Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO
Abstract
:1. Introduction
2. Results and Discussion
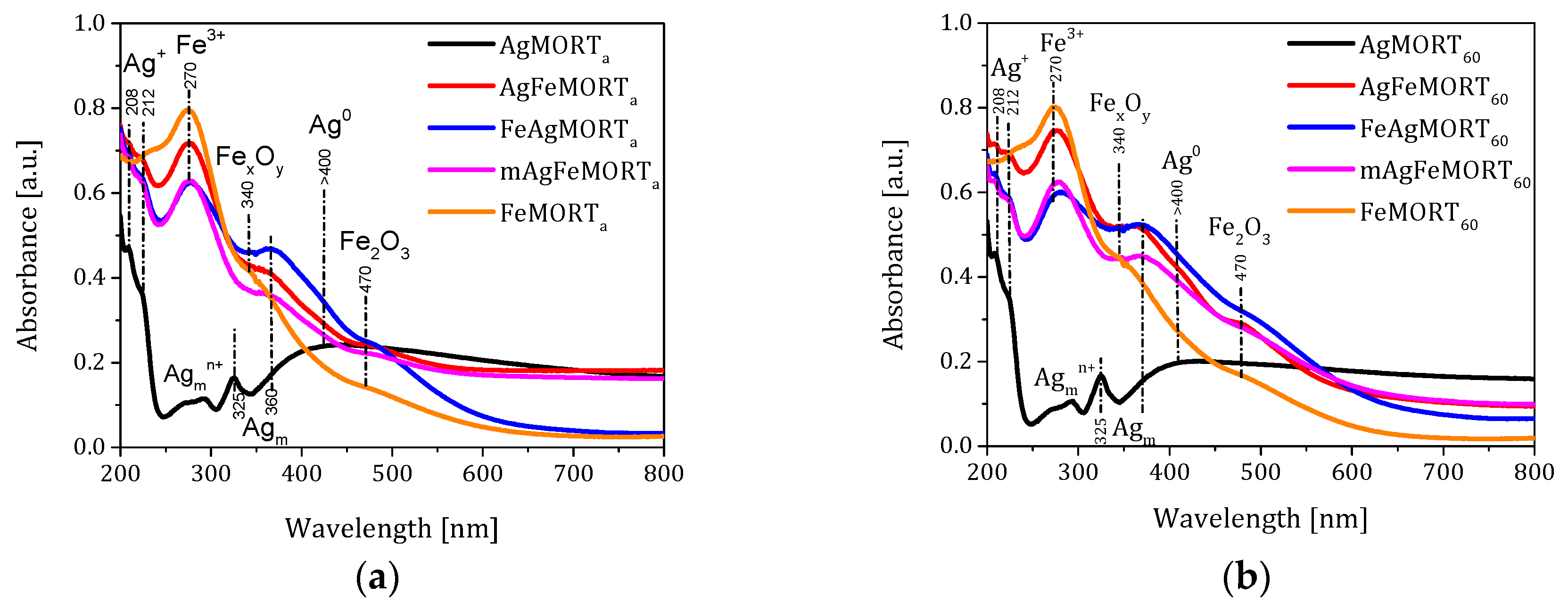
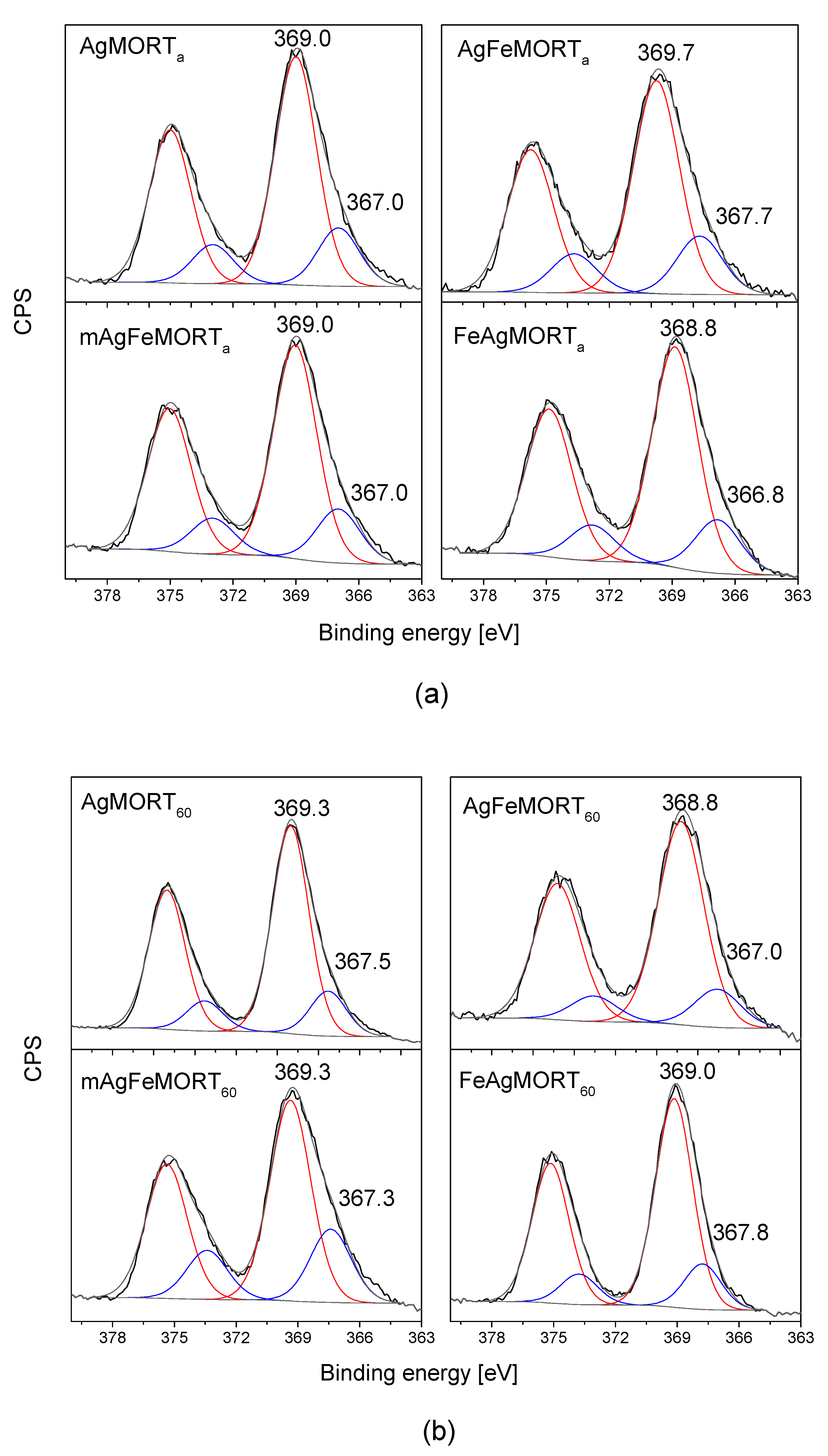
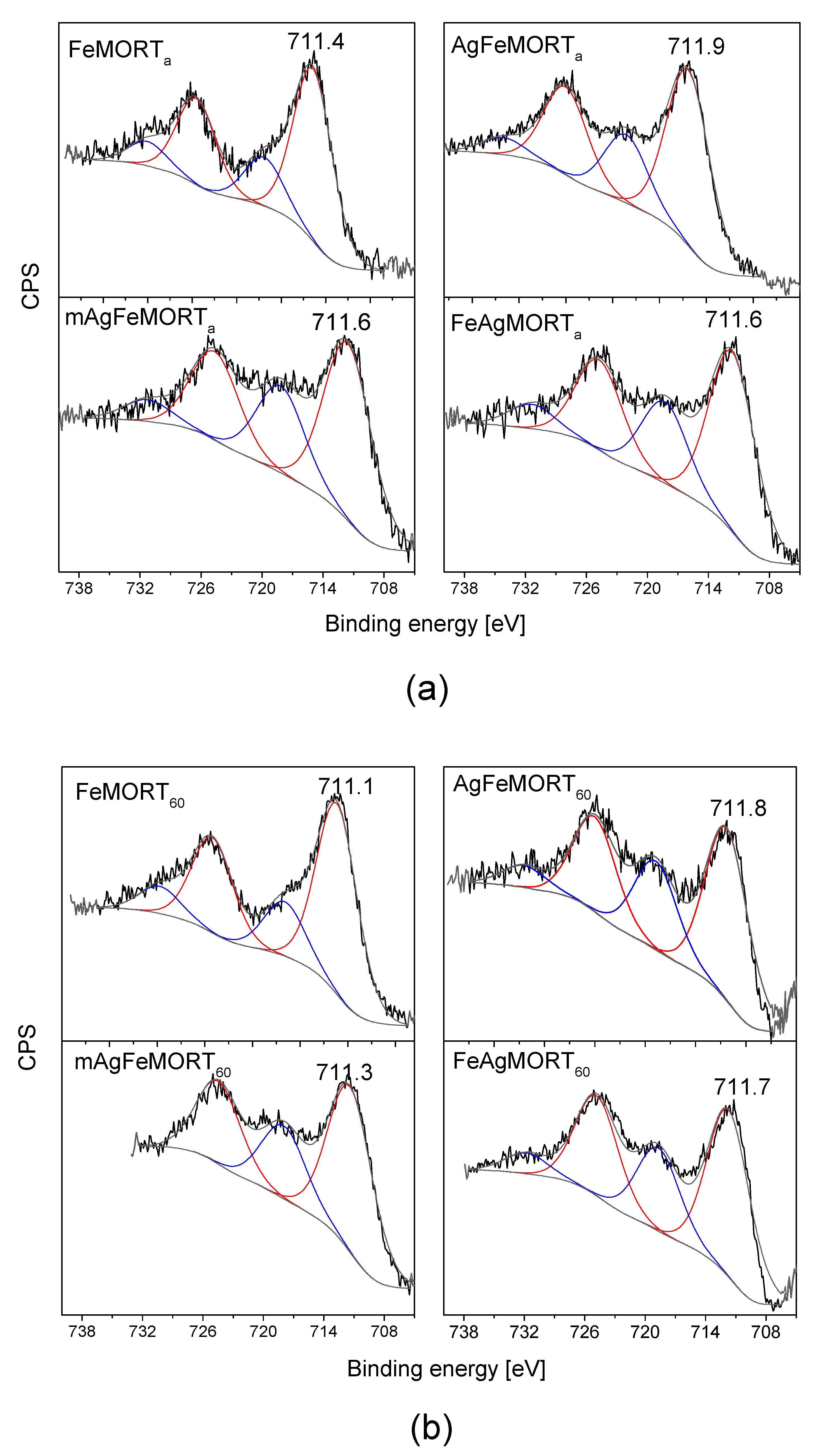
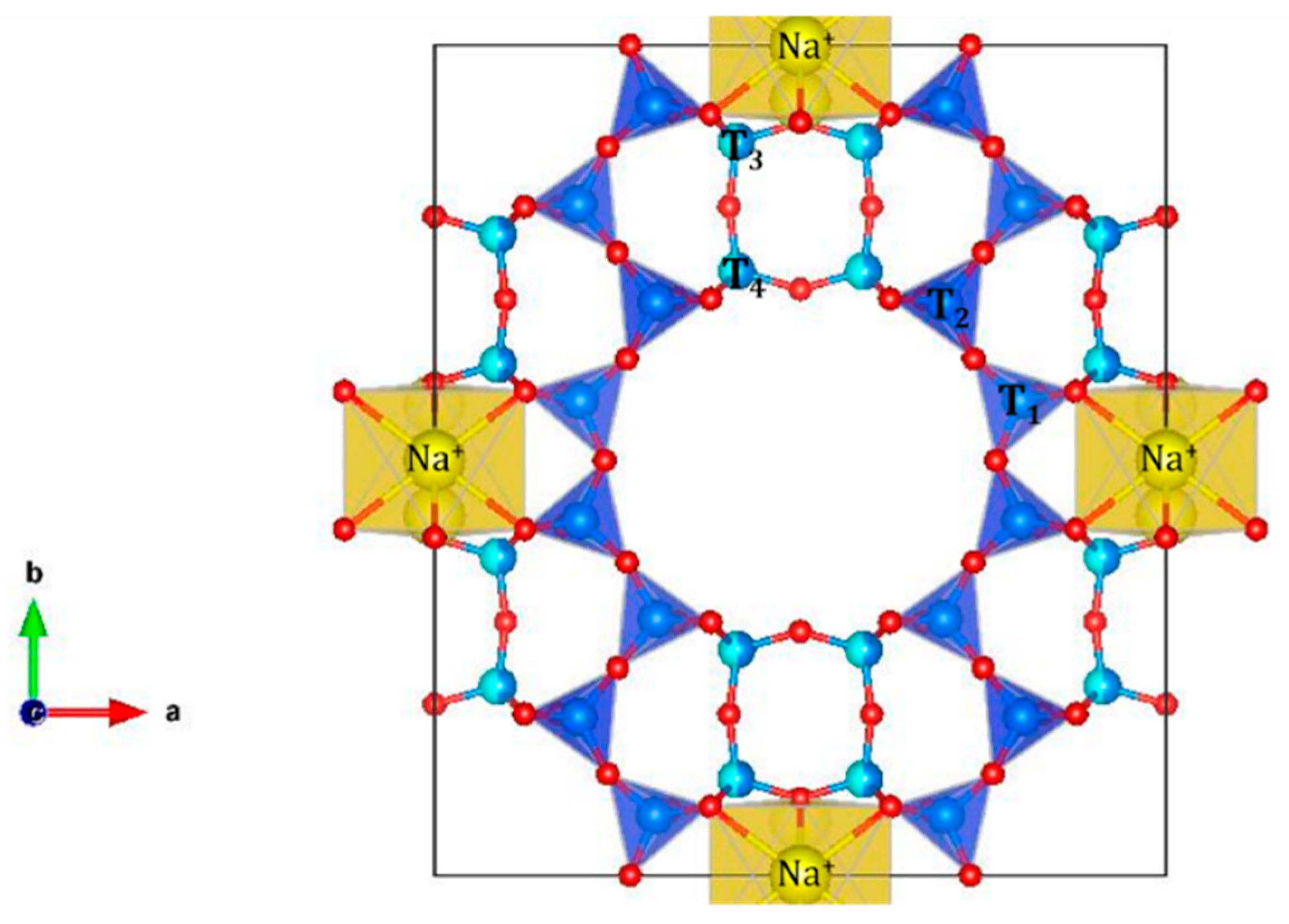
2.1. Physicochemical Properties
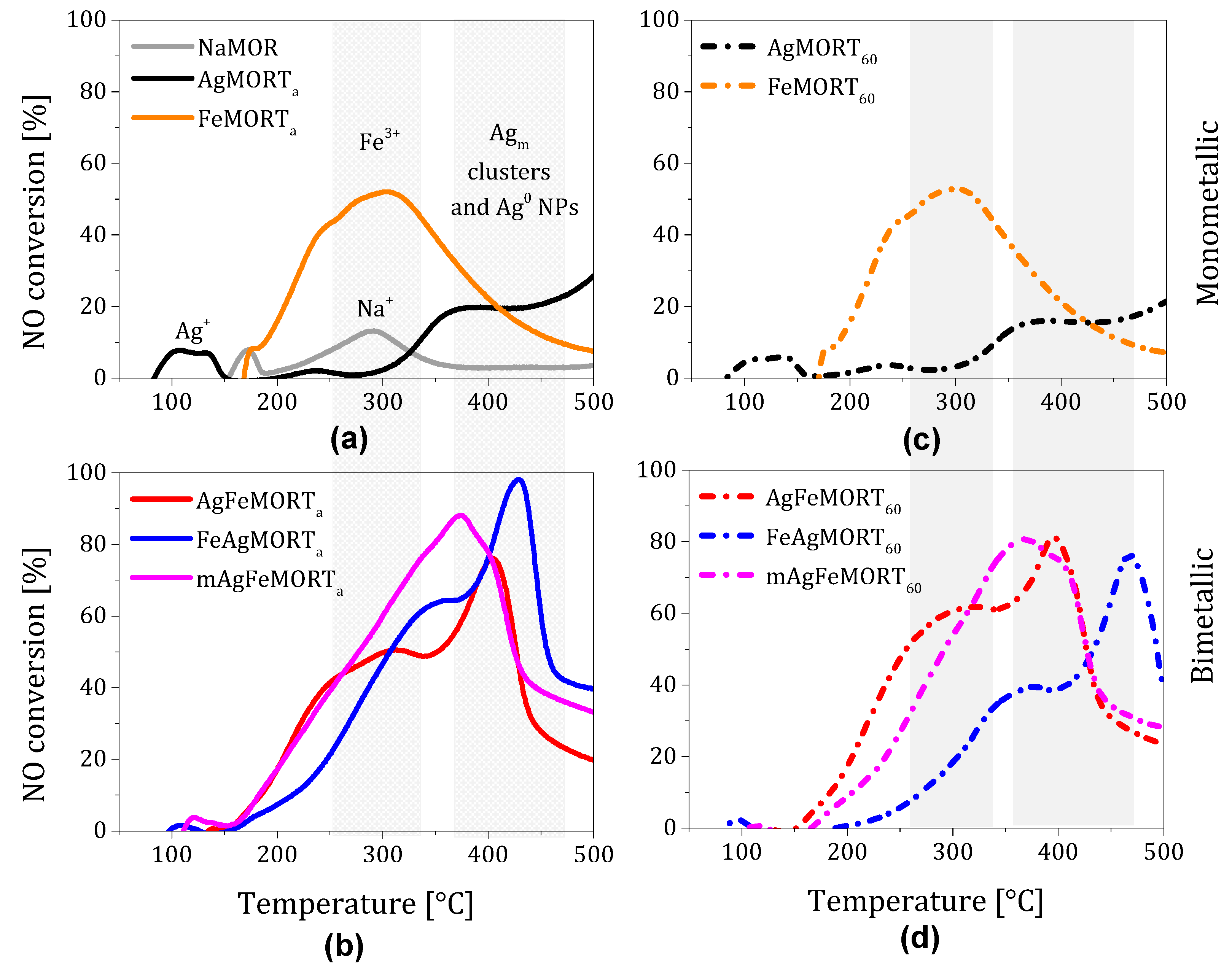
2.2. Catalytic Conversion of NO with C3H6
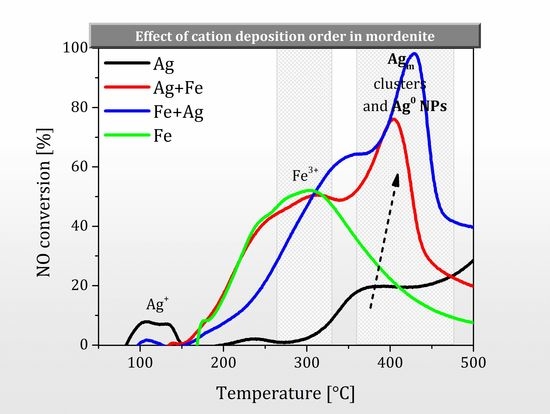
2.3. Effect of Cations Deposition Order on NO Conversion
3. Materials and Methods
3.1. Sample Preparation
3.2. Sample Characterization
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sillman, S. Tropospheric Ozone and Photochemical Smog. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2003; pp. 407–431. [Google Scholar]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Gilot, P.; Guyon, M.; Stanmore, B.R. A review of NOx reduction on zeolitic catalysts under diesel exhaust conditions. Fuel 1997, 76, 507–515. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Grange, P.; Delmon, B. Catalytic removal of NO. Catal. Today 1998, 46, 233–316. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic Materials for DeNOx Selective Catalytic Reduction. ChemCatCham 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NOx selective catalytic reduction by hydrocarbons (HC-SCR). Appl. Catal. A Gen. 2015, 504, 542–548. [Google Scholar] [CrossRef]
- Traa, Y.; Burger, B.; Weitkamp, J. Zeolite-based materials for the selective catalytic reduction of NOx with hydrocarbons. Microporous Mesoporous Mater. 1999, 30, 3–41. [Google Scholar] [CrossRef]
- Worch, D.; Suprun, W.; Gläser, R. Supported transition metal-oxide catalysts for HC-SCR DeNOx with propene. Catal. Today 2011, 176, 309–313. [Google Scholar] [CrossRef]
- Topsøe, N.Y. Catalysis for NOx abatement. Selective catalytic ceduction of NOx by ammonia: Fundamental and industrial aspects. Cattech 1997, 1, 125–134. [Google Scholar]
- Aspromonte, S.G.; Miró, E.E.; Boix, A.V. Effect of Ag-Co interactions in the mordenite on the NOx SCR with butane and toluene. Catal. Commun. 2012, 28, 105–110. [Google Scholar] [CrossRef]
