Operando Dual Beam FTIR Study of Hydroxyl Groups and Zn Species over Defective HZSM-5 Zeolite Supported Zinc Catalysts
Abstract
1. Introduction
2. Result and Discussion
2.1. Defective ZSM-5 Zeolites with Different SiO2/Al2O3 Ratios
2.2. Defective HZSM-5 Supported Zn Catalysts Prepared by Chemical Vapor Deposition (CLD) with Zn(C2H5)2
2.3. Properties of Zn Species Located in Hydroxyl Nests Versus in Si(OH)Al
2.3.1. Acid Properties of Zn Species Located in Hydroxyl Nests Versus in Si(OH)Al
2.3.2. Catalytic Performance of Zn Species Located in Hydroxyl Nests Versus in Si(OH)Al
3. Materials and Methods
3.1. Materials
3.2. Zn/ZSM-5 Prepared by Chemical Liquid Deposition (CLD) with Zn(C2H5)2
3.3. Characterization
3.4. Catalytic Tests
3.4.1. Pulse Micro-Reactor
3.4.2. Home-Built operando Dual Beam FTIR-MS
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Barrer, R.M.; Makki, M.B. Molecular sieve sorbents from clinoptilolite. Can. J. Chem. 1964, 42, 1481–1487. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Padovan, M. Silicalite characterization. Structure, adsorptive capacity, and IR spectroscopy of the framework and hydroxyl modes. J. Phys. Chem. 1992, 96, 4985–4990. [Google Scholar] [CrossRef]
- Bordiga, S.; Ugliengo, P.; Damina, A.; Lamberti, C.; Spoto, G.; Zecchin, A.; Spano, G.; Buzzoni, R.; Dalloro, L.; Rivetti, F. Hydroxyls nests in defective silicalites and strained structures derived upon dehydroxylation: Vibrational properties and theoretical modelling. Top. Catal. 2001, 15, 43–52. [Google Scholar] [CrossRef]
- Trzpit, M.; Soulard, M.; Patarin, J.; Desbiens, N.; Cailliez, F.; Boutin, A.; Demachy, I.; Fuchs, A.H. The effect of local defects on water adsorption in silicalite-1 zeolite: a joint experimental and molecular simulation study. Langmuir 2007, 23, 10131–10139. [Google Scholar] [CrossRef] [PubMed]
- Deruiter, R.; Kentgens, A.P.M.; Grootendorst, J.; Jansen, J.C.; Vanbekkum, H. Calciantion and deboronation of B-MFI single-crystals. Zeolites 1993, 13, 128–138. [Google Scholar] [CrossRef]
- Heitmann, G.P.; Dahlhoff, G.; Niederer, J.P.M.; Holderich, W.F. Active Sites of a [B]-ZSM-5 zeolite catalyst for the Beckmann rearrangement of cyclohexanone oxime to caprolactam. J. Catal. 2000, 194, 122–129. [Google Scholar] [CrossRef]
