High CO Methanation Performance of Two-Dimensional Ni/MgAl Layered Double Oxide with Enhanced Oxygen Vacancies via Flash Nanoprecipitation
Abstract
:1. Introduction
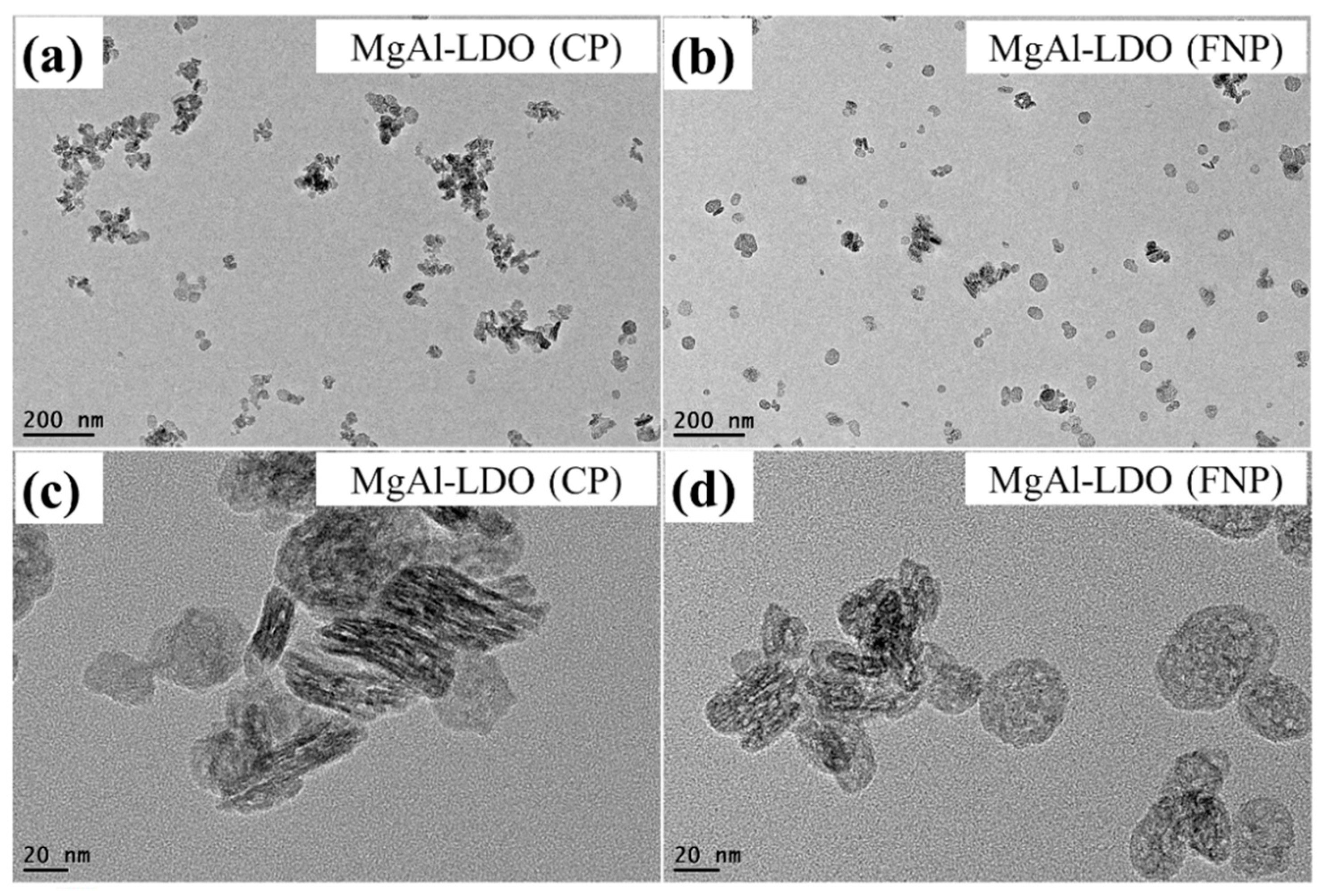
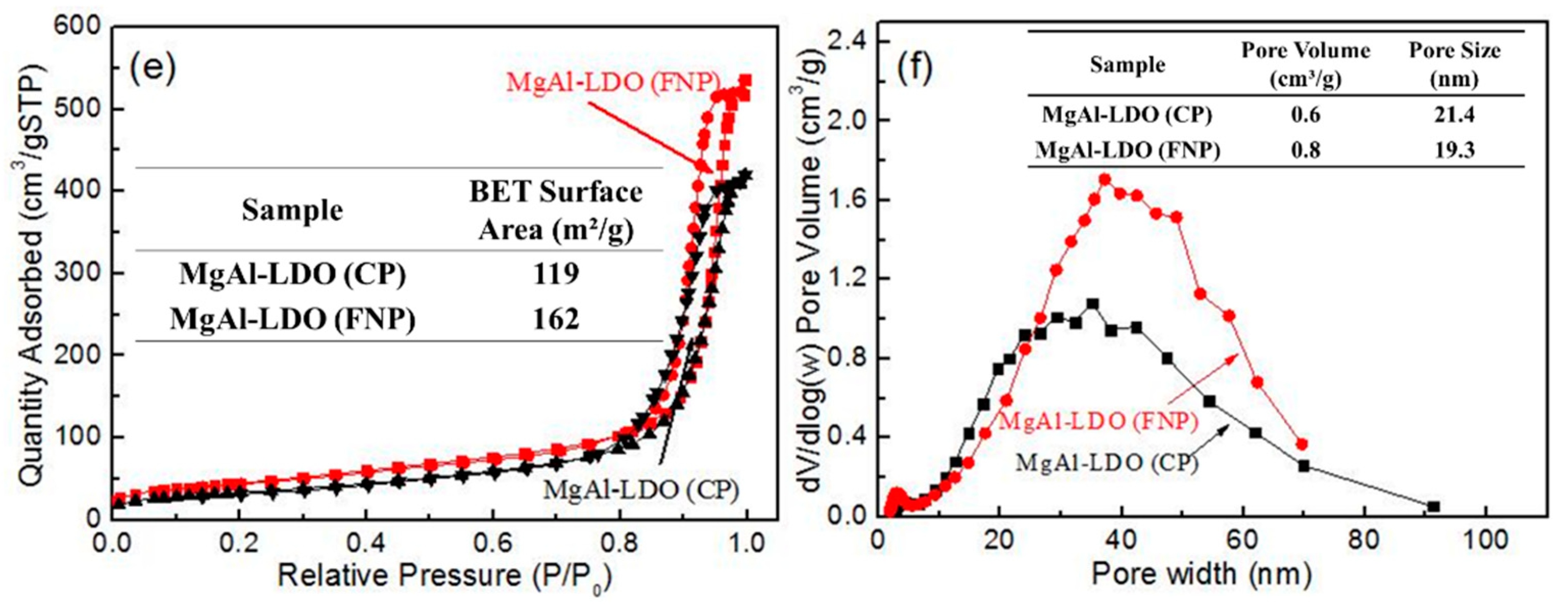
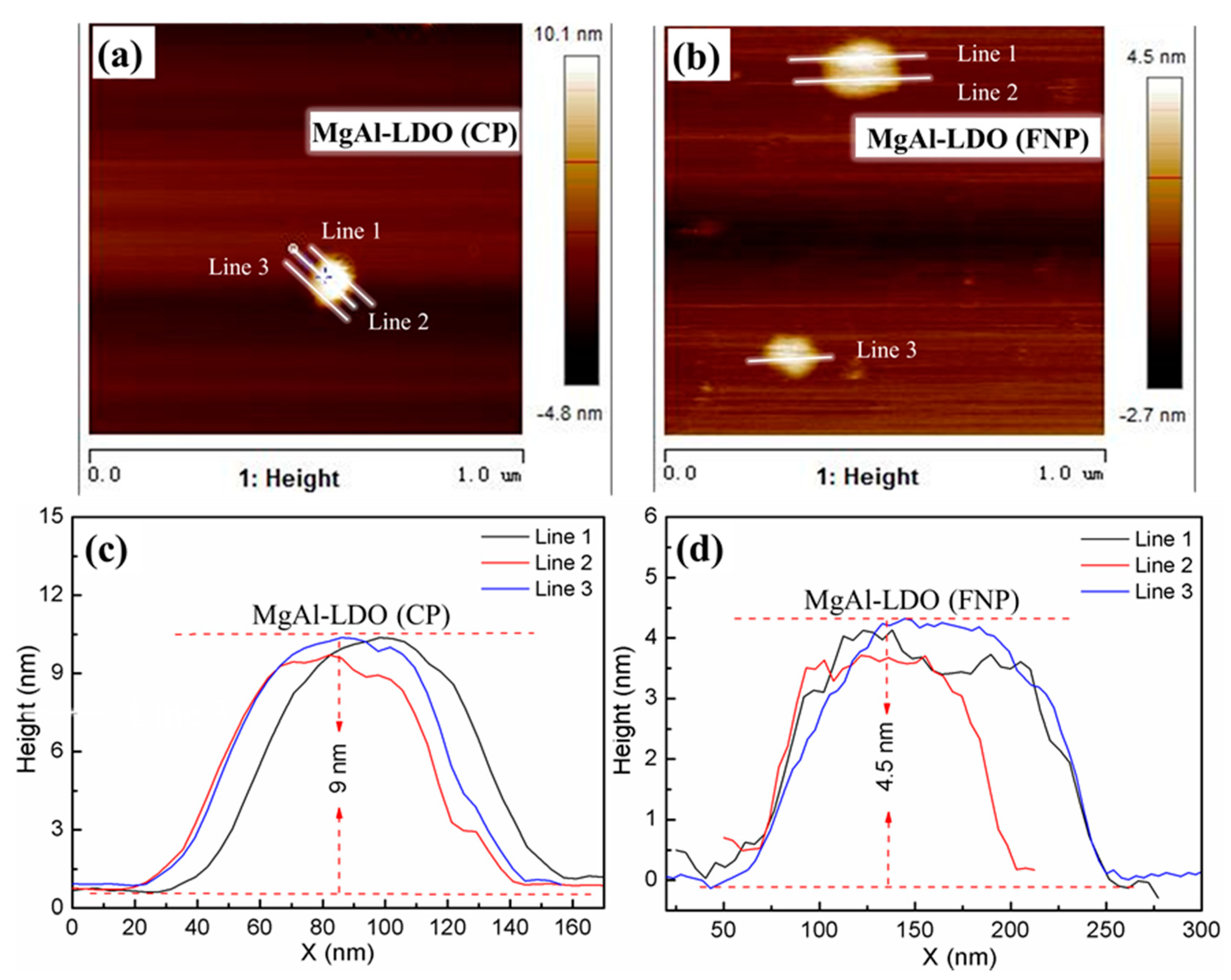
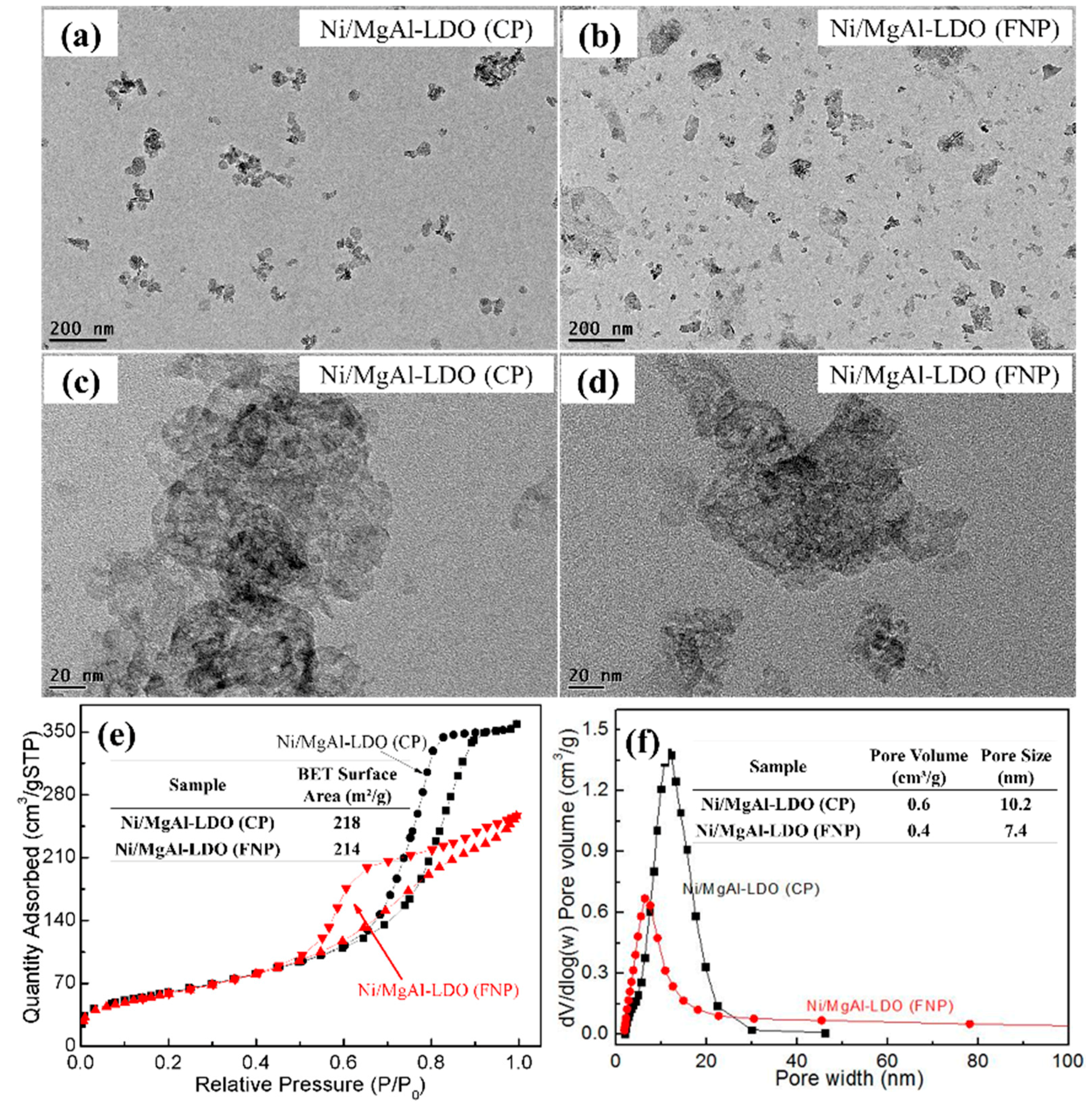
2. Results and Discussion
3. Materials and Methods
3.1. Coprecipitation Method
3.2. Flashnano-Precipitation (FNP) Method
3.3. Catalyst Characterization
3.4. Activity Measurement
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tao, M.; Meng, X.; Xin, Z.; Bian, Z.; Lv, Y.; Gu, J. Synthesis and characterization of well dispersed nickel-incorporated SBA-15 and its high activity in syngas methanation reaction. Appl. Catal. A Gen. 2016, 516, 127–134. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, F.; Lu, X.; Liu, Y.; Li, H.; Zhong, F.; Xu, G.; Su, F. Enhanced catalytic performances of Ni/Al2O3 catalyst via addition of V2O3 for CO methanation. Appl. Catal. A Gen. 2014, 488, 37–47. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Tian, Z.; Zhu, M.; Wang, F.; Li, J.; Dai, B.; Yu, F.; Qiu, H.; Gao, H. Clarification of Active Sites at Interfaces between Silica Support and Nickel Active Components for Carbon Monoxide Methanation. Catalysts 2018, 8, 293. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F.; Wang, X.; Lu, X.; Li, H.; Xu, G.; Su, F. CO methanation on ordered mesoporous Ni–Cr–Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Liu, Q.H.; Dong, X.F.; Lin, W.M. Highly selective CO methanation over amorphous Ni–Ru–B/ZrO2 catalyst. Chin. Chem. Lett. 2009, 20, 889–892. [Google Scholar] [CrossRef]