- De Lucas, A.; Valverde, J.L.; Dorado, F.; Romero, A.; Asencio, I. Influence of the ion exchanged metal (Cu, Co, Ni and Mn) on the selective catalytic reduction of NOx over mordenite and ZSM-5. J. Mol. Catal. A Chem. 2005, 225, 47–58. [Google Scholar] [CrossRef]
- Oliviera, M.L.M.; Silva, C.M.; Moreno-Tost, R.; Farias, T.L.; Jiménez-López, A.; Rodríguez-Castellón, E. A study of copper-exchanged mordenite natural and ZSM-5 zeolites as SCR-NOx catalysts for diesel road vehicles: Simulation by neural networks approach. Appl. Catal. B Environ. 2009, 88, 420–429. [Google Scholar] [CrossRef]
- Palomares, A.E.; Franch, C.; Corma, A. Determining the characteristics of a Co-zeolite to be active for the selective catalytic reduction of NOx with hydrocarbons. Catal. Today 2011, 176, 239–241. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Palomares, E. Selective catalytic reduction of NO(x) on Cu-beta zeolites. Appl. Catal. B Environ. 1997, 11, 233–242. [Google Scholar] [CrossRef]
- De La Torre, U.; Pereda-Ayo, B.; Moliner, M.; González-Velasco, J.R.; Corma, A. Cu-zeolite catalysts for NOxremoval by selective catalytic reduction with NH3 and coupled to NO storage/reduction monolith in diesel engine exhaust aftertreatment systems. Appl. Catal. B Environ. 2016, 187, 419–427. [Google Scholar] [CrossRef]
- Sachtler, W.M.H.; Zhang, Z. Zeolite-Supported Transition Metal Catalysts. Adv. Catal. 1993, 39, 129–220. [Google Scholar]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Petranovskii, V.; Fuentes, S.; Paukshtis, E.; Sugi, Y.; Licea-Claverie, A. Role of mordenite acid properties in silver cluster stabilization. Mater. Sci. Eng. A 2000, 276, 236–242. [Google Scholar] [CrossRef]
- Fonseca, A.M.; Neves, I.C. Study of silver species stabilized in different microporous zeolites. Microporous Mesoporous Mater. 2013, 181, 83–87. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Bogdanchikova, N.E. Metal clusters and nanoparticles assembled in zeolites: An example of stable materials with controllable particle size. Mater. Sci. Eng. C 2002, 19, 327–331. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wang, X.; Sachtler, W.M.H. Reduction of NOx over various Fe/zeolite catalysts. Appl. Catal. A Gen. 2000, 194, 159–168. [Google Scholar] [CrossRef]
