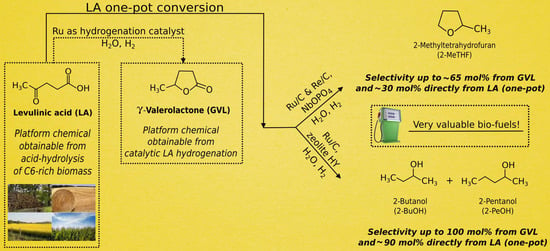
Cascade Strategy for the Tunable Catalytic Valorization of Levulinic Acid and γ-Valerolactone to 2-Methyltetrahydrofuran and Alcohols
Abstract
:1. Introduction
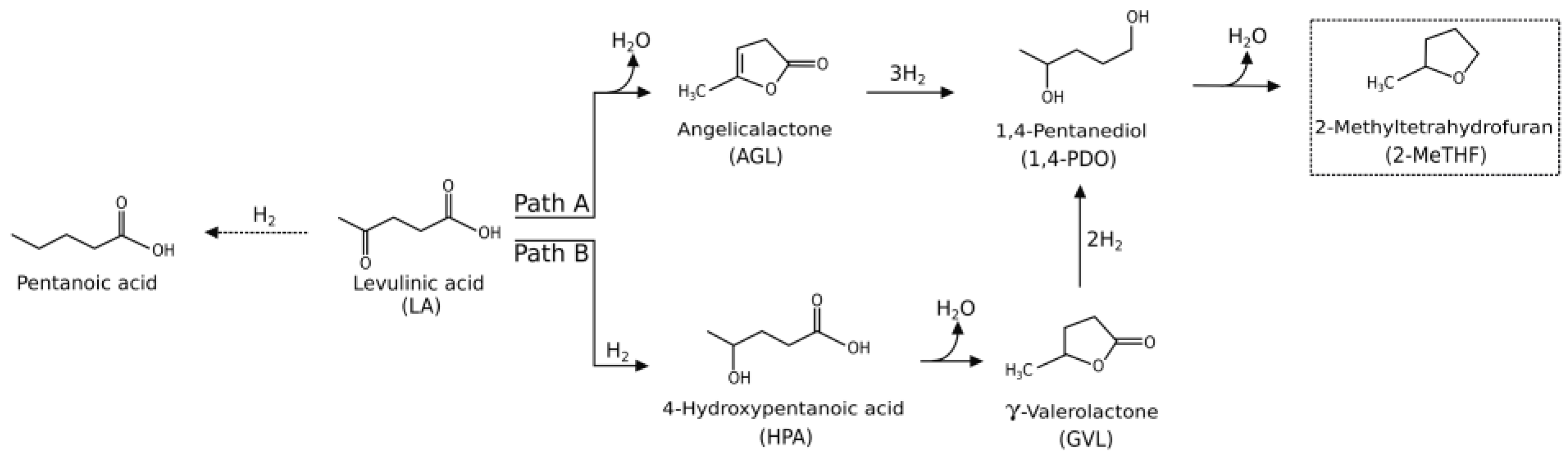
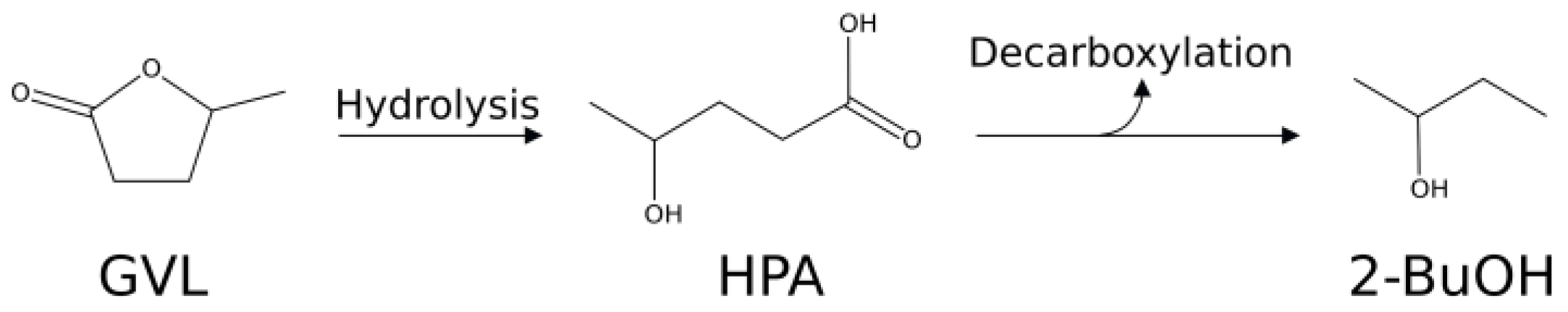
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Hydrogenation Reactions
3.3. Analysis of the Reaction Products
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel strategies for the production of fuels, lubricants, and chemicals from biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility. A review. Biofuels Bioprod. Biorefin. 2017, 11, 195–214. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, C.; Raspolli Galletti, A.M.; Fulignati, S.; Licursi, D. Amberlyst A-70: A surprisingly active catalyst for the MW-assisted dehydration of fructose and inulin to HMF in water. Catal. Commun. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Antonetti, C.; Melloni, M.; Licursi, D.; Fulignati, S.; Ribechini, E.; Rivas, S.; Parajó, J.C.; Cavani, F.; Raspolli Galletti, A.M. Microwave-assisted dehydration of fructose and inulin to HMF catalyzed by Niobium and Zirconium phosphate catalysts. Appl. Catal. B 2018, 206, 364–377. [Google Scholar] [CrossRef]
- Girisuta, B.; Heeres, H.J. Levulinic acid from biomass: Synthesis and applications. In Production of Platform Chemicals from Sustainable Resources; Fang, Z., Smith, R.L., Qi, X., Eds.; Springer: Singapore, 2017; Chapter 5; pp. 143–169. ISBN 978-9811041716. [Google Scholar]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Raspolli Galletti, A.M. New frontiers in the catalytic synthesis of levulinic acid: From sugars to raw and waste biomass as starting feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic reactions of gamma-valerolactone: A platform to fuels and value-added chemicals. Appl. Catal. B 2015, 179, 292–304. [Google Scholar] [CrossRef]
- Zhang, Z. Synthesis of γ-Valerolactone from carbohydrates and its applications. ChemSusChem 2016, 9, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yao, Q.; Fu, Y. Conversion of levulinic acid and alkyl levulinates to biofuels and high-value chemicals. Green Chem. 2017, 19, 5527–5547. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone-A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Yan, K.; Lafleur, T.; Wu, G.; Liao, J.; Ceng, C.; Xie, X. Highly selective production of value-added γ-valerolactone from biomass-derived levulinic acid using the robust Pd nanoparticles. Appl. Catal. A 2013, 468, 52–58. [Google Scholar] [CrossRef]