- Heitmann, G.P.; Dahlhoff, G.; Holderich, W.F. Catalytically active sites for the beckmann rearrangement of cyclohexanone oxime to ε-caprolactam. J. Catal. 1999, 186, 12–19. [Google Scholar] [CrossRef]
- Forni, L.; Fornasari, G.; Giordano, G.; Lucarelli, C.; Katovic, A.; Trifiro, E.; Perri, C.; Nagy, J.B. Vapor phase Beckmann rearrangement using high silica zeolite catalyst. Phys. Chem. Chem.Phys. 2004, 6, 1842–1847. [Google Scholar] [CrossRef]
- Graaff, W.N.P.; Li, G.; Mezari, B.; Pidko, E.A.; Hensen, E.J.M. Synthesis of Sn-Beta with exclusive and high framework Sn content. Chem. Cat. Chem. 2015, 7, 1152–1160. [Google Scholar]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations. Nature 2001, 412, 423–425. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Valencia, S. Water-resistant solid Lewis acid catalysts: Meerwein-Ponndorf-Verley and Oppenauer reactions catalyzed by tin-beta zeolite. J. Catal. 2003, 215, 294–304. [Google Scholar] [CrossRef]
- Jia, Y.M.; Wang, J.W.; Zhang, K.; Liu, S.B.; Chen, G.L.; Yang, Y.F.; Ding, C.M.; Liu, P. Catalytic conversion of methanol to aromatics over nanosized HZSM-5 zeolite modified by ZnSiF6·6H2O. Catal. Sci. Technol. 2012, 7, 1–3. [Google Scholar]
- Bleken, F.L.; Barbera, K.; Bonino, F.; Olsbye, U.; Lillerud, K.P.; Bordiga, S.; Beato, P.; Janssens, T.V.W.; Svelle, S. Catalyst deactivation by coke formation in microporous and desilicated zeolite H-ZSM-5 during the conversion of methanol to hydrocarbons. J. Catal. 2013, 307, 62–73. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar] [CrossRef]
- Venuto, P.B. Organic catalysis over zeolites: A perspective on reaction paths within micropores. Microporous. Mater. 1994, 2, 297–411. [Google Scholar] [CrossRef]
- Jin, F.; Cui, Y.G.; Rui, Z.B. Effect of sequential desilication and dealumination on catalytic performance of ZSM-5 catalyst for pyridine and 3-picoline synthesis. J. Mater. Res. 2010, 25, 272–282. [Google Scholar] [CrossRef]
- Dyballa, M.; Obenaus, U.; Blum, M. Alkali metal ion exchanged ZSM-5 catalysts: On acidity and methanol-to-olefin performance. Catal. Sci. Technol. 2018, 8, 4440–4449. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Shubbotina, I.R.; Rane, N.; Van Santen, R.A.; Hensen, E.J.M. On two alternative mechanisms of ethane activation over ZSM-5 zeolite modified by Zn2+ and Ga1+ cations. Phys. Chem. Chem.Phys. 2005, 7, 3088–3092. [Google Scholar] [CrossRef] [PubMed]