- Xu, L.; Shi, C.; Chen, B.; Zhao, Q.; Zhu, Y.; Gies, H.; Xiao, F.S.; De Vos, D.; Yokoi, T.; Bao, X.; et al. Improvement of catalytic activity over Cu–Fe modified Al-rich Beta catalyst for the selective catalytic reduction of NOx with NH3. Microporous Mesoporous Mater. 2016, 236, 211–217. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Monteverdi, S.; Ghanbaja, J.; Bettahar, M.M. Study of Ni-Ag/SiO2 catalysts prepared by reduction in aqueous hydrazine. J. Colloid Interface Sci. 2008, 317, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, G.; Li, P.; Wang, J.; Zhang, P. Promotion of formaldehyde oxidation over Ag catalyst by Fe doped MnOx support at room temperature. Catal. Today 2016, 277, 257–265. [Google Scholar] [CrossRef]
- Das, R.; Gupta, M.; Srivastava, S.K. Magnetic instability and f – d hybridization in CeFe2 on substituting Cr, Ag, and Au for Fe. J. Magn. Magn. Mater. 2017, 433, 162–168. [Google Scholar] [CrossRef]
- Ciesielski, K.; Chajewski, G.; Samsel–Czekała, M.; Hackemer, A.; Pikul, A.P.; Kaczorowski, D. Low-temperature electronic properties and band structures of LaTE2Si2 (TE=Fe, Co, Ag and Au). Solid State Commun. 2017, 257, 32–35. [Google Scholar] [CrossRef]
- Grandjean, D.; Coutiño-Gonzalez, E.; Cuong, N.T.; Fron, E.; Baekelant, W.; Aghakhani, S.; Schlexer, P.; Dacapito, F.; Banerjee, D.; Roeffaers, M.B.J.; et al. Origin of the bright photoluminescence of few-atom silver clusters confined in LTA zeolites. Science 2018, 361, 686–690. [Google Scholar] [CrossRef]
- Izadkhah, B.; Nabavi, S.R.; Niaei, A.; Salari, D.; Mahmuodi Badiki, T.; Çaylak, N. Design and optimization of Bi-metallic Ag-ZSM5 catalysts for catalytic oxidation of volatile organic compounds. J. Ind. Eng. Chem. 2012, 18, 2083–2091. [Google Scholar] [CrossRef]
- Li, Z.; Flytzani-Stephanopoulos, M. On the Promotion of Ag–ZSM-5 by Cerium for the SCR of NO by Methane. J. Catal. 1999, 182, 313–327. [Google Scholar] [CrossRef]
