The Promotional Effects of ZrO2 and Au on the CuZnO Catalyst Regarding the Durability and Activity of the Partial Oxidation of Methanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Durability of Catalysts
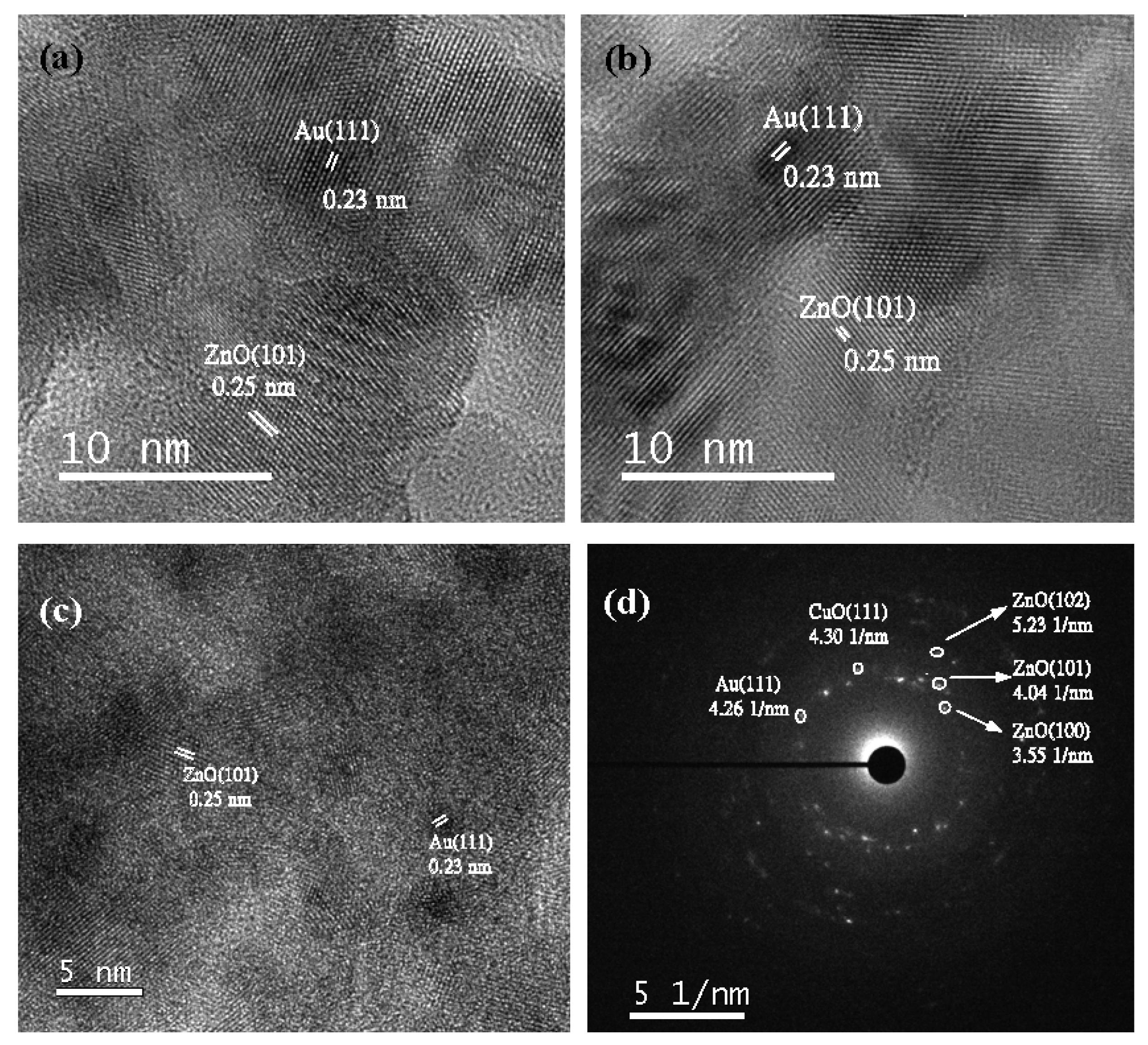
2.2. Physiochemical Properties of the CZ Catalysts with ZrO2 and Gold Promoter
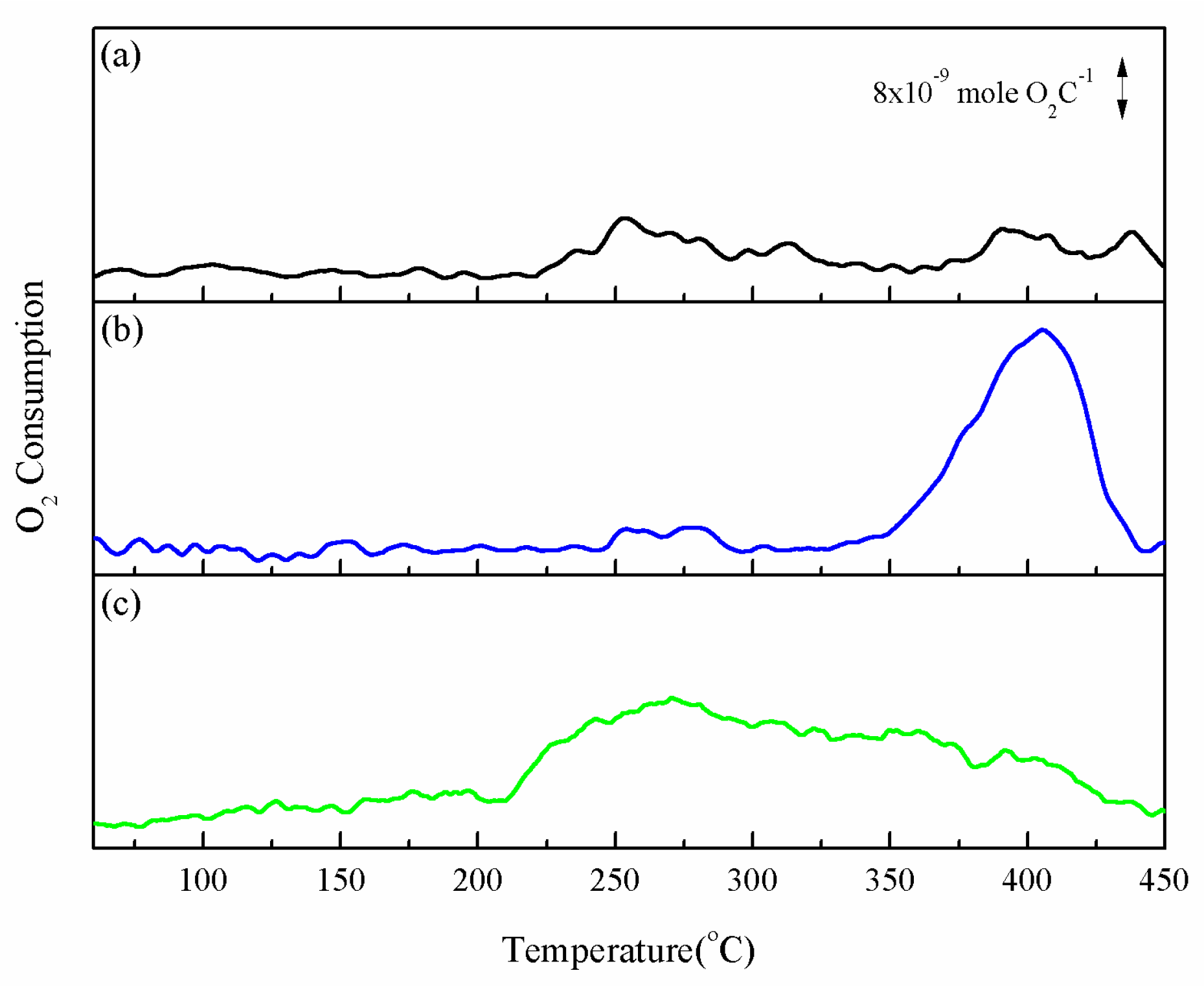
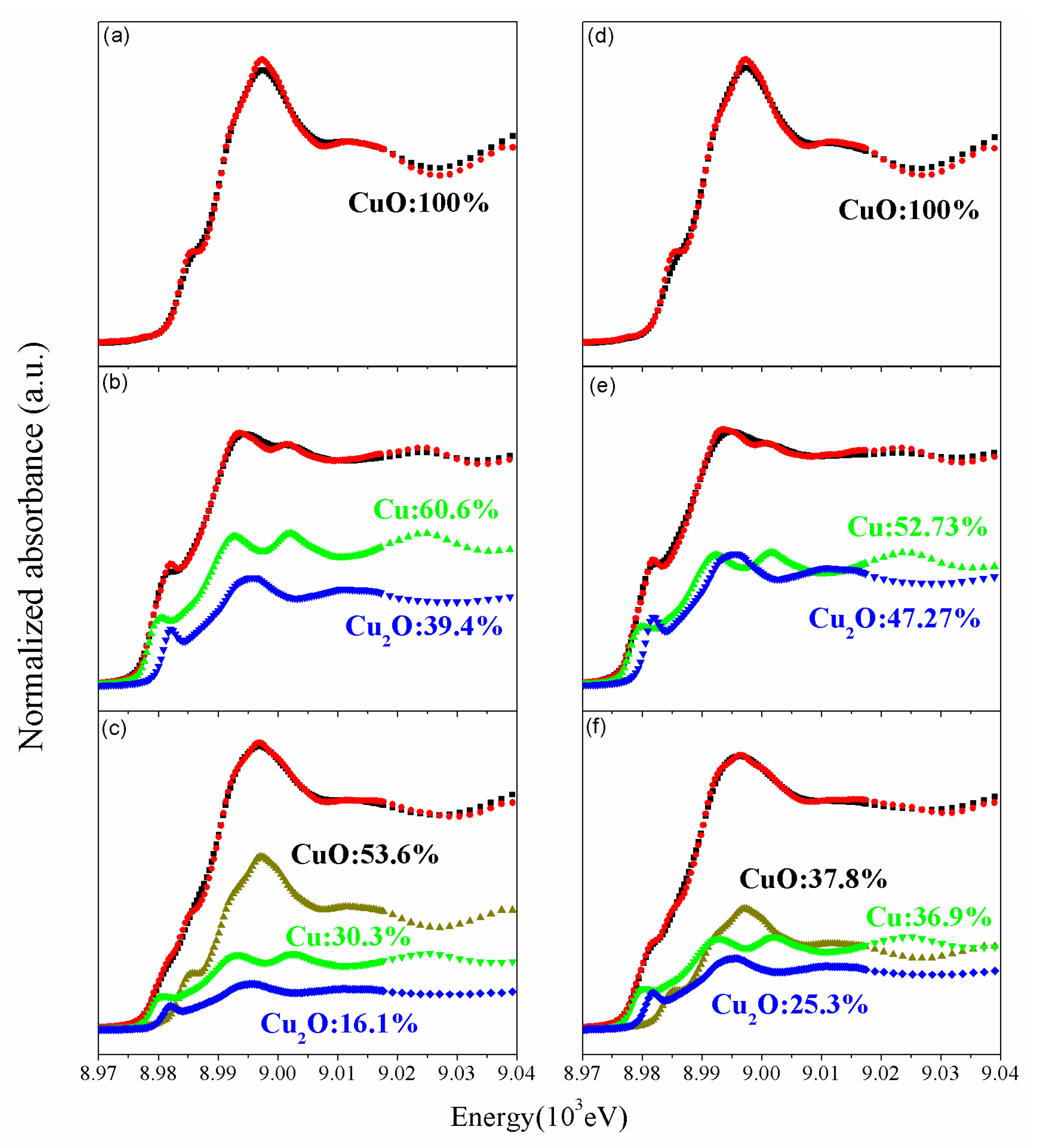
2.3. Reducibility of Catalyst
2.4. Oxygen Mobility of Catalyst
2.5. In-Situ DRIFTS
3. Materials and Methods
3.1. Preparation of Catalyst
3.2. Characterization of Catalysts
3.3. Catalytic Activity and Durability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alejo, L.; Lago, R.; Peña, M.A.; Fierro, J.L.G. Partial oxidation of methanol to produce hydrogen over CnZn-based catalysts. Appl. Catal. A: Gen. 1997, 162, 281–297. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Osaki, T. Selective production of hydrogen by partial oxidation of methanol over catalysts derived from CuZnAl-layered double hydroxides. Catal. Lett. 1999, 62, 159–167. [Google Scholar] [CrossRef]
- Agrell, J.; Hasselbo, K.; Jansson, K.; Järås, S.G.; Boutonnet, M. Production of hydrogen by partial oxidation of methanol over Cu/ZnO catalysts prepared by microemulsion technique. Appl. Catal. A: Gen. 2001, 211, 239–250. [Google Scholar] [CrossRef]
- Mo, L.; Zheng, X.; Yeh, C.-T. Selective production of hydrogen from partial oxidation of methanol over silver catalysts at low temperatures. Chem. Commun. 2004, 1426–1427. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.; Yeh, C.-T. Ignition of methanol partial oxidation over supported platinum catalyst. Catal. Today 2007, 129, 293–296. [Google Scholar] [CrossRef]
- Huang, T.-J.; Wang, S.-W. Hydrogen production via partial oxidation of methanol over copper-zinc catalysts. Appl. Catal. 1986, 24, 287–297. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takaki, K.; Takehira, K. Active Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation method in steam reforming of methanol. Appl. Catal. A: Gen. 2004, 263, 249–253. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takehira, K. Production of hydrogen from methanol over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation: Steam reforming and oxidative steam reforming. J. Mol. Catal. A: Chem. 2007, 268, 185–194. [Google Scholar] [CrossRef]
