Recent Progress in Application of Molybdenum-Based Catalysts for Epoxidation of Alkenes
Abstract
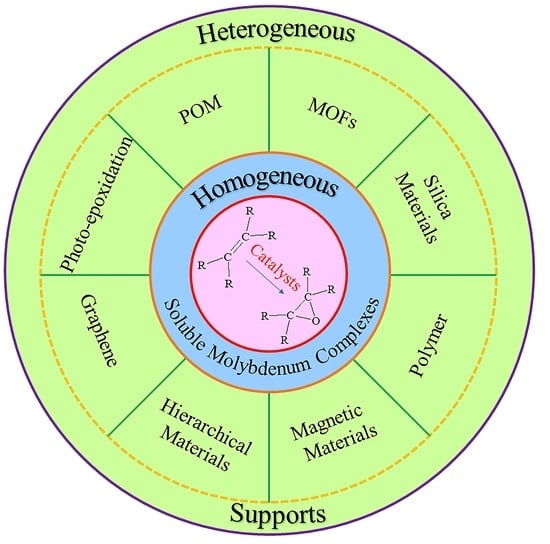
:1. Introduction
2. Homogeneous Molybdenum Complexes
3. Heterogeneous Molybdenum-Based Catalysts
3.1. Polyoxometalates (POMs) Catalysts
3.2. Molybdenum-Containing Metal Organic Frameworks (MOFs)
3.3. Silica Supported Molybdenum-Based Catalysts
3.4. Polymer Supported Molybdenum-Based Catalysts
3.5. Magnetic Molybdenum-Based Catalysts
3.6. Hierarchical Molybdenum-Based Catalysts
3.7. Graphene-Based Molybdenum-Containing Catalysts
3.8. Photocatalyzed Epoxidation Catalysts
3.9. Others
4. Reaction Conditions and Mechanisms
4.1. Oxidizing Agents
4.2. Solvents
4.3. Mechanism
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, K.; Song, T.; Wang, D.; Zou, Y.; Li, J.; Zhang, X.; Tang, Z.; Hu, W. Bimetal-organic frameworks for functionality optimization: MnFe-MOF-74 as a stable and efficient catalyst for the epoxidation of alkenes with H2O2. Nanoscale 2018, 10, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, B.; Su, Y.; Huang, H. Enantioselective epoxidation of electron-deficient alkenes catalyzed by Manganese complexes with chiral N4 ligands derived from rigid chiral diamines. Adv. Synth. Catal. 2017, 359, 2535–2541. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Chen, G.; Chen, M.; Bai, R.; Jia, M.; Yu, J. Synthesis of anatase-free nano-sized hierarchical TS-1 zeolites and their excellent catalytic performance in alkene epoxidation. J. Mater. Chem. A 2018, 6, 9473–9479. [Google Scholar] [CrossRef]
- Grosso-Giordano, N.A.; Schroeder, C.; Okrut, A.; Solovyov, A.; Schottle, C.; Chasse, W.; Marinkovic, N.; Koller, H.; Zones, S.I.; Katz, A. Outer-sphere control of catalysis on surfaces: A comparative study of Ti(IV) single-sites grafted on amorphous versus crystalline silicates for alkene epoxidation. J. Am. Chem. Soc. 2018, 140, 4956–4960. [Google Scholar] [CrossRef] [PubMed]
- Ballistreri, F.; Toscano, R.; Amato, M.; Pappalardo, A.; Gangemi, C.; Spidalieri, S.; Puglisi, R.; Trusso Sfrazzetto, G. A new Mn-Salen micellar nanoreactor for enantioselective epoxidation of alkenes in water. Catalysts 2018, 8, 129. [Google Scholar] [CrossRef]
- Lu, J.; Liang, L.; Weck, M. Micelle-based nanoreactors containing Ru-porphyrin for the epoxidation of terminal olefins in water. J. Mol. Catal. A Chem. 2016, 417, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Fu, Q.; Chen, W.; Feng, Q.; Chen, Z.; Zhang, J.; Cheong, W.C.; Yu, R.; Gu, L.; Dong, J.; et al. Carbon nitride supported Fe2 cluster catalysts with superior performance for alkene epoxidation. Nat. Commun. 2018, 9, 2353. [Google Scholar] [CrossRef] [PubMed]
- Starokon, E.V.; Malykhin, S.E.; Parfenov, M.V.; Zhidomirov, G.M.; Kharitonov, A.S. Oxidation of lower alkenes by α-oxygen (FeIII–O•−)α on the FeZSM-5 surface: The epoxidation or the allylic oxidation? Mol. Catal. 2017, 443, 43–51. [Google Scholar] [CrossRef]
- Huber, S.; Cokoja, M.; Kühn, F.E. Historical landmarks of the application of molecular transition metal catalysts for olefin epoxidation. J. Organomet. Chem. 2014, 751, 25–32. [Google Scholar] [CrossRef]
- Mizuno, N.; Yamaguchi, K.; Kamata, K. Epoxidation of olefins with hydrogen peroxide catalyzed by polyoxometalates. Coord. Chem. Rev. 2005, 249, 1944–1956. [Google Scholar] [CrossRef]
- Ballistreri, F.P.; Gangemi, C.M.; Pappalardo, A.; Tomaselli, G.A.; Toscano, R.M.; Trusso Sfrazzetto, G. (Salen)Mn(III) catalyzed asymmetric epoxidation reactions by hydrogen peroxide in water: A green protocol. Int. J. Mol. Sci. 2016, 17, 1112. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas Kashani, S.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I. Ruthenium nanoparticles immobilized on nano-silica functionalized with thiol-based dendrimer: A nanocomposite material for oxidation of alcohols and epoxidation of alkenes. Catal. Lett. 2018, 148, 1110–1123. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Cornwall, R.G.; Shi, Y. Organocatalytic asymmetric epoxidation and aziridination of olefins and their synthetic applications. Chem. Rev. 2014, 114, 8199–8256. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Synthetic and mechanistic aspects of metal-catalysed epoxidations with hydroperoxides. J. Mol. Catal. 1980, 7, 107–126. [Google Scholar] [CrossRef]