- Ramírez-Garza, R.E.; Rodríguez-Iznaga, I.; Simakov, A.; Farías, M.H.; Castillón-Barraza, F.F. Cu-Ag/mordenite catalysts for NO reduction: Effect of silver on catalytic activity and hydrothermal stability. Mater. Res. Bull. 2018, 97, 369–378. [Google Scholar] [CrossRef]
- Jouini, H.; Mejri, I.; Petitto, C.; Martinez-Ortigosa, J.; Vidal-Moya, A.; Mhamdi, M.; Blasco, T.; Delahay, G. Characterization and NH3-SCR reactivity of Cu-Fe-ZSM-5 catalysts prepared by solid state ion exchange: The metal exchange order effect. Microporous Mesoporous Mater. 2018, 260, 217–226. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Pankina, G.V.; Kkazantsev, R.V.; Eliseev, O.L. Potassium as a Structural Promoter for an Iron/Activated Carbon Catalyst: Unusual Effect of Component Deposition Order on Magnetite Particle Size and Catalytic Behavior in Fischer-Tropsch Synthesis. ChemCatChem 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Tunyogi, A.; Tanczikó, F.; Osváth, Z.; Pászti, F. Structural characterization of Fe/Ag bilayers by RBS and AFM. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2008, 266, 4916–4920. [Google Scholar] [CrossRef]
- Cook, K.M.; Perez, H.D.; Bartholomew, C.H.; Hecker, W.C. Effect of promoter deposition order on platinum-, ruthenium-, or rhenium-promoted cobalt Fischer-Tropsch catalysts. Appl. Catal. A Gen. 2014, 482, 275–286. [Google Scholar] [CrossRef]
- Park, J.B.; Conner, S.F.; Chen, D.A. Bimetallic Pt-Au clusters on TiO2(110): Growth, surface composition, and metal-support interactions. J. Phys. Chem. C 2008, 112, 5490–5500. [Google Scholar] [CrossRef]
- Bartolomeu, R.; Mendes, N.; Fernandes, A. NOx SCR with decane using Ag–MFI catalysts: On the effect of silver content and co-cation presence. Catal. Sci. Technol. 2016, 6, 3038. [Google Scholar] [CrossRef]
- Yahiro, H.; Iwamoto, M. Copper ion-exchanged zeolite catalysts in deNOx reaction. Appl. Catal. A Gen. 2001, 222, 163–181. [Google Scholar] [CrossRef]
- Zhukov, Y.M.; Efimov, A.Yu.; Shelyapina, M.G.; Petranovskii, V.; Zhizhin, E.V.; Burovikhina, A.; Zvereva, I.A. Effect of preparation method on the valence state and encirclement of copper exchange ions in mordenites. Microporous Mesoporous Mater. 2016, 224, 415–419. [Google Scholar] [CrossRef]