- Li, C.-L.; Lin, Y.-C. Catalytic partial oxidation of methanol over copper–zinc based catalysts: A comparative study of alumina, zirconia, and magnesia as promoters. Catal. Lett. 2010, 140, 69–76. [Google Scholar] [CrossRef]
- Sanches, S.G.; Flores, J.H.; de Avillez, R.R.; Pais da Silva, M.I. Influence of preparation methods and Zr and Y promoters on Cu/ZnO catalysts used for methanol steam reforming. Int. J. Hydrog. Energy 2012, 37, 6572–6579. [Google Scholar] [CrossRef]
- Zhang, X.R.; Shi, P.; Zhao, J.; Zhao, M.; Liu, C. Production of hydrogen for fuel cells by steam reforming of methanol on Cu/ZrO2/Al2O3 catalysts. Fuel Process. Technol. 2003, 83, 183–192. [Google Scholar] [CrossRef]
- Matter, P.H.; Braden, D.J.; Ozkan, U.S. Steam reforming of methanol to H2 over nonreduced Zr-containing CuO/ZnO catalysts. J. Catal. 2004, 223, 340–351. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ishibe, H. High temperature steam reforming of methanol over Cu/ZnO/ZrO2 catalysts. Appl. Catal. B: Environ. 2009, 91, 524–532. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Ni, C.; Sun, T.; Zhao, S.; Wang, S.; Wang, A.; Hu, Y. CeO2–ZrO2-promoted CuO/ZnoO catalyst for methanol steam reforming. Int. J. Hydrog. Energy 2013, 38, 4397–4406. [Google Scholar] [CrossRef]
- Oguchi, H.; Nishiguchi, T.; Matsumoto, T.; Kanai, H.; Utani, K.; Matsumura, Y.; Imamura, S. Steam reforming of methanol over Cu/CeO2/ZrO2 catalysts. Appl. Catal. A: Gen. 2005, 281, 69–73. [Google Scholar] [CrossRef]
- Park, J.E.; Yim, S.-D.; Kim, C.S.; Park, E.D. Steam reforming of methanol over Cu/ZnO/ZrO2/Al2O3 catalyst. Int. J. Hydrog. Energy 2014, 39, 11517–11527. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chang, C.-T.; Chiang, S.-J.; Liaw, B.-J.; Chen, Y.-Z. Oxidative steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts. Int. J. Hydrog. Energy 2010, 35, 7675–7683. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Grisel, R.J.H.; Nieuwenhuys, B.E. A comparative study of the oxidation of CO and CH4 over Au/Mox/Al2O3 catalysts. Catal. Today 2001, 64, 69–81. [Google Scholar] [CrossRef]
