Rational Design of High Surface Area Mesoporous Ni/CeO2 for Partial Oxidation of Propane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural Properties
2.2. TPR Analysis
2.3. Catalytic Characteristics
2.3.1. Influence of Operating Temperature and Ni Loading
2.3.2. Influence of GHSV
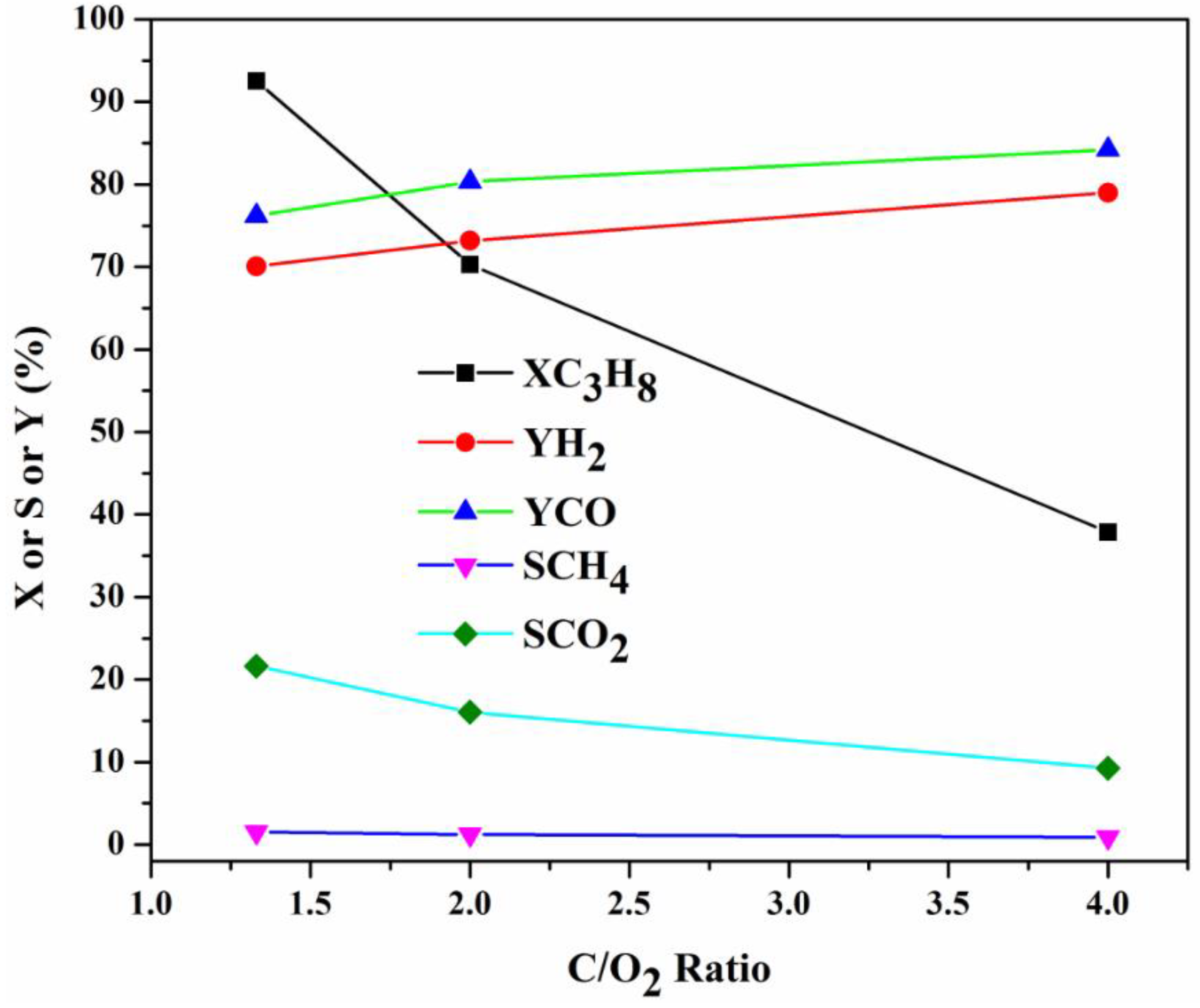
2.3.3. Influence of Feed Gas Composition
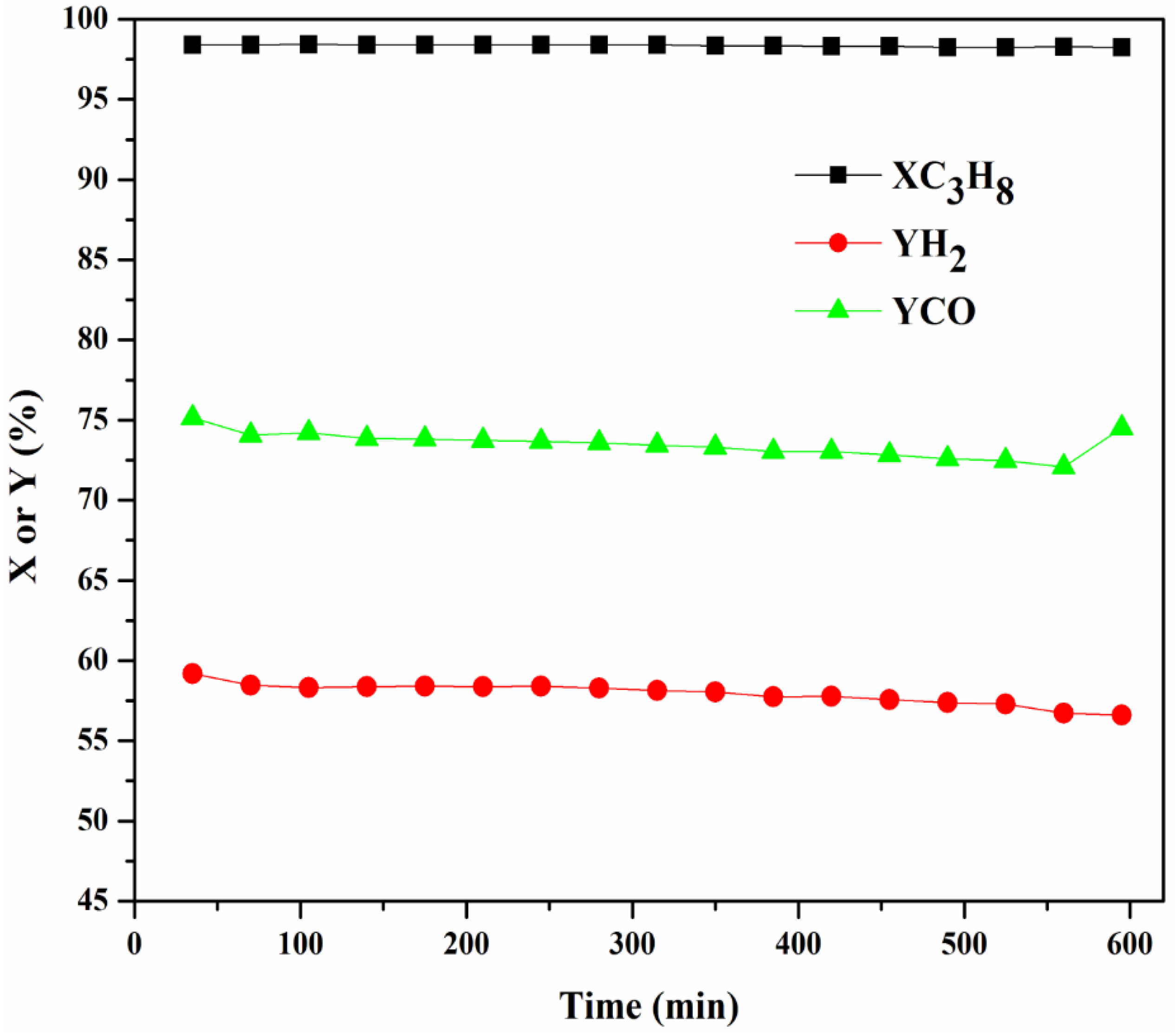
2.3.4. Stability Test
2.4. TPO Analysis
2.5. SEM
3. Experimental Section
3.1. Catalyst Synthesis
3.2. Catalytic Performance
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arandiyan, H.; Li, J.; Ma, L.; Hashemnejad, S.M.; Mirzaei, M.Z.; Chen, J.; Chang, H.; Liu, C.; Wang, C.; Chen, L. Methane reforming to syngas over LaNixFe1−xO3 (0 ≤ x ≤ 1) mixed-oxide perovskites in the presence of CO2 and O2. J. Ind. Eng. Chem. 2012, 18, 2103–2114. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Xinmei, L.; Qian, L.; Yan, Z.-F. CO2-CH4 Reforming over Nickel Catalysts Supported on Mesoporous Nanocrystalline Zirconia with High Surface Area. Energy Fuels 2007, 21, 581–589. [Google Scholar] [CrossRef]
- Arandiyan, H.; Scott, J.; Wang, Y.; Dai, H.; Sun, H.; Amal, R. Meso-Molding Three-Dimensional Macroporous Perovskites: A New Approach to Generate High-Performance Nanohybrid Catalysts. ACS Appl. Mater. Interfaces 2016, 8, 2457–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbo, P.; Migliardini, F. Hydrogen production by catalytic partial oxidation of methane and propane on Ni and Pt catalysts. Int. J. Hydrog. Energy 2007, 32, 55–66. [Google Scholar] [CrossRef]
- Ibrahim, H.H.; Idem, R.O. Kinetic studies of the partial oxidation of isooctane for hydrogen production over a nickel–alumina catalyst. Chem. Eng. Sci. 2006, 61, 5912–5918. [Google Scholar] [CrossRef]
- Maqbool, W.; Lee, E.S. Syngas Production Process Development and Economic Evaluation for Gas-to-Liquid Applications. Chem. Eng. Technol. 2014, 37, 995–1001. [Google Scholar] [CrossRef]