- Petranovskii, V.; Gurin, V.; Bogdanchikova, N.; Sugi, Y. Controlling copper reducibility in mordenites by varying the SiO2/Al2O3 molar ratio. Mater. Lett. 2003, 57, 1781–1785. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Tabata, T. Influence of Si/Al ratio on the activity and durability of Pd-ZSM-5 catalysts for nitrogen oxide reduction by methane. Appl. Catal. B Environ. 2000, 26, 275–284. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Schwieger, W.; Unger, A. Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion-exchange method. Appl. Catal. B Environ. 2008, 84, 277–288. [Google Scholar] [CrossRef]
- Panahi, P.N.; Salari, D.; Niaei, A.; Mousavi, S.M. NO reduction over nanostructure M-Cu/ZSM-5 (M: Cr, Mn, Co and Fe) bimetallic catalysts and optimization of catalyst preparation by RSM. J. Ind. Eng. Chem. 2013, 19, 1793–1799. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Li, Z.; Flytzani-Stephanopoulos, M. Selective catalytic reduction of nitric oxide by methane over cerium and silver ion-exchanged ZSM-5 zeolites. Appl. Catal. A Gen. 1997, 165, 15–34. [Google Scholar] [CrossRef]
- Efimov, A.Yu.; Petranovskii, V.; Fedotov, M.A.; Khtipun, M.K. On the role of triethanolamine in the charnell synthesis of zeolites. J. Struct. Chem. 1993, 34, 548–551. [Google Scholar] [CrossRef]
- Rao, P.R.; Matsukata, M. Dry-gel conversion technique for synthesis of zeolite BEA. Chem. Commun. 1996, 2, 1441–1442. [Google Scholar] [CrossRef]
- Luo, W.; Yang, X.; Wang, Z.; Huang, W.; Chen, J.; Jiang, W.; Wang, L.; Cheng, X.; Deng, Y.; Zhao, D. Synthesis of ZSM-5 aggregates made of zeolite nanocrystals through a simple solvent-free method. Microporous Mesoporous Mater. 2017, 243, 112–118. [Google Scholar] [CrossRef]
- Inagaki, S.; Nakatsuyama, K.; Saka, Y.; Kikuchi, E.; Kohara, S.; Matsuaka, M. Elucidation of medium-range structure in a dry gel-forming *BEA-type zeolite. J. Phys. Chem. C 2007, 111, 10285–10293. [Google Scholar] [CrossRef]