- Morales-delaRosa, S.; Campos-Martin, J.M.; Terreros, P.; Fierro, J.L.G. Catalytic epoxidation of cyclohexene with tert-butylhydroperoxide using an immobilized molybdenum catalyst. Top. Catal. 2015, 58, 325–333. [Google Scholar] [CrossRef]
- Sheldon, R.A. Molybdenum-catalysed epoxidation of olefins with alkyl hydroperoxides I. Kinetic and product studies. Recl. Trav. Chim. Pays-Bas 1973, 92, 253–266. [Google Scholar] [CrossRef]
- Chong, A.O.; Sharpless, K.B. Mechanism of the molybdenum and vanadium catalyzed epoxidation of olefins by alkyl hydroperoxides. J. Org. Chem. 1977, 42, 1587–1590. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Townsend, J.M.; Williams, D.R. On the mechanism of epoxidation of olefins by covalent peroxides of molybdenum(V1). J. Am. Chem. Soc. 1972, 94, 295–296. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Michaelson, R.C. High stereo-and regioselectivities in the transition metal catalyzed epoxidations of olefinic alcohols by tert-butyl hydroperoxide. J. Am. Chem. Soc. 1973, 95, 6136–6137. [Google Scholar] [CrossRef]
- Fusi, A.; Ugo, R.; Zanderighi, G.M. Homogeneous catalysis by transition metal complexes IV. The use of mixed catalysts in the oxidation of cyclohexene. J. Catal. 1974, 34, 175–190. [Google Scholar] [CrossRef]
- Trifirò, F.; Forzatti, P.; Preite, S.; Pasquon, I. Liquid phase epoxidation of cyclohexene by tert-butyl hydroperoxide on a Mo-based catalyst. J. Less Common Met. 1974, 36, 319–328. [Google Scholar] [CrossRef]
- Kotov, S.V.; Balbolov, E. Comparative evaluation of the activity of some homogeneous and polymeric catalysts for the epoxidation of alkenes by organic hydroperoxides. J. Mol. Catal. A Chem. 2001, 176, 41–48. [Google Scholar] [CrossRef]
- Bai, S.; Li, B.; Peng, J.; Zhang, X.; Yang, Q.; Li, C. Promoted activity of Cr(Salen) in a nanoreactor for kinetic resolution of terminal epoxides. Chem. Sci. 2012, 3, 2864–2867. [Google Scholar] [CrossRef] [Green Version]
- Kuck, J.W.; Reich, R.M.; Kuhn, F.E. Molecular epoxidation reactions catalyzed by Rhenium, Molybdenum, and Iron complexes. Chem. Rec. 2016, 16, 349–364. [Google Scholar] [CrossRef]
- Judmaier, M.E.; Holzer, C.; Volpe, M.; Mosch-Zanetti, N.C. Molybdenum(VI) dioxo complexes employing Schiff base ligands with an intramolecular donor for highly selective olefin epoxidation. Inorg. Chem. 2012, 51, 9956–9966. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, M.; Ataie, S.; Mahmoudi, H.; Janczak, J. Synthesis, structure characterization and study of a new molybdenum Schiff base complex as an epoxidation catalyst with very high turnover numbers. Inorg. Chem. Commun. 2017, 84, 63–67. [Google Scholar] [CrossRef]
- Antunes, M.M.; Amarante, T.R.; Valente, A.A.; Almeida Paz, F.A.; Gonçalves, I.S.; Pillinger, M. A linear trinuclear oxidodiperoxido-molybdenum(VI) complex with single triazole bridges: Catalytic activity in epoxidation, alcoholysis, and acetalization reactions. ChemCatChem 2018, 10, 2782–2791. [Google Scholar] [CrossRef]
- Reich, R.M.; Kaposi, M.; Pöthig, A.; Kühn, F.E. Kinetic studies of fluorinated aryl molybdenum(ii) tricarbonyl precursors in epoxidation catalysis. Catal. Sci. Technol. 2016, 6, 4970–4977. [Google Scholar] [CrossRef]
- Zare, M.; Moradi-Shoeili, Z.; Ashouri, F.; Bagherzadeh, M. Heterogeneous SBA-15-supported oxoperoxomolybdenum(VI) complex for enhanced olefin epoxidation. Catal. Commun. 2017, 88, 9–12. [Google Scholar] [CrossRef]
- Golmohamadpour, A.; Bahramian, B.; Shafiee, A.; Ma’mani, L. Molybdenum complex supported on amine-functionalized natural sepiolite-type clay mineral as a recyclable inorganic-organic hybrid catalyst for epoxidation of alkenes. Mater. Chem. Phys. 2018, 218, 326–335. [Google Scholar] [CrossRef]
- Yu, F. A highly efficient heterogeneous catalyst of cobalt-based coordination polymers for aerobic epoxidation of cyclohexene. CrystEngComm 2018, 20, 5074–5078. [Google Scholar] [CrossRef]
- Liang, J.; Chen, R.P.; Wang, X.Y.; Liu, T.T.; Wang, X.S.; Huang, Y.B.; Cao, R. Postsynthetic ionization of an imidazole-containing metal-organic framework for the cycloaddition of carbon dioxide and epoxides. Chem. Sci. 2017, 8, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Simaioforidou, A.; Papastergiou, M.; Margellou, A.; Petrakis, D.; Louloudi, M. Activated vs. pyrolytic carbon as support matrix for chemical functionalization: Efficient heterogeneous non-heme Mn(II) catalysts for alkene oxidation with H2O2. J. Mol. Catal. A Chem. 2017, 426, 516–525. [Google Scholar] [CrossRef]