- Meshkani, F.; Rezaei, M.; Andache, M. Investigation of the catalytic performance of Ni/MgO catalysts in partial oxidation, dry reforming and combined reforming of methane. J. Ind. Eng. Chem. 2014, 20, 1251–1260. [Google Scholar] [CrossRef]
- Silva, C.R.B.; da Conceição, L.; Ribeiro, N.F.P.; Souza, M.M.V.M. Partial oxidation of methane over Ni–Co perovskite catalysts. Catal. Commun. 2011, 12, 665–668. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kwak, H.J.; Kim, S.M.; Nam, S.-W.; Lim, H.T.; Hong, S.-A.; Yoon, J.K. Optimal conditions for partial oxidation of propane over ceria-promoted nickel/calcium hydroxyapatite. Korean J. Chem. Eng. 2007, 24, 226–232. [Google Scholar] [CrossRef]
- Pirez, C.; Fang, W.; Capron, M.; Paul, S.; Jobic, H.; Dumeignil, F.; Jalowiecki-Duhamel, L. Steam reforming, partial oxidation and oxidative steam reforming for hydrogen production from ethanol over cerium nickel based oxyhydride catalyst. Appl. Catal. A Gen. 2016, 518, 78–86. [Google Scholar] [CrossRef]
- Marschall, K.J.; Mleczko, L. Experimental Investigations of Catalytic Partial Oxidation of Methane to Synthesis Gas in Various Types of Fluidized-Bed Reactors. Chem. Eng. Technol. 2000, 23, 31–37. [Google Scholar] [CrossRef]
- Ding, C.; Ai, G.; Zhang, K.; Yuan, Q.; Han, Y.; Ma, X.; Wang, J.; Liu, S. Coking resistant Ni/ZrO2@SiO2 catalyst for the partial oxidation of methane to synthesis gas. Int. J. Hydrogen Energy 2015, 40, 6835–6843. [Google Scholar] [CrossRef]
- Praharso, A.A.A.; Trimm, D.L.; Cant, N.W. Supported Rh catalysts for the indirect partial oxidation of isooctane. Korean J. Chem. Eng. 2003, 20, 468–470. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of promoted nickel catalysts supported on mesoporous nanocrystalline gamma alumina for carbon dioxide methanation reaction. J. Ind. Eng. Chem. 2014, 20, 4176–4182. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Hong, X.; Li, B. Zirconia promoted metallic nickel catalysts for the partial oxidation of methane to synthesis gas. Catal. Commun. 2009, 10, 940–944. [Google Scholar] [CrossRef]
