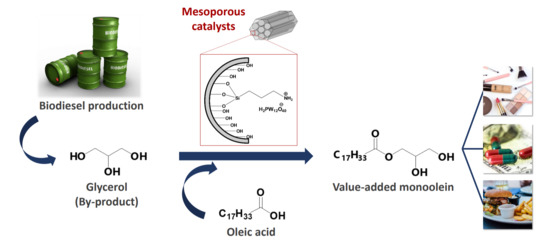
Effect of Surface Modifications of SBA-15 with Aminosilanes and 12-Tungstophosphoric Acid on Catalytic Properties in Environmentally Friendly Esterification of Glycerol with Oleic Acid to Produce Monoolein
Abstract
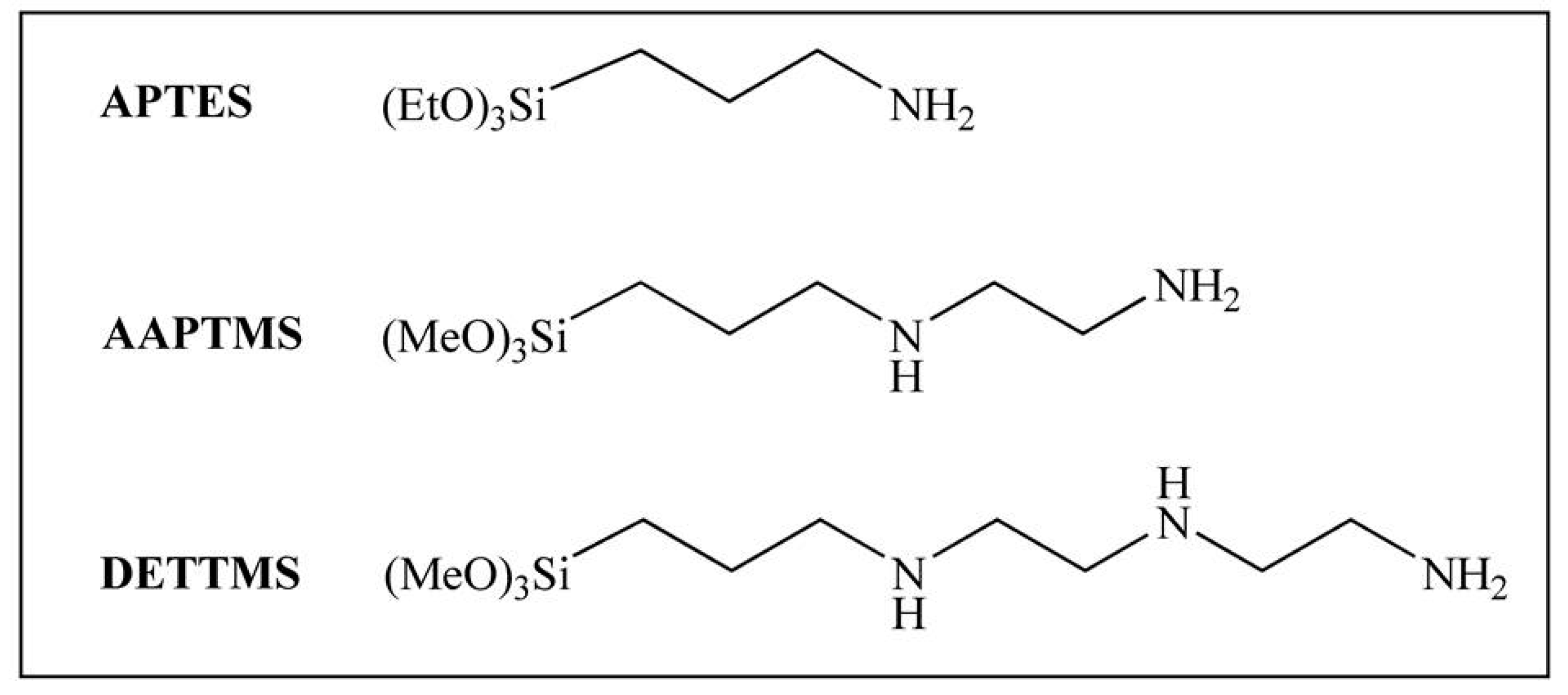
:1. Introduction
2. Results and Discussion
2.1. Materials Characterization
2.1.1. X-ray Powder Diffraction (XRD)
2.1.2. N2 Adsorption-Desorption
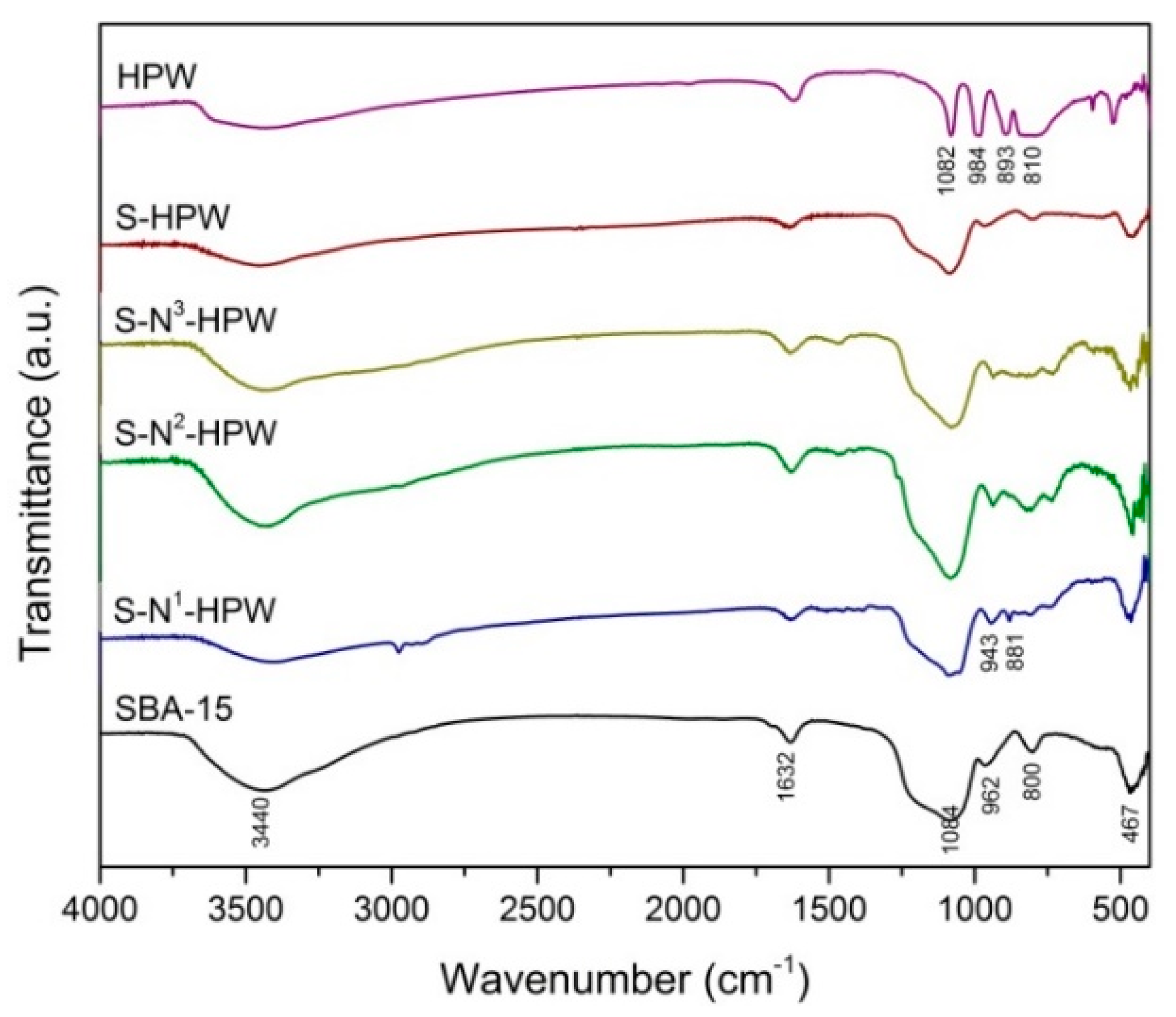
2.1.3. Fourier Transform Infrared Spectroscopy
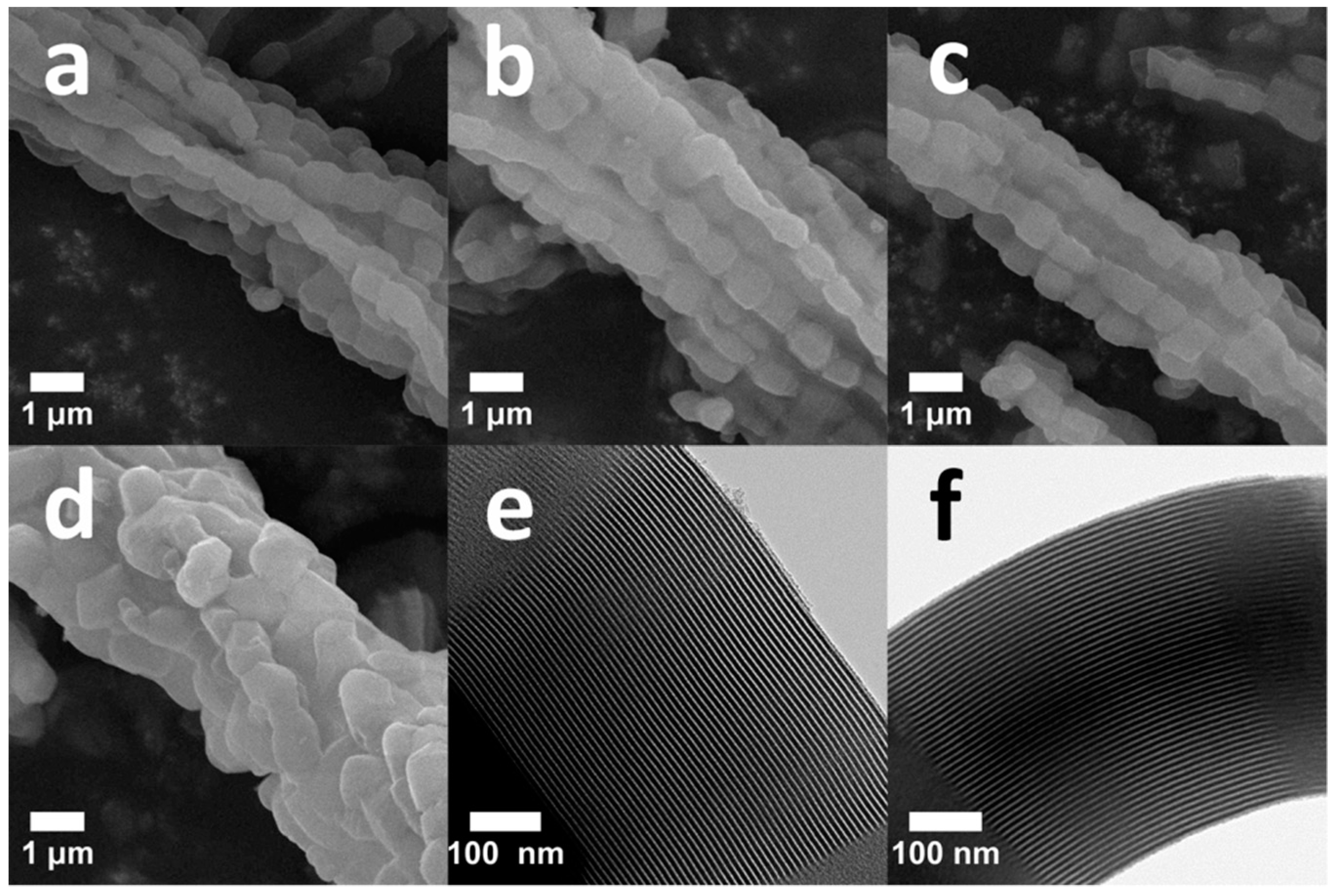
2.1.4. Morphological Analysis
2.1.5. Elemental Analysis and Acidity
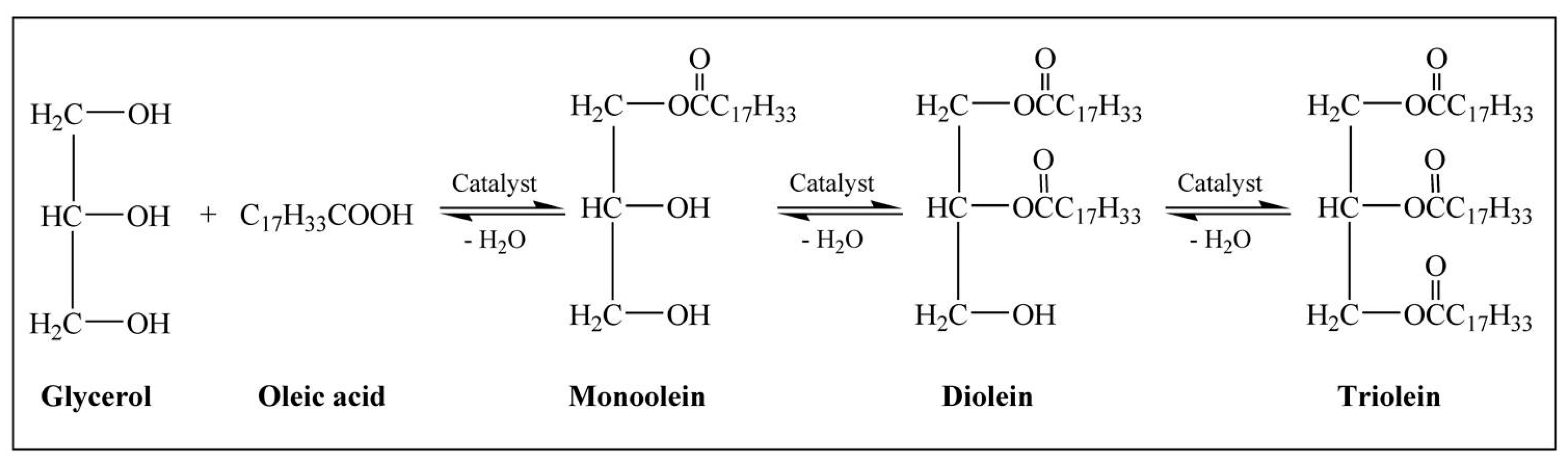
2.2. Catalytic Performance of the Synthesized Catalysts
2.3. Effect of Reaction Parameters
2.3.1. Effect of Reaction Temperature
2.3.2. Effect of Catalyst Loading
2.3.3. Effect of Glycerol/Oleic Acid Molar Ratio
2.4. Reusability of the Catalysts
2.5. Comparison of Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Amino-Functionalized SBA-15 Materials
3.3. Synthesis of Protonated Amino-Functionalized SBA-15 Materials
3.4. Materials Characterization
3.5. Catalytic Esterification of Glycerol with Oleic Acid
3.6. Catalyst Reusability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- An, S.; Sun, Y.; Song, D.; Zhang, Q.; Guo, Y.; Shang, Q. Arenesulfonic acid-functionalized alkyl-bridged organosilica hollow nanospheres for selective esterification of glycerol with lauric acid to glycerol mono- and dilaurate. J. Catal. 2016, 342, 40–54. [Google Scholar] [CrossRef]
- Hamerski, F.; Corazza, M.L. Ldh-catalyzed esterification of lauric acid with glycerol in solvent-free system. Appl. Catal. A 2014, 475, 242–248. [Google Scholar] [CrossRef]