- Sudholt, A.; Tripathi, R.; Mayer, D.; Glaude, P.A.; Battin-Leclerc, F.; Pitsch, H. The oxidation of the novel lignocellulosic biofuel γ-valerolactone in a low pressure flame. Proc. Combust. Inst. 2017, 36, 577–585. [Google Scholar] [CrossRef]
- Lilga, M.A.; Padmaperuma, A.B.; Auberry, D.L.; Job, H.M.; Swita, M.S. Ketonization of levulinic acid and γ-valerolactone to hydrocarbon fuel precursors. Catal. Today 2018, 302, 80–86. [Google Scholar] [CrossRef]
- Fegyverneki, D.; Orha, L.; Láng, G.; Horváth, I.T. Gamma-valerolactone-based solvents. Tetrahedron 2010, 66, 1078–1081. [Google Scholar] [CrossRef]
- Yan, K.; Lafleur, T.; Wu, X.; Chai, J.; Wu, C.; Xie, X. Cascade upgrading of γ-valerolactone to biofuels. Chem. Commun. 2015, 51, 6984–6987. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem. 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Wang, A.; Lu, Y.; Yi, Z.; Ejaz, A.; Hu, K.; Zhang, L.; Yan, K. Selective production of γ-valerolactone and valeric acid in one-pot bifunctional metal catalysts. ChemistrySelect 2018, 3, 1097–1101. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Antonetti, C.; Ribechini, E.; Colombini, M.P.; Nassi, N.; Bonari, E. From giant reed to levulinic acid and gamma-valerolactone: A high yield catalytic route to valeric biofuels. Appl. Energy 2013, 102, 157–162. [Google Scholar] [CrossRef]
- Antonetti, C.; Bonari, E.; Licursi, D.; Nassi, N.; Raspolli Galletti, A.M. Hydrothermal conversion of giant reed to furfural and levulinic acid: Optimization of the process under microwave irradiation and investigation of distinctive agronomic parameters. Molecules 2015, 20, 21232–21253. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Raspolli Galletti, A.M.; Antonetti, C.; Licursi, D.; Santos, V.; Parajó, J.C. A biorefinery cascade conversion of hemicellulose-free Eucalyptus Globulus wood: Production of concentrated levulinic acid solutions for γ-Valerolactone sustainable preparation. Catalysts 2018, 8, 169. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; De María, P.D.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A biomass-derived solvent with broad application in organic chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Kar, Y.; Deveci, H. Importance of p-series fuels for flexible-fuel vehicles (FFVs) and alternative fuels. Energy Source A Recovery Util. Environ. Eff. 2006, 28, 909–921. [Google Scholar] [CrossRef]
- Da Silva Trindade, W.R.; Dos Santos, R.G. Review on the characteristics of butanol, its production and use as fuel in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 642–651. [Google Scholar] [CrossRef]