- Biscardi, J.A.; Iglesia, E. Non-oxidative reactions of propane on Zn/Na-ZSM5. Phys. Chem. Chem.Phys. 1999, 1, 5753–5759. [Google Scholar] [CrossRef]
- Penzien, J.; Abraham, A.; van Bokhoven, J.A.; Jentiys, A.; Muller, T.E.; Sievers, C.; Lercher, J.A. Generation and characterization of well-defined Zn2+ Lewis acid sites in ion exchanged zeolite BEA. J. Phys. Chem. B 2004, 108, 4116–4126. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Radovan, M. Silicalite Characterization. 2. IR spectroscopy of the interaction of CO with internal and external hydroxyl groups. J. Phys. Chem. 1992, 96, 4991–4997. [Google Scholar] [CrossRef]
- Liu, J.X.; He, N.; Zhou, W.; Lin, L.; Liu, G.D.; Liu, C.Y.; Wang, J.L.; Xin, Q.; Xiong, G.; Guo, H.C. Isobutane aromatization over a complete Lewis acid Zn/HZSM-5 zeolite catalyst: Performance and mechanism. Catal. Sci. Technol. 2018, 8, 4018–4029. [Google Scholar] [CrossRef]
- Bordiga, S.; Roggero, I.; Ugliengo, P.; Zecchina, A.; Bolis, V.; Artioli, G.; Buzzoni, R.; Marra, G.; Rivetti, F.; Spano, G.; et al. Characterisation of defective silicalites. J. Chem. Soc. Dalton Trans. 2000, 21, 3921–3929. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Scarano, D.; Petrini, G.; Leofanti, G.; Padovan, M. Low-temperature Fourier-transform infrared investigation of the interaction of CO with nanosized ZSM5 and silicalite. J. Chem. Soc. Faraday Trans. 1992, 88, 2959–2969. [Google Scholar] [CrossRef]
- Bolis, V.; Busco, C.; Bordiga, S.; Ugliengo, P.; Lamberti, C.; Zecchina, A. Calorimetric and IR spectroscopic study of the interaction of NH3 with variously prepared defective silicalites: Comparison with ab initio computational data. Appl. Surf. Sci. 2002, 196, 56–70. [Google Scholar] [CrossRef]
- Li, Y.N.; Liu, S.L.; Xie, S.J.; Xu, L.Y. Promoted metal utilization capacity of alkali-treated zeolite: Preparation of Zn/ZSM-5 and its application in 1-hexene aromatization. Appl. Catal. A Gen. 2009, 360, 8–16. [Google Scholar] [CrossRef]
