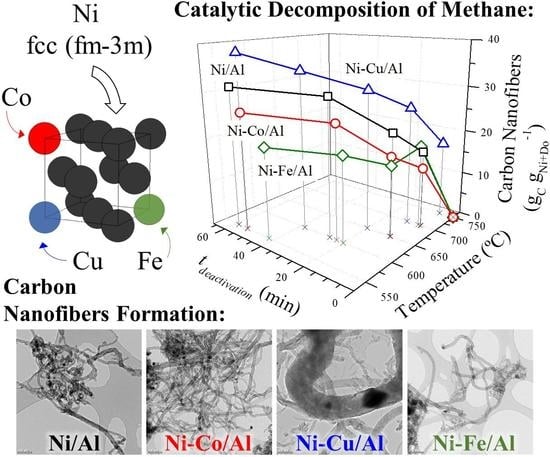
Co-, Cu- and Fe-Doped Ni/Al2O3 Catalysts for the Catalytic Decomposition of Methane into Hydrogen and Carbon Nanofibers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
2.2. Activity of of Ni and Ni–Do Catalysts in the CDM Reaction
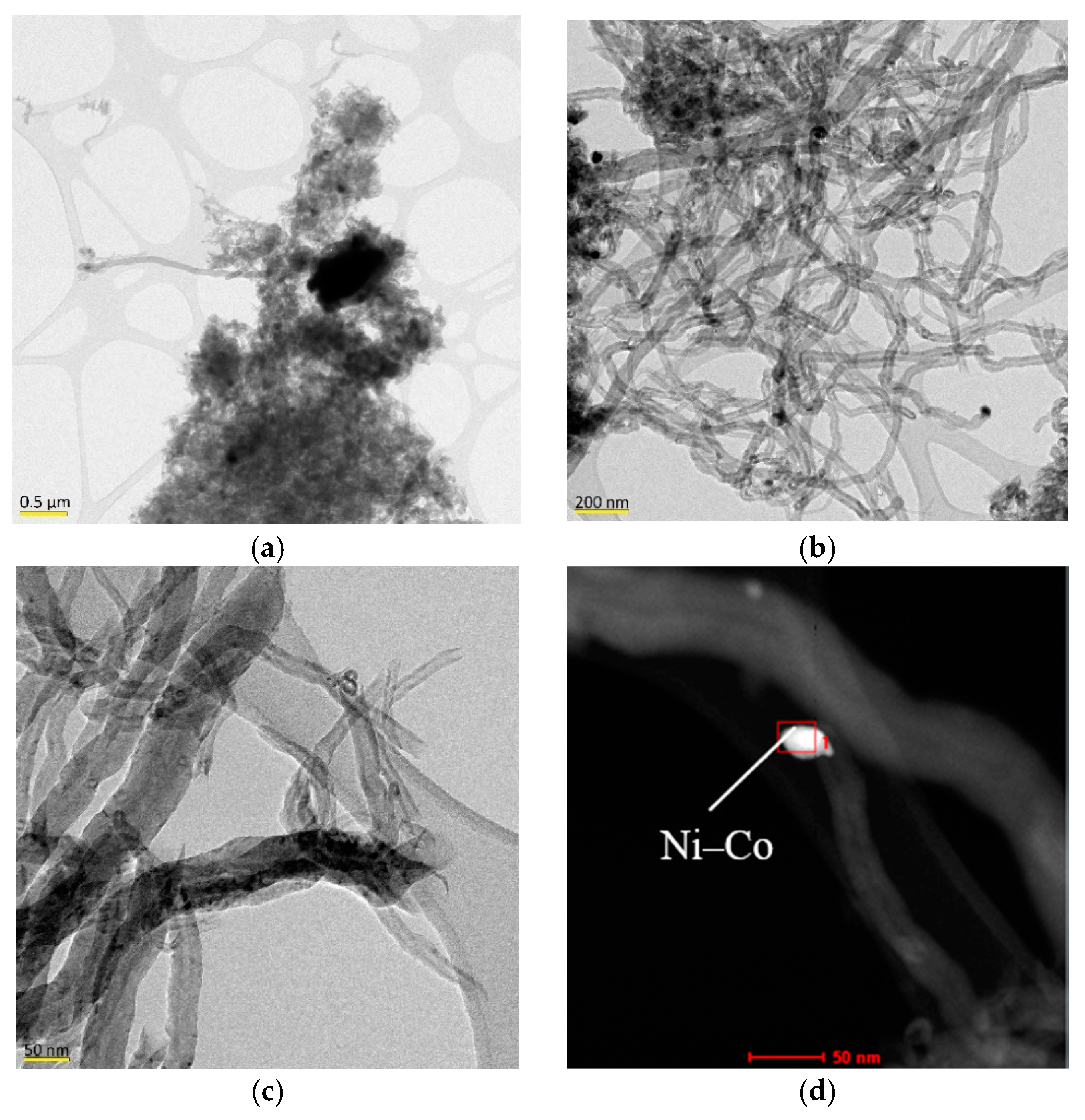
2.3. Characterization of the Carbon Nanofilaments
3. Materials and Methods
3.1. Preparation of the Catalysts
3.2. Experimental Facilities and CDM Tests
3.3. Characterization Techniques
4. Conclusions
- Co, Cu, and Fe were studied as dopants of Ni in Ni/Al2O3 catalysts for the obtention of carbon nanofibers in the CDM reaction. Dopant addition had different catalytic effects depending on the metal used: while Cu had a positive effect on the catalyst activity, Co and Fe presented a negative one, although all shortened the time to achieve the maximum carbon formation rate at lower operating temperatures (550–600 °C).
- Cu-doped catalysts yielded higher carbon formations and longer activity times at any operating temperature in the range 550–750 °C. Likewise, the Cu catalyst was active at temperatures above 700 °C, where undoped, Co-, and Fe-doped catalysts did not show activity.
- In the case of Fe doping, the formation of Ni3Fe was observed, but this did not improve the behavior of the undoped catalyst in the CDM although it was active in this reaction with the formation of narrow carbon nanofilaments. As Cu, Fe also improved the performance of the Ni catalyst at 700 °C presenting a slightly higher carbon formation than the undoped catalyst.
- Characterization of the bimetallic catalysts showed the formation of Ni alloys and the presence of a bimodal distribution of particle sizes. Cu achieved an expansion of the Ni crystalline structure (in addition to increasing its weight fraction) in the larger nickel particles which was found effective in the additional formation of large carbon nanofibers. For the doping content studied (5 mol. %), only Cu formed an alloy with a lattice constant high enough to be able to favor the carbon diffusion against surface diffusion, resulting in a higher carbon formation and the obtention of denser carbon nanofibers.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xueqing, Z.; Anja, B.-H. Modeling and simulations in photoelectrochemical water oxidation: From single level to multiscale modeling. ChemSusChem 2016, 9, 1223–1242. [Google Scholar]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Muradov, N. Hydrogen via methane decomposition: An application for decarbonization of fossil fuels. Int. J. Hydrogen Energy 2001, 26, 1165–1175. [Google Scholar] [CrossRef]