- Damstrup, M.L.; Jensen, T.; Sparsø, F.V.; Kiil, S.Z.; Jensen, A.D.; Xu, X. Solvent optimization for efficient enzymatic monoacylglycerol production based on a glycerolysis reaction. J. Am. Oil Chem. Soc. 2005, 82, 559–564. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Lipase-catalyzed syntheses of monoacylglycerols. Enzyme Microb. Technol. 1995, 17, 578–586. [Google Scholar] [CrossRef]
- Dosuna-Rodríguez, I.; Gaigneaux, E.M. Glycerol acetylation catalysed by ion exchange resins. Catal. Today 2012, 195, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Tangestanifard, M.; Ghaziaskar, H. Arenesulfonic acid-functionalized bentonite as catalyst in glycerol esterification with acetic acid. Catalysts 2017, 7, 211. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Gholami, Z.; Ayoub, M.; Gholami, F. Selective monolaurin synthesis through esterification of glycerol using sulfated zirconia-loaded SBA-15 catalyst. Chem. Eng. Commun. 2015, 203, 496–504. [Google Scholar] [CrossRef]
- Wee, L.H.; Lescouet, T.; Fritsch, J.; Bonino, F.; Rose, M.; Sui, Z.; Garrier, E.; Packet, D.; Bordiga, S.; Kaskel, S.; et al. Synthesis of monoglycerides by esterification of oleic acid with glycerol in heterogeneous catalytic process using tin-organic framework catalyst. Catal. Lett. 2013, 143, 356–363. [Google Scholar] [CrossRef]
- Machado, M.S.; Pérez-Pariente, J.; Sastre, E.; Cardoso, D.; de Guereñu, A.M. Selective synthesis of glycerol monolaurate with zeolitic molecular sieves. Appl. Catal. A 2000, 203, 321–328. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Miquel, S.; Primo, J. Catalysts for the production of fine chemicals: Production of food emulsifiers, monoglycerides, by glycerolysis of fats with solid base catalysts. J. Catal. 1998, 173, 315–321. [Google Scholar] [CrossRef]