- Haruta, M. Catalysis of gold nanoparticles deposited on metal oxides. Cattech 2002, 6, 102–115. [Google Scholar] [CrossRef]
- Wolf, A.; Schüth, F. A systematic study of the synthesis conditions for the preparation of highly active gold catalysts. Appl. Catal. A: Gen. 2002, 226, 1–13. [Google Scholar] [CrossRef]
- Hvolbæk, B.; Janssens, T.V.W.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Nørskov, J.K. Catalytic activity of Au nanoparticles. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Ng, K.L.; Huang, H.-Y. The effect of gold on the copper-zinc oxides catalyst during the partial oxidation of methanol reaction. Int. J. Hydrog. Energy 2011, 36, 15203–15211. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Kim, D.H.; Kung, M.C.; Kozlova, A.; Yuan, S.D.; Kung, H.H. Synergism between Pt/Al2O3 and Au/TiO2 in the low temperature oxidation of propene. Catal. Lett. 2004, 98, 11–15. [Google Scholar] [CrossRef]
- Konova, P.; Naydenov, A.; Venkov, C.; Mehandjiev, D.; Andreeva, D.; Tabakova, T. Activity and deactivation of Au/TiO2 catalyst in CO oxidation. J. Mol. Catal. A: Chem. 2004, 213, 235–240. [Google Scholar] [CrossRef]
- Naknam, P.; Luengnaruemitchai, A.; Wongkasemjit, S. Au/ZnO and Au/ZnO−Fe2O3 prepared by deposition−precipitation and their activity in the preferential oxidation of CO. Energy Fuels 2009, 23, 5084–5091. [Google Scholar] [CrossRef]
- Bond, G.C.; Louis, C.; Thompson, D.T. Catalysis by Gold; Imperial College Press: London, UK, 2006. [Google Scholar]
- Lee, H.H. Deactivation of catalysts by R. Hughes, academic press (London), June 1984; 265p. AIChE J. 1985, 31, 523. [Google Scholar] [CrossRef]
- Schrum, E.D.; Reitz, T.L.; Kung, H.H. Deactivation of CuO/ZnO and CuO/ZrO2 catalysts for oxidative methanol reforming. Stud. Surf. Sci. Catal. 2001, 139, 229–235. [Google Scholar]