- Ichi-oka, H.A.; Higashi, N.O.; Yamada, Y.; Miyake, T.; Suzuki, T. Carbon nanotube and nanofiber syntheses by the decomposition of methane on group 8–10 metal-loaded mgo catalysts. Diam. Relat. Mater. 2007, 16, 1121–1125. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to cox-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Harb, M.; Saih, Y.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.-L.; Li, J.; Wei, N.; Gary, D.; et al. Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials. Appl. Catal. B Environ. 2017, 208, 44–59. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Siakavelas, G.; Charisiou, N.D.; Avraam, D.G.; Tzounis, L.; Kousi, K.; Goula, M.A. Comparative study of Ni, Co, Cu supported on γ-alumina catalysts for hydrogen production via the glycerol steam reforming reaction. Fuel Process. Technol. 2016, 152, 156–175. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Muradov, N.; Vezirolu, T. From hydrocarbon to hydrogen-carbon to hydrogen economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Avdeeva, L.B.; Goncharova, O.V.; Kochubey, D.I.; Zaikovskii, V.I.; Plyasova, L.M.; Novgorodov, B.N.; Shaikhutdinov, S.K. Coprecipitated Ni-alumina and Ni-Cu-alumina catalysts of methane decomposition and carbon deposition. II. Evolution of the catalysts in reaction. Appl. Catal. A Gen. 1996, 141, 117–129. [Google Scholar] [CrossRef]
- Takenaka, S.; Shigeta, Y.; Tanabe, E.; Otsuka, K. Methane decomposition into hydrogen and carbon nanofibers over supported Pd–Ni catalysts. J. Catal. 2003, 220, 468–477. [Google Scholar] [CrossRef]
- Hermes, N.A.; Lansarin, M.A.; Perez-Lopez, O.W. Catalytic decomposition of methane over M–Co–Al catalysts (M = Mg, Ni, Zn, Cu). Catal. Lett. 2011, 141, 1018–1025. [Google Scholar] [CrossRef]
- Nuernberg, G.D.B.; Fajardo, H.V.; Foletto, E.L.; Hickel-Probst, S.M.; Carreño, N.L.V.; Probst, L.F.D.; Barrault, J. Methane conversion to hydrogen and nanotubes on Pt/Ni catalysts supported over spinel mgal2o4. Catal. Today 2011, 176, 465–469. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Yang, Y.; Jiang, S. Ni–SiO2 and Ni–Fe–SiO2 catalysts for methane decomposition to prepare hydrogen and carbon filaments. Int. J. Hydrogen Energy 2012, 37, 9058–9066. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; Aboul-Gheit, A.K. Various nickel doping in commercial Ni–Mo/Al2O3 as catalysts for natural gas decomposition to COx-free hydrogen production. Renew. Energy 2013, 57, 671–678. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Y.; Liu, G.; Li, Y. Production of hydrogen and nanocarbon from catalytic decomposition of methane over a Ni–Fe/Al2O3 catalyst. Energy Fuels 2013, 27, 4448–4456. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Khan, W.U.; Al-Fatesh, A.S.; Abasaeed, A.E.; Naeem, M.A. Production of hydrogen and carbon nanofibers from methane over Ni–Co–Al catalysts. Int. J. Hydrogen Energy 2015, 40, 1774–1781. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.S. Direct decomposition of methane over SBA-15 supported Ni, Co and Fe based bimetallic catalysts. Appl. Surf. Sci. 2015, 330, 418–430. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Methane decomposition over Ni–Fe/Al2O3 catalysts for production of COx-free hydrogen and carbon nanofiber. Int. J. Hydrogen Energy 2016, 41, 1574–1584. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Zaikovskii, V.I.; Buyanov, R.A.; Molchanov, V.V.; Plyasova, L.M. Morphology of carbon from methane on nickel-containing catalysts. Catal. Today 1995, 24, 265–267. [Google Scholar] [CrossRef]
- Wang, H.; Baker, R.T.K. Decomposition of methane over a Ni−Cu−MgO catalyst to produce hydrogen and carbon nanofibers. J. Phys. Chem. B 2004, 108, 20273–20277. [Google Scholar] [CrossRef]
- Suelves, I.; Lázaro, M.J.; Moliner, R.; Echegoyen, Y.; Palacios, J.M. Characterization of NiAl and NiCuAl catalysts prepared by different methods for hydrogen production by thermo catalytic decomposition of methane. Catal. Today 2006, 116, 271–280. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Suelves, I.; Lázaro, M.J.; Moliner, R.; Palacios, J.M. Hydrogen production by thermocatalytic decomposition of methane over Ni-Al and Ni-Cu-Al catalysts: Effect of calcination temperature. J. Power Sources 2007, 169, 150–157. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Echegoyen, Y.; Suelves, I.; Palacios, J.M.; Moliner, R. Decomposition of methane over Ai-SiO2 and Ni-Cu-SiO2 catalysts: Effect of catalyst preparation method. Appl. Catal. A Gen. 2007, 329, 22–29. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Suelves, I.; Lázaro, M.J.; Sanjuán, M.L.; Moliner, R. Thermo catalytic decomposition of methane over Ni–Mg and Ni–Cu–Mg catalysts: Effect of catalyst preparation method. Appl. Catal. A Gen. 2007, 333, 229–237. [Google Scholar] [CrossRef]
- Lua, A.C.; Wang, H.Y. Decomposition of methane over unsupported porous nickel and alloy catalyst. Appl. Catal. B Environ. 2013, 132–133, 469–478. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Pant, K.K. Synthesis of hydrogen and carbon nanotubes over copper promoted Ni/SiO2 catalyst by thermocatalytic decomposition of methane. J. Nat. Gas Sci. Eng. 2013, 13, 52–59. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Synthesis of Ni and Ni–Cu supported on carbon nanotubes for hydrogen and carbon production by catalytic decomposition of methane. Appl. Catal. B Environ. 2015, 164, 61–69. [Google Scholar] [CrossRef]