- Li, S.; Jin, H.; Gao, L.; Zhang, X. Exergy analysis and the energy saving mechanism for coal to synthetic/substitute natural gas and power cogeneration system without and with CO2 capture. Appl. Energy 2014, 130, 552–561. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Chen, X.; Jin, J.; Sha, G.; Li, C.; Zhang, B.; Su, D.; Williams, C.T.; Liang, C. Silicon–nickel intermetallic compounds supported on silica as a highly efficient catalyst for CO methanation. Catal. Sci. Technol. 2013, 4, 53–61. [Google Scholar] [CrossRef]
- Lv, Y.; Xin, Z.; Meng, X.; Tao, M.; Bian, Z.; Gu, J.; Gao, W. Essential role of organic additives in preparation of efficient Ni/KIT-6 catalysts for CO methanation. Appl. Catal. A Gen. 2018, 558, 99–108. [Google Scholar] [CrossRef]
- Dan, J.; Huang, X.; Li, P.; Zhang, Y.; Zhu, M.; Guo, X.; Dai, B.; Wang, Q.; Yu, F. Two-Dimensional Porous Silica Nanomesh from Expanded Multilayered Vermiculite via Mixed Acid Leaching. Nanosci. Nanotechnol. Lett. 2016, 8, 1028–1032. [Google Scholar] [CrossRef]
- Li, P.; Zhu, M.; Dan, J.; Kang, L.; Lai, L.; Cai, X.; Zhang, J.; Yu, F.; Tian, Z.; Dai, B. Two-dimensional porous SiO2 nanomesh supported high dispersed Ni nanoparticles for CO methanation. Chem. Eng. J. 2017, 326, 774–780. [Google Scholar] [CrossRef]
- Xin, H.; Yu, F.; Zhu, M.-Y.; Ouyang, F.-H.; Dai, B.; Dan, J.-M. Hydrochlorination of acetylene using expanded multilayered vermiculite (EML-VMT)-supported catalysts. Chin. Chem. Lett. 2015, 26, 1101–1104. [Google Scholar]