- Nishi, K.; Komai, S.; Inagaki, K.; Satsuma, A.; Hattori, T. Structure and catalytic properties of Ga-MFI in propane aromatization. Appl. Catal. A Gen. 2002, 223, 187–193. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, W.G.; So, J.S.; Moore, J.S.; Liu, Y.J.; Dixit, R.S.; Pendergast, J.G.; Sievers, C.; Sholl, D.S.; Nair, S.; et al. Propane dehydrogenation catalyzed by gallosilicate MFI zeolites with perturbed acidity. J. Catal. 2017, 345, 113–123. [Google Scholar] [CrossRef]
- Rodríguez-González, L.; Hermes, F.; Bertmer, M.; Rodríguez-Castellón, E.; Jiménez-López, A. The acid properties of H-ZSM-5 as studied by NH3-TPD and 27Al-MAS-NMR spectroscopy. Appl. Catal. A Gen. 2007, 328, 174–182. [Google Scholar] [CrossRef]
- Zhang, J.G.; Qian, W.Z.; Kong, C.Y.; Wei, F. Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/Zsm-5 Catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zhou, J.; Chen, Y.; He, Q.J.; Ruan, M.L.; Guo, L.M.; Shi, J.L.; Chen, H.R. Fabrication of mesoporous zeolite microspheres by a one-pot dual-functional templating approach. J. Mater. Chem. 2009, 19, 7614–7616. [Google Scholar] [CrossRef]
- Zhang, W.P.; Bao, X.H.; Guo, X.W.; Wang, X.S. A high-resolution solid-state NMR study on nano-structured HZSM-5 zeolite. Catal. Lett. 1999, 60, 89–94. [Google Scholar] [CrossRef]
- Almutairi, S.M.T.; Mezari, B.; Magusin, P.C.M.M.; Pidko, E.A.; Hensen, E.J.M. Structure and reactivity of Zn-modified ZSM-5 zeolites: The importance of clustered cationic Zn complexes. ACS Catal. 2012, 2, 71–83. [Google Scholar] [CrossRef]
- Wang, K.; Cao, G.; Kennedy, G.J.; Afeworki, M.; Bare, R.E.; Hall, R.B. Pore Modification of H-SAPO-34 using dialkyl zinc: Structural characterization and reaction pathway. J. Phys. Chem. C 2011, 115, 18611–18617. [Google Scholar] [CrossRef]
- Sohn, J.R.; Decanio, S.J.; Fritz, P.O.; Lunsford, J.H. Acid catalysis by dealuminated zeolite Y. 2. The roles of aluminum. J. Phys. Chem. 1986, 90, 4847–4851. [Google Scholar] [CrossRef]
- Chen, X.H.; Dong, M.; Niu, X.J.; Wang, K.; Chen, G.; Fan, W.B.; Wang, J.G.; Qin, Z.F. Influence of Zn species in HZSM-5 on ethylene aromatization. Chinese J. Catal. 2015, 36, 880–888. [Google Scholar] [CrossRef]
- Liu, J.X.; Wang, J.L.; Zhou, W.; Miao, C.L.; Xiong, G.; Xin, Q.; Guo, H.C. Construction of an operando dual-beam fourier transform infrared spectrometer and its application in the observation of isobutene reactions over nano-sized HZSM-5 zeolite. Chin. J. Catal. 2017, 38, 13–19. [Google Scholar] [CrossRef]