- Kim, S.-B.; Kim, Y.-K.; Lim, Y.-S.; Kim, M.-S.; Hahm, H.-S. Reaction mechanism of partial oxidation of methane to synthesis gas over supported ni catalysts. Korean J. Chem. Eng. 2003, 20, 1023–1025. [Google Scholar] [CrossRef]
- Peymani, M.; Alavi, S.M.; Rezaei, M. Preparation of highly active and stable nanostructured Ni/CeO2 catalysts for syngas production by partial oxidation of methane. Int. J. Hydrogen Energy 2016, 41, 6316–6325. [Google Scholar] [CrossRef]
- Yu, C.; Hu, J.; Zhou, W.; Fan, Q. Novel Ni/CeO2-Al2O3 composite catalysts synthesized by one-step citric acid complex and their performance in catalytic partial oxidation of methane. J. Energy Chem. 2014, 23, 235–243. [Google Scholar] [CrossRef]
- La Parola, V.; Pantaleo, G.; Venezia, A. Effects of Synthesis on the Structural Properties and Methane Partial Oxidation Activity of Ni/CeO2 Catalyst. Catalysts 2018, 8, 220. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Arandiyan, H.; Younis, A.; Scott, J.; Qu, B.; Wan, T.; Lin, X.; Chen, J.; Chu, D. Design and synthesis of CeO2 nanowire/MnO2 nanosheet heterogeneous structure for enhanced catalytic properties. Mater. Today Commun. 2017, 11, 103–111. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Arandiyan, H.; Scott, J.; Wan, T.; Chu, D. Correlating morphology and doping effects with the carbon monoxide catalytic activity of Zn doped CeO2 nanocrystals. Catal. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87. [Google Scholar] [CrossRef]
- Rezaei, M.; Meshkani, F.; Ravandi, A.B.; Nematollahi, B.; Ranjbar, A.; Hadian, N.; Mosayebi, Z. Autothermal reforming of methane over Ni catalysts supported on nanocrystalline MgO with high surface area and plated-like shape. Int. J. Hydrogen Energy 2011, 36, 11712–11717. [Google Scholar] [CrossRef]
- Zhan, Z.; Barnett, S.A. Use of a catalyst layer for propane partial oxidation in solid oxide fuel cells. Solid State Ionics 2005, 176, 871–879. [Google Scholar] [CrossRef]