- Patel, A.; Singh, S. A green and sustainable approach for esterification of glycerol using 12-tungstophosphoric acid anchored to different supports: Kinetics and effect of support. Fuel 2014, 118, 358–364. [Google Scholar] [CrossRef]
- Müller, A.; Peters, F.; Pope, M.T.; Gatteschi, D. Polyoxometalates: Very large clusters-nanoscale magnets. Chem. Rev. 1998, 98, 239–272. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, M.N. Acid catalysis by heteropoly acids. Appl. Catal. A 2003, 256, 19–35. [Google Scholar] [CrossRef]
- Misono, M. Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state. Chem. Commun. 2001, 1141–1152. [Google Scholar] [CrossRef]
- Obalı, Z.; Doğu, T. Activated carbon–tungstophosphoric acid catalysts for the synthesis of tert-amyl ethyl ether (TAEE). Chem. Eng. J. 2008, 138, 548–555. [Google Scholar] [CrossRef]
- Jović, A.; Bajuk-Bogdanović, D.; Nedić Vasiljević, B.; Milojević-Rakić, M.; Krajišnik, D.; Dondur, V.; Popa, A.; Uskoković-Marković, S.; Holclajtner-Antunović, I. Synthesis and characterization of 12-phosphotungstic acid supported on BEA zeolite. Mater. Chem. Phys. 2017, 186, 430–437. [Google Scholar] [CrossRef]
- Labaki, M.; Mokhtari, M.; Brilhac, J.F.; Thomas, S.; Pitchon, V. Simulation of NO and NO2 sorption–desorption–reduction behaviours on pt-impregnated hpw supported on TiO2. Appl. Catal. B 2007, 76, 386–394. [Google Scholar] [CrossRef]
- Hoo, P.-Y.; Abdullah, A.Z. Direct synthesis of mesoporous 12-tungstophosphoric acid SBA-15 catalyst for selective esterification of glycerol and lauric acid to monolaurate. Chem. Eng. J. 2014, 250, 274–287. [Google Scholar] [CrossRef]