- Borón, P.; Chmielarz, L.; Gurgul, J.; Latka, K.; Gil, B.; Marszalek, B.; Dzwigaj, S. Influence of iron state and acidity of zeolites on the catalytic activity of FeHBEA, FeHZSM-5 and FeHMOR in SCR of NO with NH3 and N2O decomposition. Microporous Mesoporous Mater. 2015, 203, 73–85. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Rodríguez-Fuentes, G.; Petranovskii, V. Ammonium modified natural clinoptilolite to remove manganese, cobalt and nickel ions from wastewater: Favorable conditions to the modification and selectivity to the cations. Microporous Mesoporous Mater. 2018, 255, 200–210. [Google Scholar] [CrossRef]
- Schuricht, F.; Reschetilowski, W. Simultaneous selective catalytic reduction (SCR) of NOx and N2O over Ag/ZSM-5—Catalytic studies and mechanistic implications. Microporous Mesoporous Mater. 2012, 164, 135–144. [Google Scholar] [CrossRef]
- Aspromonte, S.G.; Serra, R.M.; Miró, E.E.; Boix, A.V. AgNaMordenite catalysts for hydrocarbon adsorption and deNOx processes. Appl. Catal. A Gen. 2011, 407, 134–144. [Google Scholar] [CrossRef]
- Concepción-Rosabal, B.; Rodríguez-Fuentes, G.; Bogdanchikova, N.; Bosch, P.; Avalos, M.; Lara, V.H. Comparative study of natural and synthetic clinoptilolites containing silver in different states. Microporous Mesoporous Mater. 2005, 86, 249–255. [Google Scholar] [CrossRef]
- Sayah, E.; Brouri, D.; Massiani, P. A comparative in situ TEM and UV-visible spectroscopic study of the thermal evolution of Ag species dispersed on Al2O3 and NaX zeolite supports. Catal. Today 2013, 218, 10–17. [Google Scholar] [CrossRef]
- Gurin, V.S.; Bogdanchikova, N.E.; Petranovskii, V.P. Self-assembling of silver and copper small clusters within the zeolite cavities: Prediction of geometry. Mater. Sci. Eng. C 2001, 18, 37–44. [Google Scholar] [CrossRef]
- Chávez-Rivas, F.; Rodriguez-Fuentes, G.; Berlier, G.; Rodríguez-Iznaga, I.; Petranovskii, V.; Zamorano-Ulloa, R.; Coluccia, S. Evidence for controlled insertion of Fe ions in the framework of clinoptilolite natural zeolites. Microporous Mesoporous Mater. 2013, 167, 76–81. [Google Scholar] [CrossRef]