- Weerakkody, C.; Biswas, S.; Song, W.; He, J.; Wasalathanthri, N.; Dissanayake, S.; Kriz, D.A.; Dutta, B.; Suib, S.L. Controllable synthesis of mesoporous cobalt oxide for peroxide free catalytic epoxidation of alkenes under aerobic conditions. Appl. Catal. B Environ. 2018, 221, 681–690. [Google Scholar] [CrossRef]
- Ayvalı, T.; Ye, L.; Wu, S.; Lo, B.T.W.; Huang, C.; Yu, B.; Cibin, G.; Kirkland, A.I.; Tang, C.; Bagabas, A.A.; et al. Mononuclear gold species anchored on TS-1 framework as catalyst precursor for selective epoxidation of propylene. J. Catal. 2018, 367, 229–233. [Google Scholar] [CrossRef]
- Kafshgari, L.A.; Ghorbani, M.; Azizi, A. Synthesis and characterization of manganese ferrite nanostructure by co-precipitation, sol-gel, and hydrothermal methods. Part. Sci. Technol. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Modarres, M. Heterogenized peroxopolyoxotungstate catalyst on the surface of clicked magnetite-graphene oxide nanocomposite: Magnetically recoverable epoxidation catalyst. Appl. Organomet. Chem. 2018, 32, e4142. [Google Scholar] [CrossRef]
- Song, Y.F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Chen, W.; Miras, H.N.; Song, Y.-F. Modular polyoxometalate-layered double hydroxides as efficient heterogeneous sulfoxidation and epoxidation catalysts. ChemCatChem 2018, 10, 188–197. [Google Scholar] [CrossRef]
- Boudjema, S.; Zerrouki, M.; Choukchou-Braham, A. Experimental design for modeling and multi-response optimization of catalytic cyclohexene epoxidation over polyoxometalates. J. Chin. Chem. Soc. 2018, 65, 435–444. [Google Scholar] [CrossRef]
- Rezaeifard, A.; Jafarpour, M.; Haddad, R.; Feizpour, F. {Mo72Cr30} nanocluster as a novel self-separating catalyst for hydrogen peroxide olefin epoxidation. Catal. Commun. 2017, 95, 88–91. [Google Scholar] [CrossRef]
- Ghorbanloo, M.; Bikas, R.; Małecki, G. New molybdenum(VI) complexes with thiazole-hydrazone ligand: Preparation, structural characterization, and catalytic applications in olefin epoxidation. Inorg. Chim. Acta 2016, 445, 8–16. [Google Scholar] [CrossRef]
- Gao, H.; Yan, Y.; Xu, X.; Yu, J.; Niu, H.; Gao, W.; Zhang, W.; Jia, M. Catalytic epoxidation of olefin over supramolecular compounds of molybdenum oxide clusters and a copper complex. Chin. J. Catal. 2015, 36, 1811–1817. [Google Scholar] [CrossRef]
- Taghiyar, H.; Yadollahi, B. New perspective to catalytic epoxidation of olefins by Keplerate containing Keggin polyoxometalates. Polyhedron 2018, 156, 98–104. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.K.; Devi, M.; Gonsalves, K.E.; Pradeep, C.P. Engineering multifunctionality in hybrid polyoxometalates: Aromatic sulfonium octamolybdates as excellent photochromic materials and self-separating catalysts for epoxidation. Inorg. Chem. 2017, 56, 10325–10336. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Zhang, X.; Cui, X.-B.; Xu, J.-Q. A series of compounds based on [P2W18O62]6– and transition metal mixed organic ligand complexes with high catalytic properties for styrene epoxidation. Inorg. Chem. 2018, 57, 11123–11134. [Google Scholar] [CrossRef]
- An, X.; Wu, S.; Tang, Q.; Lan, H.; Tang, Y.; Liu, H.; Qu, J. Strongly coupled polyoxometalates/oxygen doped g-C3N4 nanocomposites as Fenton-like catalysts for efficient photodegradation of sulfosalicylic acid. Catal. Commun. 2018, 112, 63–67. [Google Scholar] [CrossRef]
- Jameel, U.; Zhu, M.; Chen, X.; Tong, Z. Polyoxometalate-nano gold hybrid mesostructured catalyst for green cyclohexene epoxidation. Curr. Org. Chem. 2017, 21, 2585–2596. [Google Scholar] [CrossRef]
- Moghadam, M.; Mirkhani, V.; Tangestaninejad, S.; Mohammadpoor-Baltork, I.; Javadi, M.M. Molybdenum Schiff base-polyoxometalate hybrid compound: A heterogeneous catalyst for alkene epoxidation with tert-BuOOH. Polyhedron 2010, 29, 648–654. [Google Scholar] [CrossRef]
- Stubbs, A.W.; Braglia, L.; Borfecchia, E.; Meyer, R.J.; Román-Leshkov, Y.; Lamberti, C.; Dincă, M. Selective catalytic olefin epoxidation with MnII-exchanged MOF-5. ACS Catal. 2017, 8, 596–601. [Google Scholar] [CrossRef]