- Thielemann, J.P.; Girgsdies, F.; Schlogl, R.; Hess, C. Pore structure and surface area of silica SBA-15: Influence of washing and scale-up. Beilstein J. Nanotechnol. 2011, 2, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Roy, S.C.; Nandi, L.N.; Samuel, P.; Pillai, S.M.; Bhat, B.D.; Ravindranathan, M. Synthesis of lower olefins from methanol and subsequent conversion of ethylene to higher olefins via oligomerisation. J. Mol. Catal. A: Chem. 2004, 223, 231–235. [Google Scholar] [CrossRef]
- Xie, W.; Hu, P. Production of structured lipids containing medium-chain fatty acids by soybean oil acidolysis using SBA-15-pr-NH2–HPW catalyst in a heterogeneous manner. Org. Process Res. Dev. 2016, 20, 637–645. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, Q.; Dai, W.-L.; Fan, K. A green process for the epoxidation of dicyclopentadiene with aqueous H2O2 over highly efficient and stable HPW-NH2-SBA-15. RSC Adv. 2012, 2, 6087–6093. [Google Scholar] [CrossRef]
- Karaki, M.; Karout, A.; Toufaily, J.; Rataboul, F.; Essayem, N.; Lebeau, B. Synthesis and characterization of acidic ordered mesoporous organosilica SBA-15: Application to the hydrolysis of cellobiose and insight into the stability of the acidic functions. J. Catal. 2013, 305, 204–216. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, J. Preparation of heterogeneous mesoporous silica-supported 12-tungstophosphoric acid catalyst and its catalytic performance for cyclopentene oxidation. Chin. J. Catal. 2011, 32, 1191–1198. [Google Scholar] [CrossRef]
- Lei, J.; Chen, L.; Yang, P.; Du, X.; Yan, X. Oxidative desulfurization of diesel fuel by mesoporous phosphotungstic acid/SiO2: The effect of preparation methods on catalytic performance. J. Porous Mater. 2013, 20, 1379–1385. [Google Scholar] [CrossRef]