- Fogler, H.S. Elements of Chemical Reaction Engineering; Prentice-Hall International, Inc.: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Xie, S.; Iglesia, E.; Bell, A.T. Water-assisted tetragonal-to-monoclinic phase transformation of ZrO2 at low temperatures. Chem. Mater. 2000, 12, 2442–2447. [Google Scholar] [CrossRef]
- Petkov, V.; Ren, Y.; Shan, S.Y.; Luo, J.; Zhong, C.J. A distinct atomic structure-catalytic activity relationship in 3–10 nm supported Au particles. Nanoscale 2014, 6, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Tsung, C.-K.; Huang, W.; Zhang, X.; Yang, P. Sub-two nanometer single crystal Au nanowires. Nano Lett. 2008, 8, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Alemozafar, A.R.; Biener, M.M.; Biener, J.; Friend, C.M. Reaction of Au (111) with sulfur and oxygen: Scanning tunneling microscopic study. Top. Catal. 2005, 36, 77–90. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Wang, J.-H.; Shen, C.-C.; Yeh, C.-T. Promotion of a copper–zinc catalyst with rare earth for the steam reforming of methanol at low temperatures. J. Catal. 2011, 279, 241–245. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ishibe, H. Effect of zirconium oxide added to Cu/ZnO catalyst for steam reforming of methanol to hydrogen. J. Mol. Catal. A: Chem. 2011, 345, 44–53. [Google Scholar] [CrossRef]
- Koeppel, R.A.; Baiker, A.; Wokaun, A. Copper/zirconia catalysts for the synthesis of methanol from carbon dioxide: Influence of preparation variables on structural and catalytic properties of catalysts. Appl. Catal. A: Gen. 1992, 84, 77–102. [Google Scholar] [CrossRef]
- Wang, Y.H.; Gao, W.G.; Wang, H.; Zheng, Y.E.; Na, W.; Li, K.Z. Structure–activity relationships of Cu–ZrO2 catalysts for CO2 hydrogenation to methanol: Interaction effects and reaction mechanism. RSC Adv. 2017, 7, 8709–8717. [Google Scholar] [CrossRef]
- Sloczynski, J.; Grabowski, R.; Kozlowska, A.; Olszewski, P.K.; Stoch, J. Reduction kinetics of CuO in CuO/ZnO/ZrO2 systems. Phys. Chem. Chem. Phys. 2003, 5, 4631–4640. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Okazaki, M.; Kapoor, M.P.; Osaki, T.; Ohashi, F. Oxidative steam reforming of methanol over CuZnAl(Zr)-oxide catalysts for the selective production of hydrogen for fuel cells: Catalyst characterization and performance evaluation. J. Catal. 2000, 194, 373–384. [Google Scholar] [CrossRef]