- Wu, C.D.; Zhao, M. Incorporation of molecular catalysts in metal-organic frameworks for highly efficient heterogeneous catalysis. Adv. Mater. 2017, 29, 1605446. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L.; Safarifard, V.; Doustkhah, E.; Rostamnia, S.; Morsali, A.; Nouruzi, N.; Beheshti, S.; Akhbari, K. Taking organic reactions over metal-organic frameworks as heterogeneous catalysis. Microporous Mesoporous Mater. 2018, 256, 111–127. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Tang, H.; Ramella, D.; Luan, Y. Modification of Cu2+ into Zr-based metal-organic framework (MOF) with carboxylic units as an efficient heterogeneous catalyst for aerobic epoxidation of olefins. Mol. Catal. 2018, 456, 57–64. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Liu, J.; Ouyang, L.; Hu, R.; Wang, H.; Yang, L.; Zhu, M. Na-Ion batteries: A general metal-organic framework (MOF)-derived selenidation strategy for in situ carbon-encapsulated metal selenides as high-rate anodes for Na-Ion batteries. Adv. Funct. Mater. 2018, 28, 1707573. [Google Scholar] [CrossRef]
- Du, D.Y.; Qin, J.S.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Recent advances in porous polyoxometalate-based metal-organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Gayen, S.; Koner, S. Cu(II)/Cu(II)-Mg(II) containing pyridine-2,5-dicarboxylate frameworks: Synthesis, structural diversity, inter-conversion and heterogeneous catalytic epoxidation. Polyhedron 2018, 146, 93–98. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, J.; Xu, Q.; Wu, X.-T.; Zhu, Q.-L. Pore surface engineering of metal-organic frameworks for heterogeneous catalysis. Coord. Chem. Rev. 2018, 376, 248–276. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Abbasi, A.; Masteri-Farahani, M. Post-synthetic modification of nanoporous Cu3(BTC)2 metal-organic framework via immobilization of a molybdenum complex for selective epoxidation. J. Mol. Catal. A Chem. 2015, 399, 10–17. [Google Scholar] [CrossRef]
- Noh, H.; Cui, Y.; Peters, A.W.; Pahls, D.R.; Ortuno, M.A.; Vermeulen, N.A.; Cramer, C.J.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. An exceptionally stable metal-organic framework supported molybdenum(VI) oxide catalyst for cyclohexene epoxidation. J. Am. Chem. Soc. 2016, 138, 14720–14726. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-L.; Liu, J.; Liu, Y.-Y.; Leus, K.; Depauw, H.; Wang, A.-J.; Van Der Voort, P.; Zhang, J.; Hu, Y.-K. Synthesis, characterization and catalytic performance of Mo based metal- organic frameworks in the epoxidation of propylene by cumene hydroperoxide. Chin. Chem. Lett. 2017, 28, 1057–1061. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal-organic frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Balkus, K.J. Synthesis and modification of titanium containing wrinkled mesoporous silica for cyclohexene epoxidation. Microporous Mesoporous Mater. 2017, 243, 76–84. [Google Scholar] [CrossRef]
- Yang, F.; Wang, B.; Zhou, S.; Long, S.; Liu, X.; Kong, Y. Micropore-enriched CuO-based silica catalyst directly prepared by anionic template-induced method and its boosting catalytic activity in olefins epoxidation. Microporous Mesoporous Mater. 2017, 246, 215–224. [Google Scholar] [CrossRef]
- Debecker, D.P.; Stoyanova, M.; Rodemerck, U.; Gaigneaux, E.M. Preparation of MoO3/SiO2-Al2O3 metathesis catalysts via wet impregnation with different Mo precursors. J. Mol. Catal. A Chem. 2011, 340, 65–76. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Wang, Y.; Bian, G.; Wai, P.T.; Dong, Y. MoO3@SiO2 nanoreactors: Synthesis with a thermal decomposition strategy and catalysis on alkenes epoxidation. J. Solid State Chem. 2018, 264, 156–164. [Google Scholar] [CrossRef]
- Jia, M.; Seifert, A.; Thiel, W.R. Mesoporous MCM-41 materials modified with oxodiperoxo molybdenum complexes: Efficient catalysts for the epoxidation of cyclooctene. Chem. Mater. 2003, 15, 2174–2180. [Google Scholar] [CrossRef]
- Luts, T.; Frank, R.; Suprun, W.; Fritzsche, S.; Hey-Hawkins, E.; Papp, H. Epoxidation of olefins catalyzed by novel Mn(III) and Mo(IV)-Salen complexes immobilized on mesoporous silica gel. J. Mol. Catal. A Chem. 2007, 273, 250–258. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, P.; Shen, Y.; Zhang, W.; Li, X. Molybdenum(VI) complex with a tridentate Schiff base ligand immobilized on SBA-15 as effective catalysts in epoxidation of alkenes. Microporous Mesoporous Mater. 2015, 206, 161–169. [Google Scholar] [CrossRef]