- Stevens, J.G.; Bourne, R.A.; Twigg, M.V.; Poliakoff, M. Real-time product switching using a twin catalyst system for the hydrogenation of furfural in supercritical CO2. Angew. Chem. Int. Ed. 2010, 49, 8856–8859. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhu, Y.; Ding, G.; Cui, J.; Li, X.; Li, Y. One-step conversion of furfural into 2-methyltetrahydrofuran under mild conditions. ChemSusChem 2015, 8, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, A.F.; Cai, B.; Luo, J.Y.; Pan, H.; Huang, Y.B. Catalytic transfer hydrogenation of furfural to 2-methylfuran and 2-methyltetrahydrofuran over bimetallic copper-palladium catalysts. ChemSusChem 2016, 9, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Liao, J.; Wu, X.; Xie, X. A noble-metal free Cu-catalyst derived from hydrotalcite for highly efficient hydrogenation of biomass-derived furfural and levulinic acid. RSC Adv. 2013, 3, 3853–3856. [Google Scholar] [CrossRef]
- Christian, R.V.J.; Brown, H.D.; Hixon, R.M. Derivatives of γ-valerolactone, 1,4-pentanediol and 1,4-di-(β-cyanoethoxy)-pentane. J. Am. Chem. Soc. 1947, 69, 1961–1963. [Google Scholar] [CrossRef]
- Elliot, D.; Frye, J.G. Hydrogenated 5-Carbon Compound and Method of Making. U.S. Patent 5883266, 16 March 1999. [Google Scholar]
- Mehdi, H.; Fàbos, V.; Tuba, R.; Bodor, A.; Mika, L.T.; Horvàth, I.T. Integration of homogeneous and heterogeneous catalytic processes for a multi-step conversion of biomass: From sucrose to levulinic acid, γ-valerolactone, 1,4-pentanediol, 2-methyl-tetrahydrofuran, and alkanes. Top. Catal. 2008, 48, 49–54. [Google Scholar] [CrossRef]
- Geilen, F.M.A.; Engendahl, B.; Harwardt, A.; Marquardt, W.; Klankermayer, J.; Leitner, W. Selective and flexible transformation of biomass-derived platform chemicals by a multifunctional catalytic system. Angew. Chem. Int. Ed. 2010, 49, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Upare, P.P.; Lee, J.M.; Hwang, Y.K.; Hwang, D.W.; Lee, J.H.; Halligudi, S.B.; Hwang, J.S.; Chang, J.S. Direct hydrocyclization of biomass-derived levulinic acid to 2-methyltetrahydrofuran over nanocomposite copper/silica catalysts. ChemSusChem 2011, 4, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Bi, Q.Y.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Tunable copper-catalyzed chemoselective hydrogenolysis of biomass-derived γ-valerolactone into 1,4-pentanediol or 2-methyltetrahydrofuran. Green Chem. 2012, 14, 935–939. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Menendez, J.A.; Romero, A.A.; Serrano, E.; Garcia-Martinez, J.; Luque, R. Continuous flow nanocatalysis: Reaction pathways in the conversion of levulinic acid to valuable chemicals. Green Chem. 2013, 15, 2786–2792. [Google Scholar] [CrossRef] [Green Version]