- Fierro, G.; Lo Jacono, M.; Inversi, M.; Porta, P.; Cioci, F.; Lavecchia, R. Study of the reducibility of copper in CuO-ZnO catalysts by temperature-programmed reduction. Appl. Catal. A: Gen. 1996, 137, 327–348. [Google Scholar] [CrossRef]
- Burch, R.; Golunski, S.E.; Spencer, M.S. The role of hydrogen in methanol synthesis over copper catalysts. Catal. Lett. 1990, 5, 55–60. [Google Scholar] [CrossRef]
- Burch, R.; Golunski, S.E.; Spencer, M.S. The role of copper and zinc oxide in methanol synthesis catalysts. J. Chem. Soc. Faraday Trans. 1990, 86, 2683–2691. [Google Scholar] [CrossRef]
- Wang, L.-C.; Liu, Q.; Chen, M.; Liu, Y.-M.; Cao, Y.; Fan, K.-N. Structural evolution and catalytic properties of nanostructured Cu/ZrO2 catalysts prepared by oxalate gel-coprecipitation technique. J. Phys. Chem. C 2007, 111, 16549–16557. [Google Scholar] [CrossRef]
- Tada, S.; Katagiri, A.; Kiyota, K.; Honma, T.; Kamei, H.; Nariyuki, A.; Uchida, S.; Satokawa, S. Cu species incorporated into amorphous ZrO2 with high activity and selectivity in CO2-to-methanol hydrogenation. J. Phys. Chem. C 2018, 122, 5430–5442. [Google Scholar] [CrossRef]
- Kartusch, C.; van Bokhoven, J.A. Hydrogenation over gold catalysts: The interaction of gold with hydrogen. Gold Bull. 2009, 42, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Law, J.B.K.; Thong, J.T.L. Improving the NH3 gas sensitivity of ZnO nanowire sensors by reducing the carrier concentration. Nanotechnology 2008, 19, 205502. [Google Scholar] [CrossRef] [PubMed]
- Sinhamahapatra, A.; Jeon, J.-P.; Kang, J.; Han, B.; Yu, J.-S. Oxygen-deficient zirconia (ZrO(2−x)): A new material for solar light absorption. Sci. Rep. 2016, 6, 27218. [Google Scholar] [CrossRef] [PubMed]
- Bozo, C.; Guilhaume, N.; Herrmann, J.-M. Role of the ceria–zirconia support in the reactivity of platinum and palladium catalysts for methane total oxidation under lean conditions. J. Catal. 2001, 203, 393–406. [Google Scholar] [CrossRef]