- Baskaran, T.; Kumaravel, R.; Christopher, J.; Ajithkumar, T.G.; Sakthivel, A. An environmentally friendly route for grafting of molybdenum carbonyl onto a diaminosilane-modified SBA-15 molecular sieve and its catalytic behaviour in olefin epoxidation. New J. Chem. 2015, 39, 3758–3764. [Google Scholar] [CrossRef]
- Choudhary, V.; Jha, R.; Jana, P. Selective epoxidation of styrene to styrene oxide by TBHP using simple transition metal oxides (NiO, CoO or MoO3) as highly active environmentally-friendly catalyst. Catal. Commun. 2008, 10, 205–207. [Google Scholar] [CrossRef]
- Fernandes, C.I.; Capelli, S.C.; Vaz, P.D.; Nunes, C.D. Highly selective and recyclable MoO3 nanoparticles in epoxidation catalysis. Appl. Catal. A Gen. 2015, 504, 344–350. [Google Scholar] [CrossRef]
- da Palma Carreiro, E.; Burke, A.J. Catalytic epoxidation of olefins using MoO3 and TBHP: Mechanistic considerations and the effect of amine additives on the reaction. J. Mol. Catal. A Chem. 2006, 249, 123–128. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Arsuaga, J.M.; Sainz-Pardo, J.; de Frutos, P.; Blazquez, S. Synthesis, characterization and catalytic activity of highly dispersed Mo-SBA-15. Appl. Catal. A Gen. 2007, 331, 84–94. [Google Scholar] [CrossRef]
- Briot, E.; Piquemal, J.-Y.; Brégeault, J.-M. Synthesis and characterization of highly dispersed molybdenum species in SBA-15 mesoporous molecular sieves. New J. Chem. 2002, 26, 1443–1447. [Google Scholar] [CrossRef]
- Célestin Bakala, P.; Briot, E.; Salles, L.; Brégeault, J.-M. Comparison of liquid-phase olefin epoxidation over MoOx inserted within mesoporous silica (MCM-41, SBA-15) and grafted onto silica. Appl. Catal. A Gen. 2006, 300, 91–99. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Wang, L.; Bian, G.; Wai, P.T.; Zhang, P.; Dong, Y. A general and simple method of preparing molybdenum-incorporated silica nanoparticles as potential catalysts for epoxidation of alkenes. ChemistrySelect 2018, 3, 9084–9090. [Google Scholar] [CrossRef]
- Miao, Y.; Lu, G.; Liu, X.; Guo, Y.; Wang, Y.; Guo, Y. The molybdenum species of MoO3/SiO2 and their catalytic activities for the epoxidation of propylene with cumene hydroperoxide. J. Ind. Eng. Chem. 2010, 16, 45–50. [Google Scholar] [CrossRef]
- Chandra, P.; Doke, D.S.; Umbarkar, S.B.; Biradar, A.V. One-pot synthesis of ultrasmall MoO3 nanoparticles supported on SiO2, TiO2, and ZrO2 nanospheres: An efficient epoxidation catalyst. J. Mater. Chem. A 2014, 2, 19060–19066. [Google Scholar] [CrossRef]
- Ramanathan, A.; Wu, J.-F.; Maheswari, R.; Hu, Y.; Subramaniam, B. Synthesis of molybdenum-incorporated mesoporous silicates by evaporation-induced self-assembly: Insights into surface oxide species and corresponding olefin metathesis activity. Microporous Mesoporous Mater. 2017, 245, 118–125. [Google Scholar] [CrossRef]
- Smeets, V.; Ben Mustapha, L.; Schnee, J.; Gaigneaux, E.M.; Debecker, D.P. Mesoporous SiO2-TiO2 epoxidation catalysts: Tuning surface polarity to improve performance in the presence of water. Mol. Catal. 2018, 452, 123–128. [Google Scholar] [CrossRef]
- Fan, W.; Shi, D.; Feng, B. Immobilizing of oxo-molybdenum complex on cross-linked copolymer and its catalytic activity for epoxidation reactions. Catal. Commun. 2016, 74, 1–4. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, H.; Zhong, H.; Yan, N.; Chen, Q. Preparation of gamma-Fe2O3@C@MoO3 core/shell nanocomposites as magnetically recyclable catalysts for efficient and selective epoxidation of olefins. Dalton Trans. 2014, 43, 6041–6049. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Moradi-Shoeili, Z.; Bagherzadeh, M.; Akbayrak, S.; Özkar, S. Immobilization of a molybdenum complex on the surface of magnetic nanoparticles for the catalytic epoxidation of olefins. New J. Chem. 2016, 40, 1580–1586. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, L.; He, Q.; Xiao, H.; Zhou, X.; Ji, H. Self-assembled metalloporphyrins-inorganic hybrid flowers and their application to efficient epoxidation of olefins. J. Chem. Technol. Biotechnol. 2017, 92, 2594–2605. [Google Scholar] [CrossRef]