- Naresh, G.; Vijay Kumar, V.; Anjaneyulu, C.; Tardio, J.; Bhargava, S.K.; Patel, J.; Venugopal, A. Nano size hβ zeolite as an effective support for Ni and NiCu for COx free hydrogen production by catalytic decomposition of methane. Int. J. Hydrogen Energy 2016, 41, 19855–19862. [Google Scholar] [CrossRef]
- Berndt, F.M.; Perez-Lopez, O.W. Catalytic decomposition of methane over Ni/SiO2: Influence of Cu addition. React. Kinet. Mech. Catal. 2017, 120, 181–193. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Methane dissociation to COx-free hydrogen and carbon nanofiber over Ni–Cu/Al2O3 catalysts. Fuel 2017, 195, 88–96. [Google Scholar] [CrossRef]
- Gutta, N.; Velisoju, V.K.; Chatla, A.; Boosa, V.; Tardio, J.; Patel, J.; Akula, V. Promotional effect of Cu and influence of surface Ni–Cu alloy for enhanced H2 yields from CH4 decomposition over Cu-modified Ni supported on MCM-41 catalyst. Energy Fuels 2018, 32, 4008–4015. [Google Scholar] [CrossRef]
- Asm Handbook Volume 3: Alloy Phase Diagrams, 1st ed.; ASM International: Materials Park, OH, USA, 1992.
- Chesnokov, V.V.; Chichkan, A.S. Production of hydrogen by methane catalytic decomposition over Ni–Cu–Fe/Al2O3 catalyst. Int. J. Hydrogen Energy 2009, 34, 2979–2985. [Google Scholar] [CrossRef]
- Shah, N.; Panjala, D.; Huffman, G.P. Hydrogen production by catalytic decomposition of methane. Energy Fuels 2001, 15, 1528–1534. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Suelves, I. Screening of Ni-Cu bimetallic catalysts for hydrogen and carbon nanofilaments production via catalytic decomposition of methane. Appl. Catal. A Gen. 2018, 559, 10–19. [Google Scholar] [CrossRef]
- Villacampa, J.I.; Royo, C.; Romeo, E.; Montoya, J.A.; Del Angel, P.; Monzón, A. Catalytic decomposition of methane over Ni-Al2O3 coprecipitated catalysts: Reaction and regeneration studies. Appl. Catal. A Gen. 2003, 252, 363–383. [Google Scholar] [CrossRef]
- Pinilla, J.L.; Suelves, I.; Lázaro, M.J.; Moliner, R.; Palacios, J.M. Influence of nickel crystal domain size on the behaviour of Ni and NiCu catalysts for the methane decomposition reaction. Appl. Catal. A Gen. 2009, 363, 199–207. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.-W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Ismagilov, Z.R.; Chuvilin, A.L.; Ushakov, V.A. Carbon capacious Ni-Cu-Al2O3 catalysts for high-temperature methane decomposition. Appl. Catal. A Gen. 2003, 247, 51–63. [Google Scholar] [CrossRef]
- Pinilla, J.L.; de Llobet, S.; Moliner, R.; Suelves, I. Ni-Co bimetallic catalysts for the simultaneous production of carbon nanofibres and syngas through biogas decomposition. Appl. Catal. B Environ. 2017, 200, 255–264. [Google Scholar] [CrossRef]
- Thomassen, L. An X-ray investigation of the system Cr2O3-NiO1. J. Am. Chem. Soc. 1940, 62, 1134–1136. [Google Scholar] [CrossRef]