- Pu, Z.-Y.; Liu, X.-S.; Jia, A.-P.; Xie, Y.-L.; Lu, J.-Q.; Luo, M.-F. Enhanced activity for CO oxidation over Pr- and Cu-doped CeO2 catalysts: Effect of oxygen vacancies. J. Phys. Chem. C 2008, 112, 15045–15051. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, S.; Zhu, H.; Dai, S.; Overbury, S.H. Oxygen-assisted reduction of Au species on Au/SiO2 catalyst in room temperature CO oxidation. Chem. Commun. 2008, 0, 3308–3310. [Google Scholar] [CrossRef] [PubMed]
- Cutrufello, M.G.; Rombi, E.; Cannas, C.; Casu, M.; Virga, A.; Fiorilli, S.; Onida, B.; Ferino, I. Synthesis, characterization and catalytic activity of Au supported on functionalized SBA-15 for low temperature CO oxidation. J. Mater. Sci. 2009, 44, 6644. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, S.; Zhu, H.; Dai, S.; Overbury, S.H. DRIFTS-QMS study of room temperature CO oxidation on Au/SiO2 catalyst: Nature and role of different Au species. J. Phys. Chem. C 2009, 113, 3726–3734. [Google Scholar] [CrossRef]
- Simakov, A.; Tuzovskaya, I.; Pestryakov, A.; Bogdanchikova, N.; Gurin, V.; Avalos, M.; Farías, M.H. On the nature of active gold species in zeolites in CO oxidation. Appl. Catal. A: Gen. 2007, 331, 121–128. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Huang, H.-Y.; Huang, Y.-J. Photo-triggered catalytic reforming of methanol over gold-promoted, copper-zinc catalyst at low ignition temperature. Appl. Catal. B: Environ. 2018, 220, 264–271. [Google Scholar] [CrossRef]
- Oguchi, H.; Kanai, H.; Utani, K.; Matsumura, Y.; Imamura, S. Cu2O as active species in the steam reforming of methanol by CuO/ZrO2 catalysts. Appl. Catal. A: Gen. 2005, 293, 64–70. [Google Scholar] [CrossRef]
- Millar, G.J.; Rochester, C.H.; Waugh, K.C. Evidence for the adsorption of molecules at special sites located at copper/zinc oxide interfaces. Part 2. –A fourier-transform infrared spectroscopy study of methanol adsorption on reduced and oxidised Cu/ZnO/SiO2 catalysts. J. Chem. Soc. Faraday Trans. 1992, 88, 2257–2261. [Google Scholar] [CrossRef]