- Al-Shaal, M.G.; Dzierbinski, A.; Palkovits, R. Solvent-free γ-valerolactone hydrogenation to 2-methyltetrahydrofuran catalysed by Ru/C: A reaction network analysis. Green Chem. 2014, 16, 1358–1364. [Google Scholar] [CrossRef]
- Mizugaki, T.; Togo, K.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. One-pot transformation of levulinic acid to 2-methyltetrahydrofuran catalyzed by Pt−Mo/H-β in water. ACS Sustain. Chem. Eng. 2016, 4, 682–685. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, M.; Lee, S.K.; Yoon, J.W.; Bae, J.; Hwang, D.W.; Lee, UH.; Chang, J.S.; Hwang, Y.K. Ru nanoparticles supported graphene oxide catalyst for hydrogenation of bio-based levulinic acid to cyclic ethers. Catal. Today 2016, 265, 174–183. [Google Scholar] [CrossRef]
- Obregón, I.; Gandarias, I.; Al-Shaal, M.G.; Mevissen, C.; Arias, P.L.; Palkovits, R. The role of the hydrogen source on the selective production of γ-valerolactone and 2-methyltetrahydrofuran from levulinic acid. ChemSusChem 2016, 9, 2488–2495. [Google Scholar] [CrossRef]
- Thomas, J.J.; Barile, R.G. Conversion of cellulose hydrolysis products to fuel and chemical feedstocks. Biomass Wastes 1985, 8, 1461–1494. [Google Scholar]
- Al-Shaal, M.G.; Hausoul, P.J.C.; Palkovits, R. Efficient, solvent-free hydrogenation of α-angelica lactone catalysed by Ru/C at atmospheric pressure and room temperature. Chem. Commun. 2014, 50, 10206–10209. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaal, M.G.; Wright, W.R.H.; Palkovits, R. Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions. Green Chem. 2012, 14, 1260–1263. [Google Scholar] [CrossRef]
- Abdelrahman, O.A.; Heyden, A.; Bond, J.Q. Analysis of kinetics and reaction pathways in the aqueous-phase hydrogenation of levulinic acid to form γ-valerolactone over Ru/C. ACS Catal. 2014, 4, 1171–1181. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, J.; Xu, X.; Wang, W.; Li, J.; Zhao, Y.; Tang, K.; Song, Q.; Qi, X.; Kong, D.; et al. Continuous hydrogenation of ethyl levulinate to γ-valerolactone and 2-methyl tetrahydrofuran over alumina doped Cu/SiO2 catalyst: The potential of commercialization. Sci. Rep. 2016, 6, 28898–28907. [Google Scholar] [CrossRef] [PubMed]
- Obregón, I.; Gandarias, I.; Miletic´, N.; Ocio, A.; Arias, P.L. One-pot 2-methyltetrahydrofuran production from levulinic acid in green solvents using Ni-Cu/Al2O3 catalysts. ChemSusChem 2015, 8, 3483–3488. [Google Scholar] [CrossRef] [PubMed]
- Assima, G.P.; Zamboni, I.; Lavoie, J.M. Alcohol fuels: The thermochemical route. In Biofuels Production and Processing Technology; Riazi, R.M., Chiaramonti, D., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 14; pp. 362–406. ISBN 978-1-4987-7893-0. [Google Scholar]