- Waller, M.G.; Walluk, M.R.; Trabold, T.A. Operating envelope of a short contact time fuel reformer for propane catalytic partial oxidation. J. Power Sources 2015, 274, 149–155. [Google Scholar] [CrossRef]
- Pennemann, H.; Hessel, V.; Kolb, G.; Löwe, H.; Zapf, R. Partial oxidation of propane using micro structured reactors. Chem. Eng. J. 2008, 135 (Suppl. 1), S66–S73. [Google Scholar] [CrossRef]
- Khajenoori, M.; Rezaei, M.; Nematollahi, B. Preparation of noble metal nanocatalysts and their applications in catalytic partial oxidation of methane. J. Ind. Eng. Chem. 2013, 19, 981–986. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Wang, H.; Kondratenko, V.A.; Caro, J. Selective oxidation of CH4 and C2H6 over a mixed oxygen ion and electron conducting perovskite—A TAP and membrane reactors study. J. Mol. Catal. A Chem. 2009, 297, 142–149. [Google Scholar] [CrossRef]
- Alhamamre, Z.; Vos, S.; Trimis, D. Hydrogen production by thermal partial oxidation of hydrocarbon fuels in porous media based reformer. Int. J. Hydrogen Energy 2009, 34, 827–832. [Google Scholar] [CrossRef]
- Silberova, B.; Venvik, H.J.; Holmen, A. Production of hydrogen by short contact time partial oxidation and oxidative steam reforming of propane. Catal. Today 2005, 99, 69–76. [Google Scholar] [CrossRef]
- Li, C.-Y.; Zhang, Z.-B.; Yu, C.-C.; Shen, S.-K. Temperature-Programmed Studies on Partial Oxidation of CH4 to Syngas over a Ni/Al2O3 Catalyst. Fuel Chem. Div. Prepr. 2002, 47, 123–125. [Google Scholar]
- Smith, M.W.; Shekhawat, D.; Berry, D.A.; Haynes, D.J.; Floyd, D.L.; Spivey, J.J.; Ranasingha, O. Carbon formation on Rh-substituted pyrochlore catalysts during partial oxidation of liquid hydrocarbons. Appl. Catal. A Gen. 2015, 502, 96–104. [Google Scholar] [CrossRef]
- Claridge, J.; Green, M.H.; Tsang, S.; York, A.E.; Ashcroft, A.; Battle, P. A study of carbon deposition on catalysts during the partial oxidation of methane to synthesis gas. Catal. Lett. 1993, 22, 299–305. [Google Scholar] [CrossRef]