- Li, P.; Wen, B.; Yu, F.; Zhu, M.; Guo, X.; Han, Y.; Kang, L.; Huang, X.; Dan, J.; Ouyang, F.; et al. High efficient nickel/vermiculite catalyst prepared via microwave irradiation-assisted synthesis for carbon monoxide methanation. Fuel 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Zhu, M.; Tian, Z.; Dan, J.; Li, J.; Dai, B.; Yu, F. Ultralow-weight loading Ni catalyst supported on two-dimensional vermiculite for carbon monoxide methanation. Chin. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Li, P.; Yu, F.; Altaf, N.; Zhu, M.; Li, J.; Dai, B.; Wang, Q. Two-Dimensional Layered Double Hydroxides for Reactions of Methanation and Methane Reforming in C1 Chemistry. Materials 2018, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Jiří, R.; Günter, S.E.; Jindřich, S.; Arnošt, Z. Supported nickel catalyst from hydroxycarbonate of nickel and aluminium. Chem. Eng. Technol. 1994, 17, 41–46. [Google Scholar]
- Bian, L.; Wang, W.; Xia, R.; Li, Z.H. Ni-based catalyst derived from Ni/Al hydrotalcite-like compounds by urea hydrolysis method for CO methanation. RSC Adv. 2015, 6, 677–686. [Google Scholar] [CrossRef]
- Li, Z.; Bian, L.; Zhu, Q.; Wang, W. Ni-based catalyst derived from Ni/Mg/Al hydrotalcite-like compounds and its activity in the methanation of carbon monoxide. Kinet. Catal. 2014, 55, 217–223. [Google Scholar] [CrossRef]
- Li, P.; Zhu, M.; Tian, Z.; Han, Y.; Zhang, Y.; Zhou, T.; Kang, L.; Dan, J.; Guo, X.; Yu, F.; et al. Two-Dimensional Layered Double Hydroxide Derived from VermiculiteWaste Water Supported Highly Dispersed Ni Nanoparticles for CO Methanation. Catalysts 2017, 7, 79. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Shi, Y.; Dan, J.; Lv, Y.; Guo, X.; Dai, B. Up-scaled flash nano-precipitation production route to develop a MnOx-CeO2-Al2O3 catalyst with enhanced activity and H2O resistant performance for NOx selective catalytic reduction with NH3. Chem. Eng. Res. Des. 2018, 134, 476–486. [Google Scholar] [CrossRef]