- Shabaker, J.W.; Weiner, A.; Rundell, D.N.; Sejour, H.; Goeden, G. Unit, Method, and Renewable Materials. U.S. Patent 20100281763A1, 11 November 2010. [Google Scholar]
- Lv, J.; Rong, Z.; Sun, L.; Liu, C.; Lu, A.H.; Wang, Y.; Qu, J. Catalytic conversion of biomass-derived levulinic acid into alcohols over nanoporous Ru catalyst. Catal. Sci. Technol. 2018, 8, 975–979. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Antonetti, C.; De Luise, V.; Martinelli, M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem. 2012, 12, 688–694. [Google Scholar] [CrossRef]
- Corbel-Demailly, L.; Ly, B.K.; Minh, D.P.; Tapin, B.; Especel, C.; Epron, F.; Cabiac, A.; Guillon, E.; le Besson, M.; Pinel, C. Heterogeneous catalytic hydrogenation of biobased levulinic and succinic acids in aqueous solutions. ChemSusChem 2013, 6, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Buitrago-Sierra, R.; Serrano-Ruiz, J.C.; Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A.; Dumesic, J.A. Ce promoted Pd-Nb catalysts for γ-valerolactone ring-opening and hydrogenation. Green Chem. 2012, 14, 3318–3324. [Google Scholar] [CrossRef]
- Luo, W.; Deka, U.; Beale, A.M.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]
- Prati, L.; Jouve, A.; Villa, A. Production and upgrading of γ-valerolactone with bifunctional catalytic processes. In Production of Biofuels and Chemicals with Bifunctional Catalysts, 1st ed.; Fang, Z., Smith, R.L., Li, H., Eds.; Springer: Singapore, 2017; Volume 8, pp. 221–237. ISBN 978-981-10-5136-4. [Google Scholar]
- Freitas, F.A.; Licursi, D.; Lachter, E.R.; Raspolli Galletti, A.M.; Antonetti, C.; Brito, T.C.; Nascimento, R.S.V. Heterogeneous catalysis for the ketalisation of ethyl levulinate with 1,2-dodecanediol: Opening the way to a new class of bio-degradable surfactants. Catal. Commun. 2016, 73, 84–87. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Carlini, C.; Sbrana, G.; Armaroli, T.; Busca, G. Selective saccharides dehydration to 5-hydroxymethyl-2-furaldehyde by heterogeneous niobium catalysts. Appl. Catal. A 1999, 183, 295–302. [Google Scholar] [CrossRef]
- Amaroli, T.; Busca, G.; Carlini, C.; Giuttari, M.; Raspolli Galletti, A.M.; Sbrana, G. Active acid sites characterization of niobium phosphate catalysts and their activity in fructose dehydration to 5-hydroxymethyl-2-furaldehyde. J. Mol. Catal. A: Chem. 2000, 151, 233–243. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; West, R.M.; Dumesic, J.A. γ-Valerolactone ring-opening and decarboxylation over SiO2/Al2O3 in the presence of water. Langmuir 2010, 26, 16291–16298. [Google Scholar] [CrossRef] [PubMed]
