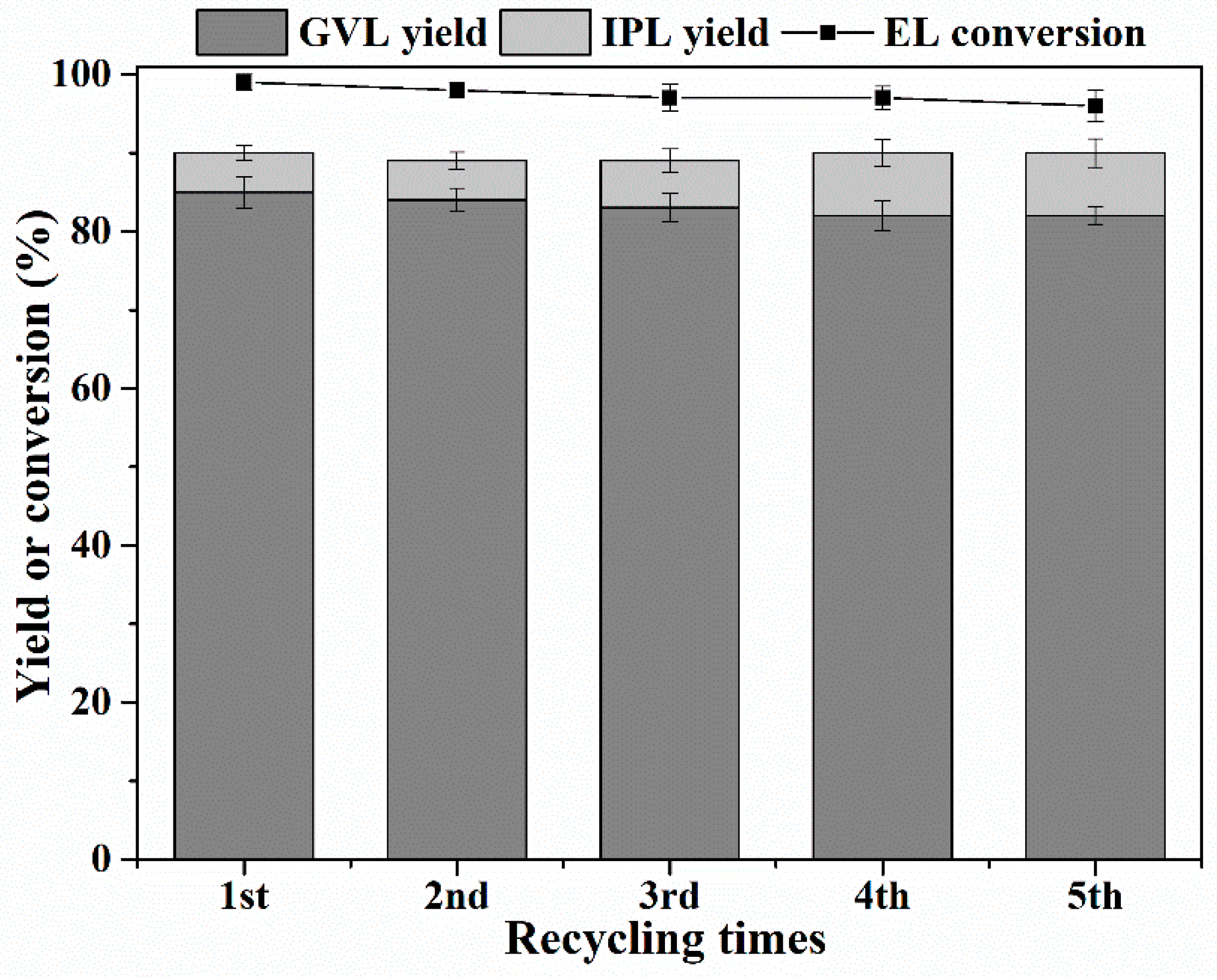
2.5. Catalyst Leaching Experiments and Recycling Study
3. Materials and Experiments
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization Techniques
3.4. Catalytic Activity Measurements
3.5. Product Analysis
3.6. Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Saravanamurugan, S.; Yang, S.; Riisager, A. Direct transformation of carbohydrates to the biofuel 5-ethoxymethylfurfural by solid acid catalysts. Green Chem. 2016, 18, 726–734. [Google Scholar] [CrossRef]
- Li, H.; Bhadury, P.S.; Riisager, A.; Yang, S. One-pot transformation of polysaccharides via multi-catalytic processes. Catal. Sci. Technol. 2014, 4, 4138–4168. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, W.; Fang, Z. Hydrophobic Pd nanocatalysts for one-pot and high-yield production of liquid furanic biofuels at low temperatures. Appl. Catal. B Environ. 2017, 215, 18–27. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Liguori, F.; Moreno-Marrodan, C.; Barbaro, P. Environmentally friendly synthesis of γ-valerolactone by direct catalytic conversion of renewable rources. ACS Catal. 2015, 5, 1882–1894. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Riisager, A.; Pandey, A.; Sangwan, R.S.; Saravanamurugan, S.; Luque, R. Zeolite and zeotype-catalysed transformations of biofuranic compounds. Green Chem. 2016, 18, 5701–5735. [Google Scholar] [CrossRef]
- Zhang, Z. Synthesis of γ-valerolactone from carbohydrates and its applications. ChemSusChem 2016, 9, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Guo, H.; Li, L.Y.; Smith, R.L., Jr. Acid-catalyzed dehydration of fructose into 5-hydroxymethylfurfural by cellulose-derived amorphous carbon. ChemSusChem 2012, 5, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, Z. One-pot catalytic conversion of carbohydrates into furfural and 5-hydroxymethylfurfural. Catal. Sci. Technol. 2016, 6, 3694–3712. [Google Scholar] [CrossRef]
- Guo, H.; Duereh, A.; Hiraga, Y.; Aida, T.M.; Qi, X.; Smith, R.L., Jr. Perfect recycle and mechanistic role of hydrogen sulfate ionic liquids as additive in ethanol for efficient conversion of carbohydrates into 5-ethoxymethylfurfural. Chem. Eng. J. 2017, 323, 287–294. [Google Scholar] [CrossRef]
- Guo, H.; Qi, X.; Hiraga, Y.; Aida, T.M.; Smith, R.L., Jr. Efficient conversion of fructose into 5-ethoxymethylfurfural with hydrogen sulfate ionic liquids as co-solvent and catalyst. Chem. Eng. J. 2017, 314, 508–514. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Van Buu, O.N.; Riisager, A. Conversion of mono- and disaccharides to ethyl levulinate and ethyl pyranoside with sulfonic acid-functionalized ionic liquids. ChemSusChem 2011, 4, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Saravanamurugan, S.; Riisager, A. Solid acid catalysed formation of ethyl levulinate and ethyl glucopyranoside from mono- and disaccharides. Catal. Commun. 2012, 17, 71–75. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Riisager, A. Zeolite catalyzed transformation of carbohydrates to alkyl levulinates. ChemCatChem 2013, 5, 1754–1757. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhou, H.; Fu, Y. Catalytic transfer hydrogenation of furfural to furfuryl alcohol over nitrogen-doped carbon-supported iron catalysts. ChemSusChem 2016, 9, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Astruc, D. The golden age of transfer hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source–recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Bigler, R.; Huber, R.; Stöckli, M.; Mezzetti, A. Iron(II)/(NH)2P2 macrocycles: Modular, highly enantioselective transfer hydrogenation catalysts. ACS Catal. 2016, 6, 6455–6464. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Riisager, A.; Saravanamurugan, S.; Song, B.; Yang, S. Acid-base bifunctional zirconium N-alkyltriphosphate nanohybrid for hydrogen transfer of biomass-derived carboxides. ACS Catal. 2016, 6, 7722–7727. [Google Scholar] [CrossRef]
- Wang, J.; Okumura, K.; Jaenicke, S.; Chuah, G.K. Post-synthesized zirconium-containing beta zeolite in Meerwein-Ponndorf-Verley reduction: Pros and cons. Appl. Catal. A 2015, 493, 112–120. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Dumesic, J.A. Exploring Meerwein-Ponndorf-Verley reduction chemistry for biomass catalysis using a first-principles approach. ACS Catal. 2013, 3, 2694–2704. [Google Scholar] [CrossRef]
- Chia, M.; Dumesic, J.A. Liquid-phase catalytic transfer hydrogenation and cyclization of levulinic acid and its esters to γ-valerolactone over metal oxide catalysts. Chem. Commun. 2011, 47, 12233–12235. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hu, L.; Sun, Y.; Zhao, G.; Hao, W.; Lin, L. Conversion of biomass-derived ethyl levulinate into γ-valerolactone via hydrogen transfer from supercritical ethanol over a ZrO2 catalyst. RSC Adv. 2013, 3, 10277–10284. [Google Scholar] [CrossRef]
- Tang, X.; Chen, H.; Hu, L.; Hao, W.; Sun, Y.; Zeng, X.; Lin, L.; Liu, S. Conversion of biomass to γ-valerolactone by catalytic transfer hydrogenation of ethyl levulinate over metal hydroxides. Appl. Catal. B Environ. 2014, 147, 827–834. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Yang, S. Direct conversion of sugars and ethyl levulinate into γ-valerolactone with superparamagnetic acid-base bifunctional ZrFeOx nanocatalysts. ACS Sustain. Chem. Eng. 2016, 4, 236–246. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Lu, Y.; Liu, Y.; Wu, Z.; Hu, D.; Yang, S. Cascade catalytic transfer hydrogenation-cyclization of ethyl levulinate to γ-valerolactone with Al-Zr mixed oxides. Appl. Catal. A Gen. 2016, 510, 11–19. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Liu, Y.; Zhao, W.; Yang, T.; Xue, W.; Yang, S. Catalytic transfer hydrogenation of ethyl levulinate into γ-valerolactone over mesoporous Zr/B mixed oxides. J. Ind. Eng. Chem. 2016, 43, 133–141. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Li, W.; Jiang, Y.; Hu, L.; Liu, S.; Sun, Y.; Lin, L. In situ generated catalyst system to convert biomass-derived levulinic acid to γ-valerolactone. ChemCatChem 2015, 7, 1372–1379. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Osada, Y.; Fujitani, T.; Yamashita, H. Catalytic transfer hydrogenation of biomass-derived levulinic acid and its esters to γ-valerolactone over ZrO2 catalyst supported on SBA-15 silica. Catal. Today 2017, 281, 418–428. [Google Scholar] [CrossRef]