- Shen, Y.R.; Jiang, P.P.; Zhang, J.; Bian, G.; Zhang, P.B.; Dong, Y.M.; Zhang, W.J. Highly dispersed molybdenum incorporated hollow mesoporous silica spheres as an efficient catalyst on epoxidation of olefins. Mol. Catal. 2017, 433, 212–223. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Furuichi, N.; Seki, H.; Yamashita, H. One-pot synthesis of molybdenum oxide nanoparticles encapsulated in hollow silica spheres: An efficient and reusable catalyst for epoxidation of olefins. J. Mater. Chem. A 2017, 5, 18518–18526. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Mirshekar, S. Covalent functionalization of graphene oxide with molybdenum-carboxylate complexes: New reusable catalysts for the epoxidation of olefins. Colloid Surf. A 2018, 538, 387–392. [Google Scholar] [CrossRef]
- Bian, G.; Jiang, P.; Jiang, K.; Shen, Y.; Kong, L.; Hu, L.; Dong, Y.; Zhang, W. MoO2 formed on mesoporous graphene oxide: Efficient and stable catalyst for epoxidation of olefins. Aust. J. Chem. 2017, 70, 1039–1047. [Google Scholar] [CrossRef]
- Martínez, H.; Cáceres, M.F.; Martínez, F.; Páez-Mozo, E.A.; Valange, S.; Castellanos, N.J.; Molina, D.; Barrault, J.; Arzoumanian, H. Photo-epoxidation of cyclohexene, cyclooctene and 1-octene with molecular oxygen catalyzed by dichloro dioxo-(4,4′-dicarboxylato-2,2′-bipyridine) molybdenum (VI) grafted on mesoporous TiO2. J. Mol. Catal. A Chem 2016, 423, 248–255. [Google Scholar] [CrossRef]
- Bian, G.; Jiang, P.; Wang, F.; Shen, Y.; Jiang, K.; Liu, L.; Zhang, W. Light driven epoxidation of olefins using a graphene oxide/g-C3N4 supported Mo (salen) complex. New J. Chem. 2018, 42, 85–90. [Google Scholar] [CrossRef]
- Mirzaee, M.; Bahramian, B.; Gholizadeh, J.; Feizi, A.; Gholami, R. Acetylacetonate complexes of vanadium and molybdenum supported on functionalized boehmite nano-particles for the catalytic epoxidation of alkenes. Chem. Eng. J. 2017, 308, 160–168. [Google Scholar] [CrossRef]
- Mirzaee, M.; Bahramian, B.; Shahraki, M.; Moghadam, H.; Mirzaee, A. Molybdenum containing catalysts grafted on functionalized hydrous zirconia nano-particles for epoxidation of alkenes. Catal. Lett. 2018, 148, 3003–3017. [Google Scholar] [CrossRef]
- Grigoropoulou, G.; Clark, J.H.; Elings, J.A. Recent developments on the epoxidation of alkenes using hydrogen peroxide as an oxidant. Green Chem. 2003, 5, 1–7. [Google Scholar] [CrossRef]
- De Faveri, G.; Ilyashenko, G.; Watkinson, M. Recent advances in catalytic asymmetric epoxidation using the environmentally benign oxidant hydrogen peroxide and its derivatives. Chem. Soc. Rev. 2011, 40, 1722–1760. [Google Scholar] [CrossRef]
- Sawada, Y.; Matsumoto, K.; Kondo, S.; Watanabe, H.; Ozawa, T.; Suzuki, K.; Saito, B.; Katsuki, T. Titanium-salan-catalyzed asymmetric epoxidation with aqueous hydrogen peroxide as the oxidant. Angew. Chem. 2006, 118, 3558–3560. [Google Scholar] [CrossRef]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Enantioselective epoxidation of styrene with TBHP catalyzed by bis(oxazoline)–vanadyl–laponite materials. Catal. Commun. 2018, 117, 90–93. [Google Scholar] [CrossRef]
- Fan, W.; Wu, P.; Tatsumi, T. Unique solvent effect of microporous crystalline titanosilicates in the oxidation of 1-hexene and cyclohexene. J. Catal. 2008, 256, 62–73. [Google Scholar] [CrossRef]
- Lignier, P.; Mangematin, S.; Morfin, F.; Rousset, J.-L.; Caps, V. Solvent and oxidant effects on the Au/TiO2-catalyzed aerobic epoxidation of stilbene. Catal. Today 2008, 138, 50–54. [Google Scholar] [CrossRef]
- Sheldon, R. Metal-catalyzed epoxidation of olefins with organic hydroperoxides II. The effect of solvent and hydroperoxide structure. J. Catal. 1973, 31, 438–443. [Google Scholar] [CrossRef]
- Wang, W.; Agustin, D.; Poli, R. Influence of ligand substitution on molybdenum catalysts with tridentate Schiff base ligands for the organic solvent-free oxidation of limonene using aqueous TBHP as oxidant. Mol. Catal. 2017, 443, 52–59. [Google Scholar] [CrossRef]
- Gunam Resul, M.F.M.; López Fernández, A.M.; Rehman, A.; Harvey, A.P. Development of a selective, solvent-free epoxidation of limonene using hydrogen peroxide and a tungsten-based catalyst. React. Chem. Eng. 2018, 3, 747–756. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, R.; Zhao, G.; Luo, X.; Yin, D. Asymmetric epoxidation of unfunctionalized olefins accelerated by thermoresponsive self-assemblies in aqueous systems. Catal. Sci. Technol. 2016, 6, 488–496. [Google Scholar] [CrossRef]