- Peres, A.P.S.; Lima, A.C.; Barros, B.S.; Melo, D.M.A. Synthesis and characterization of NiCo2O4 spinel using gelatin as an organic precursor. Mater. Lett. 2012, 89, 36–39. [Google Scholar] [CrossRef]
- Gonzalez-delaCruz, V.M.; Pereñiguez, R.; Ternero, F.; Holgado, J.P.; Caballero, A. In situ XAS study of synergic effects on Ni–Co/ZrO2 methane reforming catalysts. J. Phys. Chem. C 2012, 116, 2919–2926. [Google Scholar] [CrossRef]
- Knop, O.; Reid, K.I.G.; Sutarno; Nakagawa, Y. Chalkogenides of the transition elements. Vi. X-ray, neutron, and magnetic investigation of the spinels Co3O4, NiCo2O4, Co3S4, and NiCo2S4. Can. J. Chem. 1968, 46, 3463–3476. [Google Scholar] [CrossRef]
- Suh, I.-K.; Ohta, H.; Waseda, Y. High-temperature thermal expansion of six metallic elements measured by dilatation method and X-ray diffraction. J. Mater. Sci. 1988, 23, 757–760. [Google Scholar] [CrossRef]
- Yang, R.T.; Chen, J.P. Mechanism of carbon filament growth on metal catalysts. J. Catal. 1989, 115, 52–64. [Google Scholar] [CrossRef]
- Hume-Rothery, W.; Coles, B.R. Atomic Theory for Students of Metallurgy, 1st ed.; Institute of Metals: London, UK; Brookfield, VT, USA, 1988. [Google Scholar]
- Denton, A.R.; Ashcroft, N.W. Vegard‘s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Chicinaş, I.; Pop, V.; Isnard, O.; Le Breton, J.M.; Juraszek, J. Synthesis and magnetic properties of Ni3Fe intermetallic compound obtained by mechanical alloying. J. Alloys Compd. 2003, 352, 34–40. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Kim, M.S.; Baker, R.T.K. Deactivation of copper nickel-catalysts due to changes in surface composition. J. Catal. 1993, 140, 16–29. [Google Scholar] [CrossRef]
- Sebastián, D.; Ruiz, A.G.; Suelves, I.; Moliner, R.; Lázaro, M.J. On the importance of the structure in the electrical conductivity of fishbone carbon nanofibers. J. Mater. Sci. 2013, 48, 1423–1435. [Google Scholar] [CrossRef]
- Suelves, I.; Pinilla, J.L.; Lázaro, M.J.; Moliner, R.; Palacios, J.M. Effects of reaction conditions on hydrogen production and carbon nanofiber properties generated by methane decomposition in a fixed bed reactor using a nicual catalyst. J. Power Sources 2009, 192, 35–42. [Google Scholar] [CrossRef]
- Suelves, I.; Lazaro, M.; Moliner, R.; Corbella, B.; Palacios, J. Hydrogen production by thermo catalytic decomposition of methane on Ni-based catalysts: Influence of operating conditions on catalyst deactivation and carbon characteristics. Int. J. Hydrogen Energy 2005, 30, 1555–1567. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Torres, D.; Pinilla, J.L.; Suelves, I. Unzipping of multi-wall carbon nanotubes with different diameter distributions: Effect on few-layer graphene oxide obtention. Appl. Surf. Sci. 2017, 424, 101–110. [Google Scholar] [CrossRef]