- Ivanovaa, I.I.; Kolyagina, Y.G.; Ordomskya, V.V.; Asachenkoa, E.V.; Pasynkovab, E.M. Surface species formed during propane aromatization over Zn/MFI catalyst as determined by in situ spectroscopic techniques. J. Mol. Catal. A Chem. 2009, 305, 47–53. [Google Scholar] [CrossRef]
- Flego, C.; Peratello, S.; Perego, C.; Sabatino, L.M.F.; Bellussi, G.; Romano, U. Reaction and deactivation study of mesoporous silica-alumina (MSA) in propene oligomerisation. J. Mol. Catal. A Chem. 2003, 581, 204–205. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.X.; Lin, L.; Zhang, X.T.; He, N.; Liu, C.Y.; Guo, H.C. Enhanced dehydrogenative aromatization of propane by incorporating Fe and Pt into Zn/HZSM-5 catalyst. Ind. Eng. Chem. Res. 2018, 57, 16246–16256. [Google Scholar] [CrossRef]



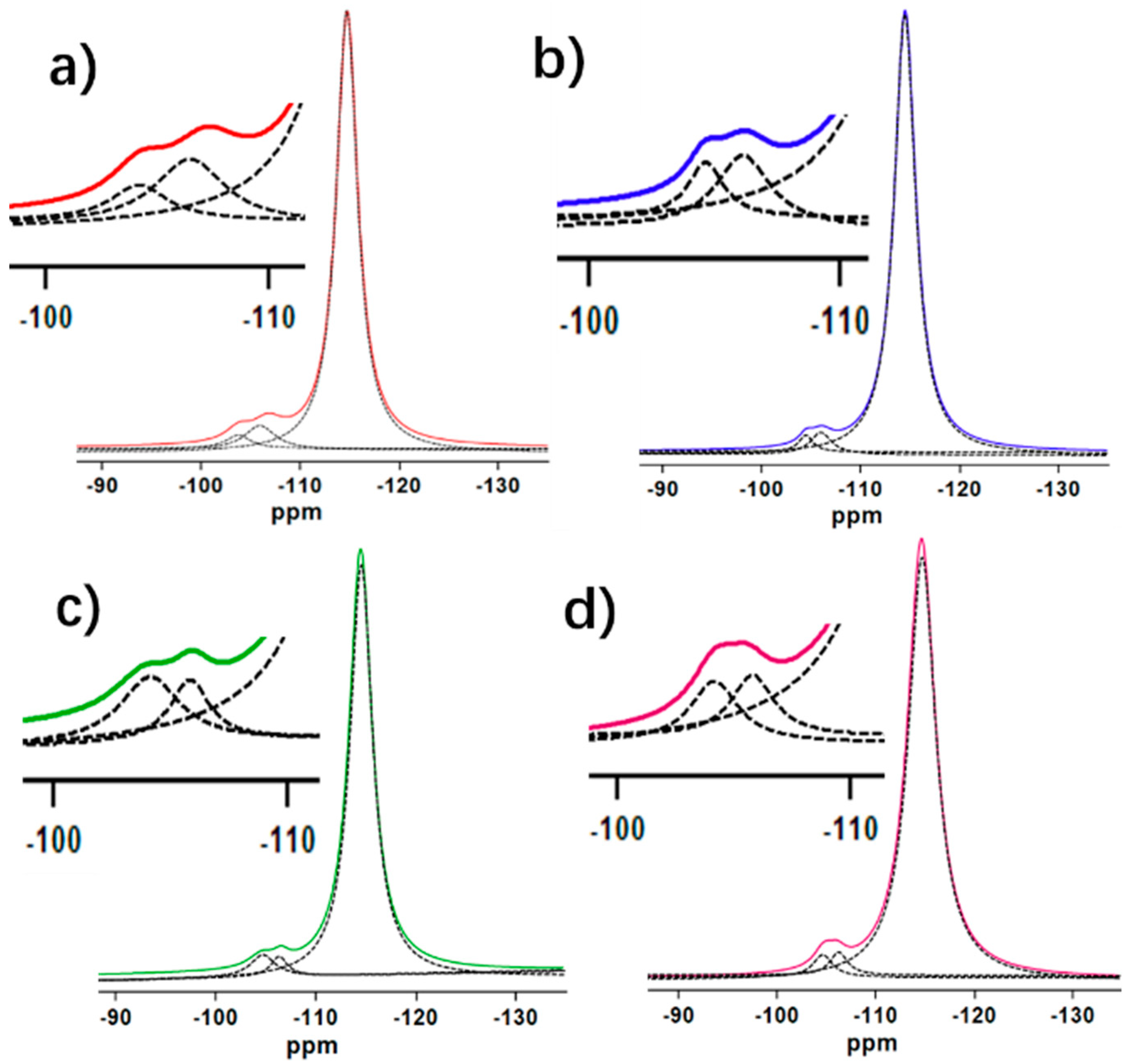



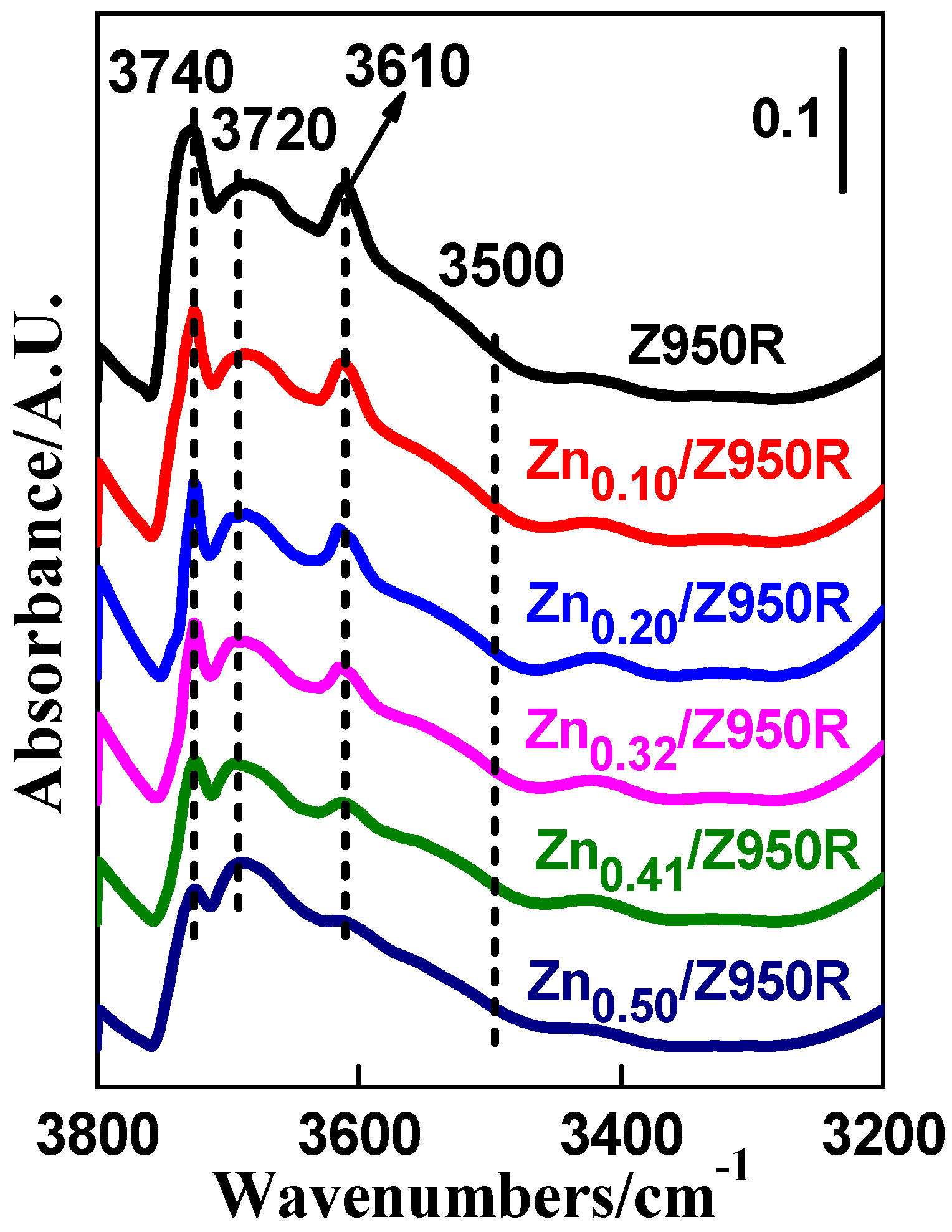





| Samples | SiO2/Al2O3 | SBET | Smicro | Vtoltal | Vmicro | Crystallinity |
|---|---|---|---|---|---|---|
| (m2/g) | (m2/g) | (cm3/g) | (cm3/g) | (%) | ||
| Z55 | 55 | 395 | 362 | 0.20 | 0.13 | 89.84 |
| Z100 | 100 | 417 | 335 | 0.24 | 0.13 | 95.01 |
| Z480 | 480 | 437 | 397 | 0.23 | 0.15 | 98.30 |
| Z950 | 950 | 426 | 382 | 0.23 | 0.14 | 98.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Zhang, X.; He, N.; Liu, J.; Xin, Q.; Guo, H. Operando Dual Beam FTIR Study of Hydroxyl Groups and Zn Species over Defective HZSM-5 Zeolite Supported Zinc Catalysts. Catalysts 2019, 9, 100. https://doi.org/10.3390/catal9010100
Lin L, Zhang X, He N, Liu J, Xin Q, Guo H. Operando Dual Beam FTIR Study of Hydroxyl Groups and Zn Species over Defective HZSM-5 Zeolite Supported Zinc Catalysts. Catalysts. 2019; 9(1):100. https://doi.org/10.3390/catal9010100
Chicago/Turabian StyleLin, Long, Xiaotong Zhang, Ning He, Jiaxu Liu, Qin Xin, and Hongchen Guo. 2019. "Operando Dual Beam FTIR Study of Hydroxyl Groups and Zn Species over Defective HZSM-5 Zeolite Supported Zinc Catalysts" Catalysts 9, no. 1: 100. https://doi.org/10.3390/catal9010100
APA StyleLin, L., Zhang, X., He, N., Liu, J., Xin, Q., & Guo, H. (2019). Operando Dual Beam FTIR Study of Hydroxyl Groups and Zn Species over Defective HZSM-5 Zeolite Supported Zinc Catalysts. Catalysts, 9(1), 100. https://doi.org/10.3390/catal9010100






