Two Possible Side Reaction Pathways during Furanic Etherification
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
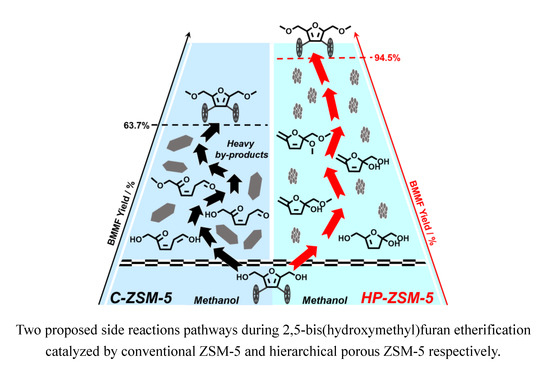
2.2. Catalytic Performance of C-ZSM-5 and HP-ZSM-5 in the Etherification of BHMF
2.3. Catalyst Reusability
2.4. Carbon Deposition Analysis
2.5. Speculated Mechanism of Side Reaction during BHMF to BMMF Process
3. Material and Methods
3.1. Materials
3.2. Synthesis of Catalysts
3.3. Catalysts Characterization
3.4. Catalytic Activity
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De Jong, E.; Vijlbrief, T.; Hijkoop, R.; Gruter, G.J.M.; van der Waal, J.C. Promising results with YXY Diesel components in an ESC test cycle using a PACCAR Diesel engine. Biomass Bioenergy 2012, 36, 151–159. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. Direct, high-yield conversion of cellulose into biofuel. Angew. Chem. Int. Ed. 2008, 47, 7924–7926. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wei, J.; Ding, N.; Sun, Y.; Zeng, X.; Hu, L.; Liu, S.; Lei, T.; Lin, L. Chemoselective hydrogenation of biomass derived 5-hydroxymethylfurfural to diols: Key intermediates for sustainable chemicals, materials and fuels. Renew. Sustain. Energy Rev. 2017, 77, 287–296. [Google Scholar] [CrossRef]
- Gruter, G.M.J. Hydroxymethylfurfural Ethers from Sugars or HMF and Branched Alcohols. U.S. 20100218416A1, 2 September 2010. [Google Scholar]
- Lee, Y.; Kim, J.; Han, J.; Kim, Y.; Jung, B.; Hwang, S.; Jegal, J. Highly selective catalytic hydrogenation and etherification of 5-hydroxymethyl-2-furaldehyde to 2,5-bis(alkoxymethyl)furans for potential biodiesel production. Synlett 2017, 28, 2299–2302. [Google Scholar]
- Cao, Q.; Liang, W.Y.; Guan, J.; Wang, L.; Qu, Q.; Zhang, X.Z.; Wang, X.C.; Mu, X.D. Catalytic synthesis of 2,5-bis-methoxymethylfuran: A promising cetane number improver for diesel. Appl. Catal. A Gen. 2014, 481, 49–53. [Google Scholar] [CrossRef]
- Gupta, D.; Saha, B. Dual acidic titania carbocatalyst for cascade reaction of sugar to etherified fuel additives. Catal. Commun. 2018, 110, 46–50. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, K.; Chen, S.Y.; Li, C.; Li, F.; Xu, H.J.; Fu, Y. A cobalt catalyst for reductive etherification of 5-hydroxymethyl-furfural to 2,5-bis (methoxymethyl)furan under mild conditions. Green Chem. 2018, 20, 1095–1105. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Riisager, A.; Pandey, A.; Sangwan, R.S.; Saravanamurugan, S.; Luque, R. Zeolite and zeotype-catalysed transformations of biofuranic compounds. Green Chem. 2016, 18, 5701–5735. [Google Scholar] [CrossRef]
- Wang, H.; Frenklach, M. Transport properties of polycyclic aromatic hydrocarbons for flame modeling. Combust. Flame 1994, 96, 163–170. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Park, H.J.; Park, K.H.; Jeon, J.K.; Kim, J.; Ryoo, R.; Jeong, K.E.; Park, S.H.; Park, Y.K. Production of phenolics and aromatics by pyrolysis of miscanthus. Fuel 2012, 97, 379–384. [Google Scholar] [CrossRef]
- Lee, H.I.; Park, H.J.; Park, Y.K.; Hur, J.Y.; Jeon, J.K.; Kim, J.M. Synthesis of highly stable mesoporous aluminosilicates from commercially available zeolites and their application to the pyrolysis of woody biomass. Catal. Today 2008, 132, 68–74. [Google Scholar] [CrossRef]
- Xian, X.; Ran, C.; Nai, C.; Yang, P.; Zhao, S.; Dong, L. Characterization of the location of coke deposited on spent HZSM-5 zeolite by special temperature-programmed oxidation and isothermal oxidation methods. Appl. Catal. A Gen. 2017, 547, 37–51. [Google Scholar] [CrossRef]
- Gelmini, A.; Albonetti, S.; Cavani, F.; Cesari, C.; Lolli, A.; Zanotti, V.; Mazzoni, R. Oxidant free one-pot transformation of bio-based 2,5-bis-hydroxymethylfuran into α-6-hydroxy-6-methyl-4-enyl-2H-pyran-3-one in water. Appl. Catal. B Environ. 2016, 180, 38–43. [Google Scholar] [CrossRef]
- Alamillo, R.; Tucker, M.; Chia, M.; Pagán Torres, Y.; Dumesic, J. The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts. Green Chem. 2012, 14, 1413–1419. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Chuntanapum, A.; Matsumura, Y. Formation of Tarry Material from 5-HMF in Subcritical and Supercritical Water. Ind. Eng. Chem. Res. 2009, 48, 9837–9846. [Google Scholar] [CrossRef]