- Kumar, M.S.; Schwidder, M.; Grünert, W.; Bentrup, U.; Brückner, A. Selective reduction of NO with Fe-ZSM-5 catalysts of low Fe content: Part II. Assessing the function of different Fe sites by spectroscopic in situ studies. J. Catal. 2006, 239, 176–186. [Google Scholar]
- Gurgul, J.; Ła̧tka, K.; Hnat, I.; Rynkowski, J.; Dzwigaj, S. Identification of iron species in FeSiBEA by DR UV-vis, XPS and Mössbauer spectroscopy: Influence of Fe content. Microporous Mesoporous Mater. 2013, 168, 1–6. [Google Scholar] [CrossRef]
- Wu, P.; Komatsu, T.; Yashima, T. Isomorphous substitution of Fe3+ in the framework of aluminosilicate mordenite by hydrothermal synthesis. Microporous Mesoporous Mater. 1998, 20, 139–147. [Google Scholar] [CrossRef]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and Reactivity of Framework and Extraframework Iron in Fe-Silicalite as Investigated by Spectroscopic and Physicochemical Methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Lopez-Salido, I.; Lim, D.C.; Kim, Y.D. Ag nanoparticles on highly ordered pyrolytic graphite (HOPG) surfaces studied using STM and XPS. Surf. Sci. 2005, 588, 6–18. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yahiro, H. Novel catalytic decomposition and reduction of NO. Catal. Today 1994, 22, 5–18. [Google Scholar] [CrossRef]
- Vilhena, F.d.S.; Serra, R.M.; Boix, A.V.; Ferrerira, G.B.; José, J.W. DFT study of Li+ and Na+ positions in mordenites and hydration stability. Comput. Theor. Chem. 2016, 1091, 115–121. [Google Scholar] [CrossRef]
- More, P.M. Effect of active component addition and support modification on catalytic activity of Ag/Al2O3 for the selective catalytic reduction of NOx by hydrocarbon—A review. J. Environ. Manag. 2017, 188, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Mori, T.; Sugiyama, H.; Uozumi, Y.; Ikeda, K.; Atadani, A.; Nagao, M. On the possibility of AgZSM-5 zeolite being a partial oxidation catalyst for methane. J. Colloid Interface Sci. 2009, 33, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Sazama, P.; Capek, L.; Drobná, H.; Sobalík, Z.; Dedecek, J.; Arve, K.; Wichterlová, B. Enhancement of decane-SCR-NOxover Ag/alumina by hydrogen. Reaction kinetics and in situ FTIR and UV-vis study. J. Catal. 2005, 232, 302–317. [Google Scholar] [CrossRef]