- Trucano, P.; Chen, R. Structure of graphite by neutron diffraction. Nature 1975, 258, 136. [Google Scholar] [CrossRef]
- Zhu, Y.-A.; Dai, Y.-C.; Chen, D.; Yuan, W.-K. First-principles study of carbon diffusion in bulk nickel during the growth of fishbone-type carbon nanofibers. Carbon 2007, 45, 21–27. [Google Scholar] [CrossRef]
- Snoeck, J.W.; Froment, G.F.; Fowles, M. Filamentous carbon formation and gasification: Thermodynamics, driving force, nucleation, and steady-state growth. J. Catal. 1997, 169, 240–249. [Google Scholar] [CrossRef]
- Hofmann, S.; Csányi, G.; Ferrari, A.C.; Payne, M.C.; Robertson, J. Surface diffusion: The low activation energy path for nanotube growth. Phys. Rev. Lett. 2005, 95. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, J.L.; Suelves, I.; Lázaro, M.J.; Moliner, R.; Palacios, J.M. Activity of nicual catalyst in methane decomposition studied using a thermobalance and the structural changes in the Ni and the deposited carbon. Int. J. Hydrogen Energy 2008, 33, 2515–2524. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Lázaro, M.J.; Moliner, R.; Suelves, I. Hydrogen and multiwall carbon nanotubes production by catalytic decomposition of methane: Thermogravimetric analysis and scaling-up of Fe–Mo catalysts. Int. J. Hydrogen Energy 2014, 39, 3698–3709. [Google Scholar] [CrossRef] [Green Version]
- Biscoe, J. An X-ray study of carbon black. J. Appl. Phys. 1942, 13, 364–371. [Google Scholar] [CrossRef]









| Elemental Analysis (wt. %) | SBETa (m2 g−1) | Smicb (m2 g−1) | 2θ Pos. (°) | d002 (nm) | Lc (nm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Metals | ||||||
| Ni/Al | 96.70 | 0.13 | 0.03 | 0.00 | 0.30 | 2.84 | 90 | 16 | 26.14 | 0.3407 | 5.6 |
| Ni–Co/Al | 95.84 | 0.14 | 0.00 | 0.00 | 1.04 | 2.98 | 96 | 19 | 26.19 | 0.3400 | 5.5 |
| Ni–Cu/Al | 97.80 | 0.22 | 0.07 | 0.00 | 0.39 | 1.52 | 130 | 39 | 26.29 | 0.3387 | 6.5 |
| Ni–Fe/Al | 96.23 | 0.13 | 0.03 | 0.00 | 0.25 | 3.36 | 91 | 15 | 26.15 | 0.3405 | 5.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, D.; Pinilla, J.L.; Suelves, I. Co-, Cu- and Fe-Doped Ni/Al2O3 Catalysts for the Catalytic Decomposition of Methane into Hydrogen and Carbon Nanofibers. Catalysts 2018, 8, 300. https://doi.org/10.3390/catal8080300
Torres D, Pinilla JL, Suelves I. Co-, Cu- and Fe-Doped Ni/Al2O3 Catalysts for the Catalytic Decomposition of Methane into Hydrogen and Carbon Nanofibers. Catalysts. 2018; 8(8):300. https://doi.org/10.3390/catal8080300
Chicago/Turabian StyleTorres, Daniel, José Luis Pinilla, and Isabel Suelves. 2018. "Co-, Cu- and Fe-Doped Ni/Al2O3 Catalysts for the Catalytic Decomposition of Methane into Hydrogen and Carbon Nanofibers" Catalysts 8, no. 8: 300. https://doi.org/10.3390/catal8080300