- de los Reyes, M.; Majewski, P.J.; Scales, N.; Luca, V. Hydrolytic stability of mesoporous zirconium titanate frameworks containing coordinating organic functionalities. ACS Appl. Mater. Interfaces 2013, 5, 4120–4128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, T.; Fang, Z. Biomass-derived mesoporous Hf-containing hybrid for efficient Meerwein-Ponndorf-Verley reduction at low temperatures. Appl. Catal. B Environ. 2018, 227, 79–89. [Google Scholar] [CrossRef]
- Gelman, F.; Blum, J.; Avnir, D. Acids and bases in one pot while avoiding their mutual destruction. Angew. Chem. Int. Ed. 2001, 40, 3647–3649. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Li, X.; Zhao, J.; Bai, S.; Liu, J.; Yang, Q. A yolk-shell nanoreactor with a basic core and an acidic shell for cascade reactions. Angew. Chem. 2012, 124, 9298–9302. [Google Scholar] [CrossRef]
- Margelefsky, E.L.; Zeidan, R.K.; Davis, M.E. Cooperative catalysis by silica-supported organic functional groups. Chem. Soc. Rev. 2008, 37, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fang, Z.; Smith, R.L., Jr.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J.; Hanley, T.L.; Luca, V. Zirconium organophosphonates as high capacity, selective lanthanide sorbents. Sep. Purif. Technol. 2014, 129, 150–158. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, T.; Liu, Y.; Ren, T.; Yuan, Z. Metal phosphonate hybrid materials: From densely layered to hierarchically nanoporous structures. Inorg. Chem. Front. 2014, 1, 360–383. [Google Scholar] [CrossRef]
- Ma, T.; Yuan, Z. Metal phosphonate hybrid mesostructures: Environmentally friendly multifunctional materials for clean energy and other applications. ChemSusChem 2011, 4, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, P.; Bhaumik, A. Organic-inorganic hybrid metal phosphonates as recyclable heterogeneous catalysts. ChemCatChem 2016, 8, 1607–1616. [Google Scholar] [CrossRef]
- Silbernagel, R.; Martin, C.H.; Clearfield, A. Zirconium (IV) phosphonate-phosphates as efficient ion-exchange materials. Inorg. Chem. 2016, 55, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Yang, T.; Zhao, W.; Saravanamurugan, S.; Yang, S. Porous zirconium-furandicarboxylate microspheres for efficient redox conversion of biofuranics. ChemSusChem 2017, 10, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Dai, W.; Sun, X.; Wu, G.; Guan, N.; Hunger, M.; Li, L. Mesoporous Zr-Beta zeolites prepared by a post-synthetic strategy as a robust Lewis acid catalyst for the ring-opening aminolysis of epoxides. Green Chem. 2015, 17, 1744–1755. [Google Scholar] [CrossRef]