- Rezaeifard, A.; Haddad, R.; Jafarpour, M.; Hakimi, M. Catalytic epoxidation activity of keplerate polyoxomolybdate nanoball toward aqueous suspension of olefins under mild aerobic conditions. J. Am. Chem. Soc. 2013, 135, 10036–10039. [Google Scholar] [CrossRef] [PubMed]
- Cindrić, M.; Pavlović, G.; Katava, R.; Agustin, D. Towards a global greener process: From solvent-less synthesis of molybdenum(vi) ONO Schiff base complexes to catalyzed olefin epoxidation under organic-solvent-free conditions. New J. Chem. 2017, 41, 594–602. [Google Scholar] [CrossRef]
- Zare, M.; Moradi-Shoeili, Z.; Esmailpour, P.; Akbayrak, S.; Özkar, S. Oxazine containing molybdenum(VI)–oxodiperoxo complex immobilized on SBA-15 as highly active and selective catalyst in the oxidation of alkenes to epoxides under solvent-free conditions. Microporous Mesoporous Mater. 2017, 251, 173–180. [Google Scholar] [CrossRef]
- Mimoun, H.; Seree de Roch, I.; Sajus, L. Epoxydation des olefines par les complexes peroxydiques covalents du molybdene-VI. Tetrahedron 1970, 26, 37–50. [Google Scholar] [CrossRef]
- Thiel, W.R.; Priermeier, T. The first olefin-substituted peroxomolybdenum complex: Insight into a new mechanism for the molybdenum-catalyzed epoxidation of olefins. Angew. Chem. Int. Ed. Engl. 1995, 34, 1737–1738. [Google Scholar] [CrossRef]
- Moreno, J.; Iglesias, J.; Melero, J.A. Mo(VI) complexes immobilized on SBA-15 as an efficient catalyst for 1-octene epoxidation. Catalysts 2017, 7, 215. [Google Scholar] [CrossRef]
- Berkessel, A.; Adrio, J.A. Kinetic studies of olefin epoxidation with hydrogen peroxide in 1,1,1,3,3,3-hexafluoro-2-propanol reveal a crucial catalytic role for solvent clusters. Adv. Synth. Catal. 2004, 346, 275–280. [Google Scholar] [CrossRef]








| Entry | Catalyst | Olefin | Oxidant | Solvent | Temperature (°C) | Time (h) | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cis-[MoO2(LX)2]-1 | Cyclooctene | TBHP | Chloroform | 50 | 1 | 100 | 99 (24 h) | [25] |
| 2 | Cis-[MoO2(LX)2]-2 | Cyclooctene | TBHP | Chloroform | 50 | 1 | 100 | 99 (24 h) | [25] |
| 3 | Cis-[MoO2(LX)2]-3 | Cyclooctene | TBHP | Chloroform | 50 | 1 | 22 | 99 (24 h) | [25] |
| 4 | Cis-[MoO2(LX)2]-4 | Cyclooctene | TBHP | Chloroform | 50 | 1 | 30 | 99 (24 h) | [25] |
| 5 | Cis-[MoO2(LX)2]-5 | Cyclooctene | TBHP | Chloroform | 50 | 1.5 | 100 | 99 (24 h) | [25] |
| 6 | [MoO2L]n | Cyclooctene | TBHP | 1,2-dichloroethane | 80 | 2 | 93 | 100 | [26] |
| 7 | (Htrz)2[Mo3O6(O2)4(trz)2]⋅H2O | Cis-cyclooctene | TBHP | α,α,α-trifluorotoluene | 70 | 6 | 94 | - | [27] |
| 8 | [MoO3(trz)0.5] | Cis-cyclooctene | TBHP | α,α,α-trifluorotoluene | 70 | 6 | 83 | - | [27] |
| 9 | [CpMo(CO)3Bz-m-CF3] | Cis-cyclooctene | TBHP | Benzene | 55 | 24 | 100 | 99 | [28] |
| 10 | [CpMo(CO)3Bz-m-(-CF3)2] | Cis-cyclooctene | TBHP | Benzene | 55 | 24 | 100 | 99 | [28] |
| Entry | Catalyst | Olefin | Oxidant | Solvent | Temperature (°C) | Time (h) | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 20% PVMo/Hmont | Cyclooctene | 60 wt% H2O2 | Acetonitrile | 70 | 9 | 91 | 96 | [40] |
| 2 | {Mo72Cr30} | Cyclooctene | 30 wt% H2O2 | Ethanol | 70 | 0.75 | 97 | ˃99 | [41] |
| 3 | [MoO2(HL)(H2O)]2[Mo6O19]·2MeCN | Cyclooctene | TBHP | 1,2-dichloroethane | 80 | 5 | 100 | 100 | [42] |
| 4 | [Cu(bipy)]4[Mo15O47]·2H2O | Cyclooctene | TBHP | Chloroform | 35 | 8 | 96 | ≈100 | [43] |
| 5 | [CuI(bix)][(CuIbix)(δ-MoVI8O26)0.5] | Cyclooctene | TBHP | Chloroform | 35 | 10 | 92 | ≈100 | [43] |
| 6 | (H2bix)[(Hbix)2(γ-Mo8O26)]2·H2O | Cyclooctene | TBHP | Chloroform | 35 | 10 | 39 | ≈100 | [43] |
| 7 | {Mo72Fe30} | Cyclooctene | 30 wt% H2O2 | Ethanol | 75 | 3.25 | 73 | 97 | [44] |
| 8 | PMo12 ⊂ Mo72Fe30 | Cyclooctene | 30 wt% H2O2 | Ethanol | 75 | 3.25 | 82 | 98 | [44] |
| 9 | SiMo12 ⊂ Mo72Fe30 | Cyclooctene | 30 wt% H2O2 | Ethanol | 75 | 3.25 | 91 | 99 | [44] |
| 10 | BW12 ⊂ Mo72Fe30 | Cyclooctene | 30 wt% H2O2 | Ethanol | 75 | 3.25 | 93 | 99 | [44] |
| 11 | 1% Au/PMo11/CPTES-SBA-15 | Cyclohexene | O2 (0.4 MPa) | - | 50 | 24 | 48.1 | 35.9 | [48] |
| 12 | [Mo(O)2(salen)-POM] | Cyclooctene | TBHP | 1,2-dichloroethane | 75 | 6 | 100 | 100 | [49] |