- Wang, M.; Yang, N.; Guo, Z.; Gu, K.; Shao, A.; Zhu, W.; Xu, Y.; Wang, J.; Prud’homme, R.K.; Guo, X. Facile Preparation of AIE-Active Fluorescent Nanoparticles through Flash Nanoprecipitation. Ind. Eng. Chem. Res. 2015, 54, 4683–4688. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, C.; Yu, F.; Shi, Y.; Cao, P.; Dan, J.; Chen, K.; Lv, Y.; Guo, X.; Dai, B. Enhanced Oxygen Vacancies in a Two-Dimensional MnAl-Layered Double Oxide Prepared via Flash Nanoprecipitation Offers High Selective Catalytic Reduction of NOx with NH3. Nanomaterials 2018, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, F.; Ji, K.; Song, Y.; Li, Z. Slurry phase methanation of carbon monoxide over nanosized Ni–Al2O3 catalysts prepared by microwave-assisted solution combustion. Appl. Catal. A Gen. 2016, 510, 74–83. [Google Scholar] [CrossRef]
- Lim, Y.S.; Moon, D.J.; Park, N.C.; Shin, J.S.; Kim, J.H.; Kim, Y.C. Autothermal reforming of propane over hydrotalcite-like catalysts containing promotor. J. Nanosci. Nanotechnol. 2007, 7, 4009–4012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Jin, S.; Peng, Z.; Liang, C. Ni/Al2O3 Catalysts Derived from Layered Double Hydroxide and Their Applications in Hydrodeoxygenation of Anisole. Communication 2016, 1, 577–584. [Google Scholar]
- Hwang, S.; Lee, J.; Hong, U.G.; Baik, J.H.; Koh, D.J.; Lim, H.; Song, I.K. Methanation of carbon dioxide over mesoporous Ni–Fe–Ru–Al2O3 xerogel catalysts: Effect of ruthenium content. J. Ind. Eng. Chem. 2013, 19, 698–703. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, Y.; Tian, Y.; Gu, F.; Zhong, Z.; Su, F. Ordered Mesoporous Ni–Fe–Al Catalysts for CO Methanation with Enhanced Activity and Resistance to Deactivation. Ind. Eng. Chem. Res. 2017, 56, 9809–9820. [Google Scholar] [CrossRef]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.Y.; Hu, S.; Sun, L.S.; Zhang, A.C. The activity and mechanism study of Fe-Mn-Ce/gamma-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Wang, J.; Liu, M.; Yuan, Z.; Chen, K.; Li, L.; Prud’Homme, R.K.; Guo, X. Biocompatible Nanoparticle based on Dextran-b-Poly(L-Lactide) Block Copolymer formed by Flash Nanoprecipitation. Chem. Lett. 2015, 44, 1688–1690. [Google Scholar] [CrossRef]
- Daab, M.; Rosenfeldt, S.; Kalo, H.; Stöter, M.; Bojer, B.; Siegel, R.; Förster, S.; Senker, J.; Breu, J. Two-Step Delamination of Highly Charged, Vermiculite-like Layered Silicates via Ordered Heterostructures. Langmuir 2017, 33, 4816–4822. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Cai, Z.; Zhou, D.; Zhang, G.; Zhang, Q.; Jia, Y.; Duan, X.; Xie, Q.; Lai, S.; Xie, T.; et al. A highly-efficient oxygen evolution electrode based on defective nickel-iron layered double hydroxide. Sci. Chin. Mater. 2018, 7, 939–947. [Google Scholar] [CrossRef]
- Mu, W.; Zhu, J.; Zhang, S.; Guo, Y.; Su, L.; Li, X.; Li, Z. Novel proposition on mechanism aspects over Fe-Mn/ZSM-5 catalyst for NH3-SCR of NOx at low temperature: Rate and direction of multifunctional electron-transfer-bridge and in-situ DRIFTs analysis. Catal. Sci. Technol. 2016, 6, 7532–7548. [Google Scholar] [CrossRef]