- Song, J.; Zhou, B.; Zhou, H.; Wu, L.; Meng, Q.; Liu, Z.; Han, B. Porous zirconium–phytic acid hybrid: A highly efficient catalyst for Meerwein–Ponndorf–Verley reductions. Angew. Chem. Int. Ed. 2015, 54, 9399–9403. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Consoli, D.F.; Gunther, W.R.; Román-Leshkov, Y. Investigation of the reaction kinetics of isolated Lewis acid sites in Beta zeolites for the Meerwein-Ponndorf-Verley reduction of methyl levulinate to γ-valerolactone. J. Catal. 2014, 320, 198–207. [Google Scholar] [CrossRef]
- Assary, R.J.; Curtiss, L.A. Theoretical studies for the formation of γ-valero-lactone from levulinic acid and formic acid by homogeneous catalysis. Chem. Phys. Lett. 2012, 541, 21–26. [Google Scholar] [CrossRef]
- Chalid, M.; Broekhuis, A.A.; Heeres, H.J. Experimental and kinetic modeling studies on the biphasic hydrogenation of levulinic acid to γ-valerolactone using a homogeneous water-soluble Ru-(TPPTS) catalyst. J. Mol. Catal. A Chem. 2011, 341, 14–21. [Google Scholar] [CrossRef]










| Entry | Catalyst | Temp (°C) | Time (h) | GVL Yield (%) | EL Conversion (%) | Reference |
|---|---|---|---|---|---|---|
| 1 | ZrO2 | 180 | 4 | 80 | 93 | [27] |
| 2 | ZrO2 | 250 | 1 | 63 | 82 | [28] |
| 3 | Zr(OH)4 | 240 | 3 | 80 | 99 | [29] |
| 4 | ZrFeO(1:3)-300 | 230 | 3 | 87 | 93 | [30] |
| 5 | Al7Zr3-300 | 220 | 4 | 83 | 96 | [31] |
| 6 | Zr1B1 | 200 | 4 | 88 | 95 | [32] |
| 7 | PPOA–Hf-1:1.5 | 160 | 6 | 85 | 100 | This work |
| Entry | Catalyst | GVL Yield (%) | IPL Yield (%) | EL Conversion (%) | Average Rate (µmol g−1h−1) b |
|---|---|---|---|---|---|
| 1 | SiO2 | <1 | <1 | 1 | <20 |
| 2 | TiO2 | <1 | <1 | 1 | <20 |
| 3 | MgO | <1 | <1 | 1 | <20 |
| 4 | HfO2 | 6 | 1 | 10 | 140 |
| 5 | Al2O3 | 1 | 5 | 8 | 23 |
| 6 | ZrO2 | 2 | - | 5 | 46 |
| 7 | PPOA | 0 | 13 | 16 | - |
| 8 | CaO | 17 | 43 | 81 | 400 |
| 9 | PPOA–Hf-1:1.5 | 85 | 5 | 100 | 1970 |
| 10 | PPOA–Zr-1:1.5 | 73 | 6 | 90 | 1690 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Li, Y.; Li, H.; Zhao, W.; Yang, S. Acid–Base Bifunctional Hf Nanohybrids Enable High Selectivity in the Catalytic Conversion of Ethyl Levulinate to γ-Valerolactone. Catalysts 2018, 8, 264. https://doi.org/10.3390/catal8070264
Wu W, Li Y, Li H, Zhao W, Yang S. Acid–Base Bifunctional Hf Nanohybrids Enable High Selectivity in the Catalytic Conversion of Ethyl Levulinate to γ-Valerolactone. Catalysts. 2018; 8(7):264. https://doi.org/10.3390/catal8070264
Chicago/Turabian StyleWu, Weibo, Yan Li, Hu Li, Wenfeng Zhao, and Song Yang. 2018. "Acid–Base Bifunctional Hf Nanohybrids Enable High Selectivity in the Catalytic Conversion of Ethyl Levulinate to γ-Valerolactone" Catalysts 8, no. 7: 264. https://doi.org/10.3390/catal8070264
APA StyleWu, W., Li, Y., Li, H., Zhao, W., & Yang, S. (2018). Acid–Base Bifunctional Hf Nanohybrids Enable High Selectivity in the Catalytic Conversion of Ethyl Levulinate to γ-Valerolactone. Catalysts, 8(7), 264. https://doi.org/10.3390/catal8070264