- Zhu, K.; Sun, J.M.; Liu, J.; Wang, L.Q.; Wan, H.Y.; Hu, J.Z.; Wang, Y.; Peden, C.H.F.; Nie, Z.M. Solvent evaporation assisted preparation of oriented nanocrystalline mesoporous MFI zeolites. ACS Catal. 2011, 1, 682–690. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gassolid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1982, 154, 2201–2218. [Google Scholar] [CrossRef]
- Kim, J.N.; Choi, M.; Ryoo, R. Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process. J. Catal. 2010, 269, 219–228. [Google Scholar] [CrossRef]
- Serrano, D.P.; García, R.A.; Vicente, G.; Linares, M.; Procházková, D.; Čejka, J. Acidic and catalytic properties of hierarchical zeolites and hybrid ordered mesoporous materials assembled from MFI protozeolitic units. J. Catal. 2011, 279, 366–380. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef] [PubMed]
- Sazama, P.; Sobalik, Z.; Dedecek, J.; Jakubec, I.; Parvulescu, V.; Bastl, Z.; Rathousky, J.; Jirglova, H. Enhancement of activity and selectivity in acid-catalyzed reactions by dealuminated hierarchical zeolites. Angew. Chem. Int. Ed. 2013, 52, 2038–2041. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.S.; Taarning, E.; Egeblad, K.; Christensen, C. Catalysis with hierarchical zeolites. Catal. Today 2011, 168, 3–16. [Google Scholar] [CrossRef]
- Koehle, M.; Lobo, R.F. Lewis acidic zeolite Beta catalyst for the Meerwein-Ponndorf-Verley reduction of furfural. Catal. Sci. Technol. 2016, 6, 3018–3026. [Google Scholar] [CrossRef]
- Gou, M.L.; Cai, J.Q.; Song, W.S.; Liu, Z.; Ren, Y.L.; Pan, B.L.; Niu, Q.S. Coking and deactivation behavior of ZSM-5 during the isomerization of styrene oxide to phenylacetaldehyde. Catal. Commun. 2017, 98, 116–120. [Google Scholar] [CrossRef]
- Gou, J.S.; Wang, Z.P.; Li, C.; Qi, X.D.; Vattipalli, V.; Cheng, Y.T.; Huber, G.; Conner, W.C.; Dauenhauer, P.J.; Mountziaris, T.J.; Fan, W. The effects of ZSM-5 mesoporosity and morphology on the catalytic fast pyrolysis of furan. Green Chem. 2017, 19, 3549–3557. [Google Scholar] [CrossRef]
- Fang, W.T.; Hu, H.L.; Dong, P.; Ma, Z.S.; He, Y.L.; Wang, L.; Zhang, Y.J. Improvement of furanic diether selectivity by adjusting Brønsted and Lewis acidity. Appl. Catal. A Gen. 2018, 565, 146–151. [Google Scholar] [CrossRef]
- Shindo, A.; Izumino, K. Structural variation during pyrolysis of furfuryl alcohol and furfural-furfuryl alcohol resins. Carbon 1994, 32, 1233–1243. [Google Scholar] [CrossRef]
- Horvat, J.; Klaić, B.; Metelko, B.; Šunjić, V. Mechanism of levulinic acid formation. Tetrahedron Lett. 1985, 26, 2111–2114. [Google Scholar] [CrossRef]
- Wahlen, J.; Moens, B.; De Vos, D.E.; Alsters, P.L.; Jacobs, P.A. Titanium silicalite 1 (TS-1) catalyzed oxidative transformations of furan derivatives with hydrogen peroxide. Adv. Synth. Catal. 2004, 346, 333–338. [Google Scholar] [CrossRef]
- Che, P.; Lu, F.; Zhang, J.; Huang, Y.; Nie, X.; Gao, J.; Xu, J. Catalytic selective etherification of hydroxyl groups in 5-hydroxymethylfurfural over H4SiW12O40/MCM-41 nanospheres for liquid fuel production. Bioresour. Technol. 2012, 119, 433–436. [Google Scholar] [CrossRef] [PubMed]




| Sample | Si/Al Molar Ratio a | SBET (m2·g−1) b | Vmicro (cm3·g−1) c | Vmeso (cm3·g−1) d | Number of Acid Sites (mmol of NH3·g−1) e | ||
|---|---|---|---|---|---|---|---|
| Weak Acid | Strong Acid | Total Acid | |||||
| Conventional ZSM-5 (C-ZSM-5) | 33 | 330 | 0.11 | 0.05 | 0.33 | 0.43 | 0.76 |
| Hierarchical porous ZSM-5 (HP-ZSM-5) | 43 | 453 | 0.11 | 0.34 | 0.14 | 0.16 | 0.30 |
| Catalysts | Time (h) | BMMF Yield (%) |
|---|---|---|
| Fresh C-ZSM-5 | 24 | 64.0 |
| Spent C-ZSM-5 | 24 | 39.6 |
| Fresh HP-ZSM-5 | 12 | 94.5 |
| Spent HP-ZSM-5 | 12 | 93.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, W.; Hu, H.; Ma, Z.; Wang, L.; Zhang, Y. Two Possible Side Reaction Pathways during Furanic Etherification. Catalysts 2018, 8, 383. https://doi.org/10.3390/catal8090383
Fang W, Hu H, Ma Z, Wang L, Zhang Y. Two Possible Side Reaction Pathways during Furanic Etherification. Catalysts. 2018; 8(9):383. https://doi.org/10.3390/catal8090383
Chicago/Turabian StyleFang, Wenting, Hualei Hu, Zhongsen Ma, Lei Wang, and Yajie Zhang. 2018. "Two Possible Side Reaction Pathways during Furanic Etherification" Catalysts 8, no. 9: 383. https://doi.org/10.3390/catal8090383