- Stahl, A.; Wang, Z.; Schwämmle, T.; Ke, J.; Li, X. Novel Fe-W-Ce Mixed Oxide for the Selective Catalytic Reduction of NOx with NH3 at Low Temperatures. Catalysts 2017, 7, 71. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin Spinel-Structured Nanosheets Rich in Oxygen Deficiencies for Enhanced Electrocatalytic Water Oxidation. Angew. Chem. 2015, 127, 7507–7512. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2016, 128, 5363–5367. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.-Z.; Lin, J.-D.; Chen, Z.-Q.; Wang, Y. Crucial support effect on the durability of Pt/MgAl2O4 for partial oxidation of methane to syngas. Appl. Catal. B Environ. 2018, 231, 292–298. [Google Scholar] [CrossRef]
- Gil, A.; Arrieta, E.; Vicente, M.A.; Korili, S.A. Synthesis and CO2 adsorption properties of hydrotalcite-like compounds prepared from aluminum saline slag wastes. Chem. Eng. J. 2018, 334, 1341–1350. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, P.; Zhang, Z.; Zhou, Y.; Zhang, J.; Zhang, H.; Shi, Z.; Han, R.P.S.; Huang, F.; Ma, D. Nickel catalyst stabilization via graphene encapsulation for enhanced methanation reaction. J. Catal. 2016, 334, 42–51. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y. Nanostructured perovskite oxides as promising substitutes of noble metals catalysts for catalytic combustion of methane. Chin. Chem. Lett. 2018, 29, 252–260. [Google Scholar] [CrossRef]





| 2D Catalysts | Ni Content | Preparation Method | Optimum Temperature (°C) | Pressure (Mpa) | Gas Hourly Space Velocity mL·g−1·h−1 | CO Conversion (%) | CH4 Selectivity (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Ni/VMT (MIAS) | 10 wt.% | MIAS | 400 | 1.5 | 12,000 | 99.6 | 93.8 | [13] |
| PIM-Ni/PVMT | 0.5 wt.% | PIM | 450 | 1.5 | 6000 | 93.5 | 64 | [14] |