- Singh, S.; Patel, A. Selective green esterification and oxidation of glycerol over 12-tungstophosphoric acid anchored to MCM-48. Ind. Eng. Chem. Res. 2014, 53, 14592–14600. [Google Scholar] [CrossRef]
- Majda, D.; Napruszewska, B.D.; Zimowska, M.; Makowski, W. Porosity of SBA-15 after functionalization of the surface with aminosilanes. Microporous Mesoporous Mater. 2016, 234, 98–106. [Google Scholar] [CrossRef]
- Dong, X.; Wang, D.; Li, K.; Zhen, Y.; Hu, H.; Xue, G. Vanadium-substituted heteropolyacids immobilized on amine- functionalized mesoporous MCM-41: A recyclable catalyst for selective oxidation of alcohols with H2O2. Mater. Res. Bull. 2014, 57, 210–220. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Suo, Y.; Zheng, G.-P.; Guan, X.-X.; Zheng, X.-C. Mesoporous solid acid catalysts of 12-tungstosilicic acid anchored to SBA-15: Characterization and catalytic properties for esterification of oleic acid with methanol. J. Taiwan Inst. Chem. Eng. 2015, 51, 186–192. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. 12-tungstophosphoric acid anchored to sba-15: An efficient, environmentally benign reusable catalysts for biodiesel production by esterification of free fatty acids. Appl. Catal. A 2011, 403, 161–172. [Google Scholar] [CrossRef]
- Heykants, E.; Verrelst, W.H.; Parton, R.F.; Jacobs, P.A. Shape-selective zeolite catalysed synthesis of monoglycerides by esterification of fatty acids with glycerol. In Studies in Surface Science and Catalysis; Chon, H., Ihm, S.-K., Uh, Y.S., Eds.; Elsevier: New York, NY, USA, 1997; Volume 105, pp. 1277–1284. [Google Scholar]