- Finocchio, E.; Daturi, M.; Binet, C.; Lavalley, J.C.; Blanchard, G. Thermal evolution of the adsorbed methoxy species on CexZr1−xO2 solid solution samples: A FT-IR study. Catal. Today 1999, 52, 53–63. [Google Scholar] [CrossRef]
- Lin, S.D.; Cheng, H.; Hsiao, T.C. In situ DRIFTS study on the methanol oxidation by lattice oxygen over Cu/ZnO catalyst. J. Mol. Catal. A: Chem. 2011, 342–343, 35–40. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Shen, C.-C.; Huang, Y.-J. Enhancement of the partial oxidation of methanol reaction over CuZn catalyst by Mn promoter. Ind. Eng. Chem. Res. 2014, 53, 12622–12630. [Google Scholar] [CrossRef]
- Zuo, Z.-J.; Gao, X.-Y.; Han, P.-D.; Liu, S.-Z.; Huang, W. Density functional theory (DFT) and kinetic monte carlo (KMC) study of the reaction mechanism of hydrogen production from methanol on ZnCu (111). J. Phys. Chem. C 2016, 120, 27500–27508. [Google Scholar] [CrossRef]
- Kulkarni, G.U.; Rao, C.N.R. EXAFS and XPS investigations of Cu/ZnO catalysts and their interaction with CO and methanol. Top. Catal. 2003, 22, 183–189. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Gong, X.-Q.; Kohanoff, J.; Sanchez, C.; Hu, P. Catalytic role of metal oxides in gold-based catalysts: A first principles study of CO oxidation on TiO2 supported Au. Phys. Rev. Lett. 2003, 91, 266102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kim, H.Y.; Henkelman, G. CO oxidation at the Au–Cu interface of bimetallic nanoclusters supported on CeO2 (111). J. Phys. Chem. Lett. 2013, 4, 2943–2947. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Huang, Y.-J. Low CO generation on tunable oxygen vacancies of non-precious metallic Cu/ZnO catalysts for partial oxidation of methanol reaction. Appl. Catal. B: Environ. 2014, 150–151, 506–514. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gervasini, A.; Bennici, S. Dispersion and surface states of copper catalysts by temperature-programmed-reduction of oxidized surfaces (s-TPR). Appl. Catal. A: Gen. 2005, 281, 199–205. [Google Scholar] [CrossRef]









| Sample | Ti | Reduction State d(Cu) a nm | After POM Reaction d(Cu) a nm | d(Cu) Increase % | Deactivation Rate Constant b ± CI c (hr−1) |
|---|---|---|---|---|---|