- Kellicutt, A.B.; Salary, R.; Abdelrahman, O.A.; Bond, J.Q. An examination of the intrinsic activity and stability of various solid acids during the catalytic decarboxylation of γ-valerolactone. Catal. Sci. Technol. 2014, 4, 2267–2279. [Google Scholar] [CrossRef]
- Villa, A.; Schiavoni, M.; Chan-Thaw, C.E.; Fulvio, P.F.; Mayes, R.T.; Dai, S.; More, K.L.; Veith, G.M.; Prati, L. Acid-functionalized mesoporous carbon: An efficient support for ruthenium-catalyzed γ-valerolactone production. ChemSusChem 2015, 8, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Schüβler, F.; D’Amico, A.; Danilina, N.; Van Bokhoven, J.A.; Lercher, J.A.; Jones, C.W.; Sievers, C. Stability of zeolites in hot liquid water. J. Phys. Chem. C 2010, 114, 19582–19595. [Google Scholar] [CrossRef]
- Pine, L.A. Decarboxylation. U.S. Patent 3476803A, 4 November 1969. [Google Scholar]
- Di, X.; Li, C.; Zhang, B.; Qi, J.; Li, W.; Su, D.; Liang, C. Role of Re and Ru in Re. Ru/C bimetallic catalysts for the aqueous hydrogenation of succinic Acid. Ind. Eng. Chem. Res. 2017, 56, 4672–4683. [Google Scholar] [CrossRef]
- Van Borm, R.; Reyniers, M.F.; Martens, J.A.; Marin, G.B. Catalytic cracking of methylcyclohexane on FAU, MFI, and bimodal porous materials: Influence of acid properties and pore topology. Ind. Eng. Chem. Res. 2010, 49, 10486–10495. [Google Scholar] [CrossRef]



| Run | Catalytic System | GVL/Re mol/mol | T (°C) | t [h] | Conv. GVL (mol %) | Selectivity (mol %) | |||
|---|---|---|---|---|---|---|---|---|---|
| 2-MeTHF | 2-BuOH | 2-PeOH | 1,4-PDO | ||||||
| 1 | Ru/C 5% | - | 210 | 6 | traces | - | - | - | - |
| 2 | Ru/C 5% + NBP (1 g) | - | 200 | 3 | 31.7 | 35.2 | 42.2 | 5.7 | 12.2 |
| 3 | Ru/C 5% + NBP (1 g) | - | 200 | 6 | 40.5 | 38.9 | 34.0 | 6.5 | 14.0 |
| 4 | Ru/C 5% + NBP (1 g) | - | 180 | 6 | 3.6 | 49.2 | 27.0 | 0.1 | 5.4 |
| 5 | Ru/C 5% + NBP (1 g) | - | 210 | 6 | 71.1 | 21.0 | 66.8 | 7.7 | 0.4 |
| 6 | Re/C 10% (10 mg) | 312.33 | 210 | 6 | traces | - | - | - | - |
| 7 | Ru/C 5% + Re/C 10% (5 mg) + NBP (1 g) | 624.66 | 200 | 3 | 35.9 | 43.0 | 31.5 | 8.7 | 14.1 |
| 8 | Ru/C 5% + Re/C 10% (10 mg) + NBP (1 g) | 312.33 | 200 | 3 | 36.3 | 47.2 | 23.0 | 6.1 | 18.8 |
| 9 | Ru/C 5% + Re/C 10% (20 mg) + NBP (1g) | 156.16 | 200 | 3 | 36.1 | 57.5 | 11.1 | 4.6 | 21.3 |
| 10 | Ru/C 5% + Re/C 10% (20 mg), no NBP | 156.16 | 200 | 3 | 27.3 | 22.1 | 6.1 | 3.6 | 63.8 |
| 11 | Ru/C 5% + Re/C 10% (20 mg) + NBP (250 mg) | 156.16 | 200 | 3 | 30.8 | 44.6 | 7.1 | 5.1 | 30.2 |
| 12 | Ru/C 5% + Re/C 10% (20 mg) + NBP (500 mg) | 156.16 | 200 | 3 | 39.3 | 64.9 | 7.3 | 3.7 | 21.5 |
| Run | Catalyst and co-catalyst | Reaction conditions T, PH2 | Conv. GVL (mol %) | Sel. 2-MeTHF (mol %) | Sel. 2-BuOH (mol %) | Sel. 2-PeOH (mol %) | Sel. 1,4-PDO (mol %) |