- Tang, S.; Lin, J.; Tan, K.L. Partial oxidation of methane to syngas over Ni/MgO, Ni/CaO and Ni/CeO2. Catal. Lett. 1998, 51, 169–175. [Google Scholar] [CrossRef]
- Yan, Q.-G.; Chao, Z.-S.; Wu, T.-H.; Weng, W.-Z.; Chen, M.-S.; Wan, H.-L. Carbon deposition on Ni/Al2O3 catalyst during partial oxidation of methane to syngas. In Studies in Surface Science and Catalysis; Corma, A., Melo, F.V., Mendioroz, S., Fierro, J.L.G., Eds.; Elsevier: New York, NY, USA, 2000; pp. 3549–3554. [Google Scholar]
- Peymani, M.; Alavi, S.M.; Rezaei, M. Synthesis Gas Production by Catalytic Partial Oxidation of Propane on Mesoporous Nanocrystalline Ni/Al2O3 Catalysts. Appl. Catal. A Gen. 2017, 529, 1–9. [Google Scholar] [CrossRef]









| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Ni Crystallite Size (nm) | |||
|---|---|---|---|---|---|---|---|
| Reduced | Spent | Reduced | Spent | Reduced | Spent | ||
| CeO2 | 138 | - | 0.45 | - | 16.3 | - | - |
| 2.5Ni/CeO2 | 41.0 | 23.6 | 0.20 | 0.079 | 19.5 | 13.5 | - |
| 5Ni/CeO2 | 39.4 | 37.7 | 0.18 | 0.088 | 18.4 | 9.42 | 8.80 |
| 7.5Ni/CeO2 | 39.6 | 41.4 | 0.18 | 0.090 | 18.2 | 8.88 | 13.0 |
| 10Ni/CeO2 | 36.4 | 46.7 | 0.16 | 0.092 | 18.3 | 7.53 | 15.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peymani, M.; Alavi, S.M.; Arandiyan, H.; Rezaei, M. Rational Design of High Surface Area Mesoporous Ni/CeO2 for Partial Oxidation of Propane. Catalysts 2018, 8, 388. https://doi.org/10.3390/catal8090388
Peymani M, Alavi SM, Arandiyan H, Rezaei M. Rational Design of High Surface Area Mesoporous Ni/CeO2 for Partial Oxidation of Propane. Catalysts. 2018; 8(9):388. https://doi.org/10.3390/catal8090388
Chicago/Turabian StylePeymani, Mohammad, Seyed Mehdi Alavi, Hamidreza Arandiyan, and Mehran Rezaei. 2018. "Rational Design of High Surface Area Mesoporous Ni/CeO2 for Partial Oxidation of Propane" Catalysts 8, no. 9: 388. https://doi.org/10.3390/catal8090388