| CZ | 180 | 6.25 | 19.50 | 212 | 0.358 ± 0.0238 |
| CZrZ | 175 | 5.40 | 12.24 | 127 | 0.202 ± 0.0248 |
| A5CZrZ | 120 | 3.97 | 9.00 | 127 | 0.191 ± 0.0253 |
| A5CZ | 125 | 3.30 | 11.78 | 257 | 0.351 ± 0.0256 |
| Sample Name | Metallic Composition a | Cu Dispersion (%) b | BET | dCuO | dCu | dZnO | Composition of Catalyst Surfaces d (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | Surface Area | (nm) c | (nm) c | (nm) c | |||||||||||
| Au | Cu | Zr | Zn | (m²/g) | Cu (111) | Cu (200) | Zn (101) | Au4f | Cu2p | Zr3d | Zn2p | Au0/Au3+ | |||
| Fresh | After POM | ||||||||||||||
| CZ | – | 29.8 | – | 70.2 | 20.51 | 34.59 | 5.65 | 6.25 | 9.03 | – | 26.24 | – | 73.76 | – | – |
| CZrZ | – | 31.2 | 15.9 | 52.9 | 21.02 | 77.52 | 4.49 | 5.40 | 6.42 | – | 27.85 | 3.38 | 68.77 | – | – |
| A1CZrZ | 1.0 | 31.4 | 15.4 | 52.2 | 26.93 | 64.06 | 4.81 | 4.33 | 6.96 | 10.07 | 27.45 | 4.39 | 58.09 | 0.65 | 0.83 |
| A3CZrZ | 3.1 | 31.6 | 15.6 | 49.7 | 28.32 | 73.21 | 4.09 | 4.28 | 5.98 | 10.18 | 28.29 | 4.89 | 56.64 | 0.85 | 1.11 |
| A5CZrZ | 4.5 | 31.7 | 16.7 | 47.1 | 27.92 | 77.03 | 3.48 | 3.97 | 5.69 | 11.59 | 26.35 | 3.72 | 58.34 | 1.37 | 1.43 |
| Catalyst | CZ | CZrZ | A1CZrZ | A3CZrZ | A5CZrZ | |
|---|---|---|---|---|---|---|
| Fractions of TPR Area (%) | β | 56 | 61 | 50 | 51 | 48 |
| α | 44 | 39 | 50 | 49 | 53 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-Y.; Chen, H.-I.; Huang, Y.-J. The Promotional Effects of ZrO2 and Au on the CuZnO Catalyst Regarding the Durability and Activity of the Partial Oxidation of Methanol. Catalysts 2018, 8, 345. https://doi.org/10.3390/catal8090345
Huang H-Y, Chen H-I, Huang Y-J. The Promotional Effects of ZrO2 and Au on the CuZnO Catalyst Regarding the Durability and Activity of the Partial Oxidation of Methanol. Catalysts. 2018; 8(9):345. https://doi.org/10.3390/catal8090345
Chicago/Turabian StyleHuang, Hsiao-Yu, Hao-I Chen, and Yuh-Jeen Huang. 2018. "The Promotional Effects of ZrO2 and Au on the CuZnO Catalyst Regarding the Durability and Activity of the Partial Oxidation of Methanol" Catalysts 8, no. 9: 345. https://doi.org/10.3390/catal8090345