| NiAl-LDO | 56.5 wt.% | Urea hydrolysis | 400 | 0.1 | 15,000 | 98 | 92 | [17] |
| NiMg8 | 22 wt.% | CP method | 400 | 0.1 | 30,000 | 98 | 90 | [18] |
| Ni/VMT-LDO | 10 wt.% | CP method | 400 | 1.5 | 20,000 | 87.9 | 90 | [19] |
| Ni/MgAl-LDO (CP) | 10 wt.% | CP method | 450 | 0.1 | 20,000 | 81.12 | 75.14 | This work |
| Ni/MgAl-LDO (FNP) | 10 wt.% | FNP method | 350 | 0.1 | 20,000 | 97 | 79.76 | This work |
| Sample | Ni (%) | Ni0/Ni (%) | Ni2+/Ni (%) | Mg (%) | Al (%) | O (%) |
|---|---|---|---|---|---|---|
| MgAl-LDO (CP) | - | - | 17.86 | 13.06 | 53.90 | |
| Ni/MgAl-LDO (CP) | 1.16 | 4.0 | 42.6 | 19.08 | 16.66 | 51.80 |
| MgAl-LDO (FNP) | - | - | 15.68 | 14.45 | 53.72 | |
| Ni/MgAl-LDO (FNP) | 1.85 | 4.7 | 48.6 | 14.77 | 18.60 | 51.44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Yu, F.; Li, J.; Chen, K.; Yao, Y.; Li, P.; Zhu, M.; Shi, Y.; Wang, Q.; Guo, X. High CO Methanation Performance of Two-Dimensional Ni/MgAl Layered Double Oxide with Enhanced Oxygen Vacancies via Flash Nanoprecipitation. Catalysts 2018, 8, 363. https://doi.org/10.3390/catal8090363
Zhang M, Yu F, Li J, Chen K, Yao Y, Li P, Zhu M, Shi Y, Wang Q, Guo X. High CO Methanation Performance of Two-Dimensional Ni/MgAl Layered Double Oxide with Enhanced Oxygen Vacancies via Flash Nanoprecipitation. Catalysts. 2018; 8(9):363. https://doi.org/10.3390/catal8090363
Chicago/Turabian StyleZhang, Mengjuan, Feng Yu, Jiangbing Li, Kai Chen, Yongbin Yao, Panpan Li, Mingyuan Zhu, Yulin Shi, Qiang Wang, and Xuhong Guo. 2018. "High CO Methanation Performance of Two-Dimensional Ni/MgAl Layered Double Oxide with Enhanced Oxygen Vacancies via Flash Nanoprecipitation" Catalysts 8, no. 9: 363. https://doi.org/10.3390/catal8090363