| Entry | Catalyst | Olefin | Oxidant | Solvent | Temperature (°C) | Time (h) | TOF (h−1) | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Peptide immobil. Mo-Salen | Cyclooctene | TBHP | Toluene | 75 | 3 | 24.5 | ≈100 | ≈100 | [67] |
| 2 | Mo-FSAP-CH3-Cl-SBA-15 | Cyclohexene | TBHP | 1,2-Dichloroethane | 80 | 4 | - | 68 | 95 | [68] |
| 3 | SBA-DA-Mo | Cyclooctene | TBHP | Mesitylene | 70 | 4 | - | 93 | 86 | [69] |
| 4 | MoO3 | Styrene | TBHP | - | 82-83 | 3 | 10.7 | 42 | 76.2 | [70] |
| 5 | MoO3 and 0.17 Pyrazole | Styrene | TBHP | Toluene | 100 | 1 | - | 44 | 91 | [72] |
| 6 | Mo-SBA-15 | 1-octene | TBHP | Decane | 80 | 6 | 97.2 | 58.5 | 99.9 | [73] |
| 7 | Mo-MCM-41(68) | Cyclooctene | TBHP | Acetonitrile | 40 | 3 | - | 66 | >99 | [75] |
| 8 | Mo-SBA-15(122) | Cyclooctene | TBHP | Acetonitrile | 40 | 3 | - | 85 | 93 | [75] |
| 9 | Mo-SiO2-bead(160) | Cyclooctene | TBHP | Acetonitrile | 40 | 3 | - | 51 | 50 | [75] |
| 10 | Mo-MSN-50 | Cyclooctene | H2O2 | Acetonitrile | 70 | 4 | - | 79 | >95 | [76] |
| 11 | MoO3/SiO2 | Propylene | CHP | tert-butyl alcohol | 80 (2.6 MPa) | 4 | - | 99 | 85.3 | [77] |
| 12 | MoO3/SiO2 | Cyclooctene | TBHP | 1,2-dichloroethane | 80 | 2 | 72.3 | 90 | 100 | [78] |
| 13 | MoO3/TiO2 | Cyclooctene | TBHP | 1,2-dichloroethane | 80 | 2 | 24.8 | 37 | 100 | [78] |
| 14 | MoO3/ZrO2 | Cyclooctene | TBHP | 1,2-dichloroethane | 80 | 2 | 25.9 | 39.6 | 100 | [78] |
| 15 | NHSG | Cyclooctene | 30 wt% H2O2 | Acetonitrile/H2O | 60 | 2 | - | 13 | 16 | [80] |
| 16 | [email protected] | Cyclooctene | 30 wt% H2O2 | Acetonitrile/H2O | 60 | 2 | - | 13.7 | 37 | [80] |
| Entry | Catalyst | Olefin | Oxidant | Solvent | Temperature (°C) | Time (h) | TOF (h−1) | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mo-AMP-CuBTC | Cyclooctene | TBHP | Chloroform | 60 | 2 | 521 | 99 | ˃99 | [58] |
| 2 | Mo-SIM | Cyclohexene | TBHP | Toluene | 60 | 7 | 420 | 93 | 99 | [59] |
| 3 | DVB-AA-Mo | Cyclooctene | TBHP | Chloroform | 70 | 4 | - | 95 | 100 | [81] |
| 4 | γ-Fe2O3@C@MoO3 | Cyclooctene | TBHP | Tetrachlormethane | 80 | 6 | - | 97.3 | 99.9 | [82] |
| 5 | MoO2(sal-phz)(CH3OH)/Chloropropyltriethoxysilane coated Fe3O4 | Cyclooctene | TBHP | 1,2-dichloroethane | 84 | 1 | 426 | 98 | 99 | [83] |
| 6 | Mo/HMSS-X | Cyclohexene | TBHP | 1,2-dichloroethane | 80 | 4 | 893 | 87 | 99 | [86] |
| 7 | MoOx@HSS-2 | Cyclooctene | TBHP | 1,2-dichloroethane | 80 | 8 | - | 80 | 99 | [87] |
| 8 | GO-Mo | Cyclooctene | TBHP | Chloroform | Reflux | 8 | 100 | 92 | ˃99 | [88] |
| 9 | rGO-Mo | Cyclooctene | TBHP | Chloroform | Reflux | 8 | 230 | 95 | ˃99 | [88] |
| 10 | m-MoO2/GO | Cyclooctene | TBHP | Chloroform | 60 | 6 | 410 | ˃99 | ˃99 | [89] |
| 11 | Mo(VI)Cl2O2Bipy/TiO2 SC-150 | Cyclohexene | O2 (UV-vis) | Acetonitrile | 19 | 56 | - | 65.9 | 83 | [90] |
| 12 | Mo-GO/g-C3N4 | Cyclooctene | O2 (AM 1.5G) | Acetonitrile | Ambient temperature | 6 | 164 | 44 | 98 | [91] |
| 13 | Sep-Am-MoO2 | Cyclooctene | TBHP | 1,2-dichloroethane | 84 | 1.25 | 74.16 | 98 | 100 | [30] |
| 14 | Mo-Im-BNPs | Cyclooctene | TBHP | Tetrachlormethane | Reflux | 1 | 126 | 97 | 97 | [92] |
| 15 | Mo-A-BNPs | Cyclooctene | TBHP | Tetrachlormethane | Reflux | 3.5 | 89 | 97 | 97 | [92] |
| 16 | PMo-AFZNP | Cyclooctene | TBHP | 1,2-dichloroethane | Reflux | 2 | 49 | 98 | 100 | [93] |
| 17 | SiMo-AFZNP | Cyclooctene | TBHP | 1,2-dichloroethane | Reflux | 3 | 35 | 98 | 100 | [93] |
| 18 | Mo-AFZNP | Cyclooctene | TBHP | 1,2-dichloroethane | Reflux | 4.5 | 12 | 90 | 100 | [93] |
| 19 | Mo-IFZNP | Cyclooctene | TBHP | 1,2-dichloroethane | Reflux | 1 | 119 | 91 | 100 | [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Jiang, P.; Wai, P.T.; Gu, Q.; Zhang, W. Recent Progress in Application of Molybdenum-Based Catalysts for Epoxidation of Alkenes. Catalysts 2019, 9, 31. https://doi.org/10.3390/catal9010031
Shen Y, Jiang P, Wai PT, Gu Q, Zhang W. Recent Progress in Application of Molybdenum-Based Catalysts for Epoxidation of Alkenes. Catalysts. 2019; 9(1):31. https://doi.org/10.3390/catal9010031
Chicago/Turabian StyleShen, Yirui, Pingping Jiang, Phyu Thin Wai, Qian Gu, and Weijie Zhang. 2019. "Recent Progress in Application of Molybdenum-Based Catalysts for Epoxidation of Alkenes" Catalysts 9, no. 1: 31. https://doi.org/10.3390/catal9010031