- Konwar, L.J.; Mäki-Arvela, P.; Kumar, N.; Mikkola, J.-P.; Sarma, A.K.; Deka, D. Selective esterification of fatty acids with glycerol to monoglycerides over –SO3H functionalized carbon catalysts. React. Kinet. Mech. Catal. 2016, 119, 121–138. [Google Scholar] [CrossRef]
- Bossaert, W.D.; De Vos, D.E.; Rhijn, W.M.V.; Bullen, J.; Grobet, P.J.; Jacobs, P.A. Mesoporous sulfonic acids as selective heterogeneous catalysts for the synthesis of monoglyceides. J. Catal. 1999, 182, 156–164. [Google Scholar] [CrossRef]
- Hermida, L.; Abdullah, A.Z.; Mohamed, A.R. Synthesis of monoglyceride through glycerol esterification with lauric acid over propyl sulfonic acid post-synthesis functionalized SBA-15 mesoporous catalyst. Chem. Eng. J. 2011, 174, 668–676. [Google Scholar] [CrossRef]
- Pérez-Pariente, J.N.; Díaz, I.; Mohino, F.; Sastre, E. Selective synthesis of fatty monoglycerides by using functionalised mesoporous catalysts. Appl. Catal. A 2003, 254, 173–188. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Yang, W.; Guo, H.; Fang, F.; Tang, Z. Immobilization of phosphortungstic acid on amino-functionalized bimetallic Zr–La-SBA-15 and its highly catalytic performance for acetylation. J. Mol. Catal. A: Chem. 2014, 393, 1–7. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, K.; Dai, C.; Shi, H.; Li, J. Adsorption of Pb2+ on amino-functionalized core–shell magnetic mesoporous sba-15 silica composite. Chem. Eng. J. 2015, 262, 897–903. [Google Scholar] [CrossRef]









| Materials | Total BET Surface Area a (m2 g−1) | Internal Surface Area b (m2 g−1) | External Surface Area b (m2 g−1) | Pore Diameter c (nm) | Pore Volume c (cm3 g−1) | Nitrogen d (mmole g−1) | Acidity e (mmole g−1) | Tungsten f (wt %) |
|---|---|---|---|---|---|---|---|---|