|---|---|---|---|---|---|---|---|
| 13 | 5% Ru/C NBP | 200 °C, 9.0 MPa | 98.9 | 8.5 | 75.7 | 15.8 | - |
| 14 | 5% Ru/C NBP | 200 °C, 5.0 MPa | 77.9 | 5.7 | 83.3 | 10.9 | 0.1 |
| 15 | 5% Ru/C NBP | 180 °C, 5.0 MPa | 26.6 | 9.7 | 60.6 | 4.2 | 20.7 |
| 16 | 5% Ru/C 10% Re/C NBP | 180 °C, 5.0 MPa | 48.3 | 37.8 | 36.6 | 7.5 | 18.1 |
| 17 | 5% Ru/C HY | 180 °C 5.0 MPa | 66.3 | 21.3 | 63.3 | 13.8 | 1.6 |
| 18 | 5% Ru/C HY | 200 °C 3.0 MPa | 100 | - | 81.3 | 18.7 | - |
| 19 | 5% Ru/C 10% Re/C HY | 200 °C 3.0 MPa | 100 | 35.7 | 47.6 | 16.7 | - |
| Run | Catalyst and Co-Catalyst | T (°C) | Conv. LA (mol %) | Sel. GVL (mol %) | Sel. 2-MeTHF (mol %) | Sel. 2-BuOH (mol %) | Sel. 2-PeOH (mol %) | Sel. 1,4-PDO (mol %) |
|---|---|---|---|---|---|---|---|---|
| 20 | 5% Ru/C, NBP | 200 | 99.7 | 73.4 | 8.8 | 9.9 | 0.9 | 1.3 |
| 21 | 5% Ru/C, NBP | 210 | 99.9 | 70.6 | 8.7 | 13.8 | 1.5 | 1.7 |
| Run | Catalyst and Co-Catalyst | Reaction Conditions T, PH2 | Conv. LA (mol %) | Sel. GVL (mol %) | Sel. 2-MeTHF (mol %) | Sel. 2-BuOH (mol %) | Sel. 2-PeOH (mol %) | Sel. 1,4-PDO (mol %) |
|---|---|---|---|---|---|---|---|---|
| 22 | 5%Ru/C NBP | 180 °C, 5.0 MPa | 100 | 36.2 | 10.1 | 37.8 | 13.0 | 2.9 |
| 23 | 5%Ru/C HY | 180 °C, 5.0 MPa | 100 | 30.8 | 3.8 | 35.0 | 28.7 | 1.7 |
| 24 | 5%Ru/C HY | 180 °C, 3.0 MPa | 100 | 46.8 | 3.6 | 36.5 | 13.1 | - |
| 25 | 5%Ru/C HY | 180 °C, 1.0 MPa | 100 | 81.1 | 0.6 | 13.6 | 4.7 | - |
| 26 | 5%Ru/C HY | 200 °C, 3.0 MPa | 100 | 7.9 | 3.3 | 68.9 | 19.9 | - |
| 27 | 5%Ru/C NBP | 200 °C 3.0 MPa | 100 | 25.8 | 7.0 | 52.0 | 15.2 | - |
| 28 | 5%Ru/C10%R/C, NBP | 180 °C 5.0 MPa | 100 | 35.1 | 27.8 | 19.5 | 6.0 | 11.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licursi, D.; Antonetti, C.; Fulignati, S.; Giannoni, M.; Raspolli Galletti, A.M. Cascade Strategy for the Tunable Catalytic Valorization of Levulinic Acid and γ-Valerolactone to 2-Methyltetrahydrofuran and Alcohols. Catalysts 2018, 8, 277. https://doi.org/10.3390/catal8070277
Licursi D, Antonetti C, Fulignati S, Giannoni M, Raspolli Galletti AM. Cascade Strategy for the Tunable Catalytic Valorization of Levulinic Acid and γ-Valerolactone to 2-Methyltetrahydrofuran and Alcohols. Catalysts. 2018; 8(7):277. https://doi.org/10.3390/catal8070277
Chicago/Turabian StyleLicursi, Domenico, Claudia Antonetti, Sara Fulignati, Michael Giannoni, and Anna Maria Raspolli Galletti. 2018. "Cascade Strategy for the Tunable Catalytic Valorization of Levulinic Acid and γ-Valerolactone to 2-Methyltetrahydrofuran and Alcohols" Catalysts 8, no. 7: 277. https://doi.org/10.3390/catal8070277