| SBA-15 | 815 | 769 | 52 | 8.06 | 1.18 | - | 0.09 | - |
| S-N1-HPW | 205 | 151 | 19 | 7.05 | 0.33 | 1.34 | 0.32 | 23.8 |
| S-N2-HPW | 177 | 116 | 20 | 7.05 | 0.30 | 1.78 | 0.41 | 27.9 |
| S-N3-HPW | 55 | 28 | 10 | 6.18 | 0.06 | 2.87 | 0.47 | 30.1 |
| S-HPW | 721 | 676 | 35 | 8.06 | 1.15 | - | 0.31 | 24.0 |
| Materials | Weight Ratios of Si/W | |||
|---|---|---|---|---|
| Region 1 | Region 2 | Region 3 | S.D. | |
| S-N1-HPW | 0.871 | 0.873 | 0.829 | 0.025 |
| S-N2-HPW | 0.769 | 0.768 | 0.770 | 0.001 |
| S-N3-HPW | 0.611 | 0.609 | 0.612 | 0.002 |
| S-HPW | 8.522 | 8.173 | 15.486 | 4.125 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratchadapiban, K.; Praserthdam, P.; Tungasmita, D.N.; Tangku, C.; Anutrasakda, W. Effect of Surface Modifications of SBA-15 with Aminosilanes and 12-Tungstophosphoric Acid on Catalytic Properties in Environmentally Friendly Esterification of Glycerol with Oleic Acid to Produce Monoolein. Catalysts 2018, 8, 360. https://doi.org/10.3390/catal8090360
Ratchadapiban K, Praserthdam P, Tungasmita DN, Tangku C, Anutrasakda W. Effect of Surface Modifications of SBA-15 with Aminosilanes and 12-Tungstophosphoric Acid on Catalytic Properties in Environmentally Friendly Esterification of Glycerol with Oleic Acid to Produce Monoolein. Catalysts. 2018; 8(9):360. https://doi.org/10.3390/catal8090360
Chicago/Turabian StyleRatchadapiban, Kullatida, Piyasan Praserthdam, Duangamol Nuntasri Tungasmita, Chutima Tangku, and Wipark Anutrasakda. 2018. "Effect of Surface Modifications of SBA-15 with Aminosilanes and 12-Tungstophosphoric Acid on Catalytic Properties in Environmentally Friendly Esterification of Glycerol with Oleic Acid to Produce Monoolein" Catalysts 8, no. 9: 360. https://doi.org/10.3390/catal8090360