- Bartolomeu, R.; Bértolo, R.; Casale, S.; Fernandes, A.; Henriques, C.; da Costa, P.; Riberio, F. Particular characteristics of silver species on Ag-exchanged LTL zeolite in K and H form. Microporous Mesoporous Mater. 2013, 169, 137–147. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B Environ. 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Melián-Cabrera, I.; Mentriut, C.; Pieterse, J.A.Z.; van den Brink, R.W.; Mul, G.; Kapteijn, F.; Moulijn, J.A. Highly active and stable ion-exchanged Fe—Ferrierite catalyst for N2O decomposition under nitric acid tail gas conditions. Catal. Commun. 2005, 6, 301–305. [Google Scholar] [CrossRef]
- Zhou, T.; Li, L.; Shen, Q.; Xie, Q.; Hao, Z. Fe-mordenite/cordierite monolith for the catalytic decomposition of nitrous oxide. Ceram. Int. 2009, 35, 3097–3101. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Hirabayashi, H.; Yahiro, H.; Mizuno, N.; Iwamoto, M. Iron ion-exchanged zeolite: The most active catalyst at 473 K for selective reduction of nitrogen monoxide by ethene in oxidizing atmosphere. Catal. Lett. 1992, 12, 193–200. [Google Scholar] [CrossRef]
- Śrębowata, A.; Zielińska, I.; Baran, R.; Słowik, G.; Dzwigaj, S. Ag-Ni bimetallic SiBEA zeolite as an efficient catalyst of hydrodechlorination of 1,2-dichloroethane towards ethylene. Catal. Commun. 2015, 69, 154–160. [Google Scholar] [CrossRef]

| Sample | Ion Exchange, First Step | Ion Exchange, Second Step | ||||
|---|---|---|---|---|---|---|
| Solution, 0.1 N | Volume, mL | Calculated Ion Exchange Capacity, Atomic % | Solution, 0.1 N | Volume, mL | Calculated Ion Exchange Capacity, Atomic % | |
| FeMOR | Fe(ClO4)2 | 104 | 3.2 | - | - | - |
| AgMOR | AgNO3 | 104 | 6.4 | - | - | - |
| AgFeMOR | AgNO3 | 52 | 6.4 | Fe(ClO4)2 | 52 | 3.2 |
| FeAgMOR | Fe(ClO4)2 | 52 | 3.2 | AgNO3 | 52 | 6.4 |
| mAgFeMOR | AgNO3 + Fe(ClO4)2 | 52 + 52 | (Ag + 2Fe) = 6.4 | - | - | - |
| Sample | Ion Exchange Temperature | Atomic % | Charge Balance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | Al | Na | Ag | Fe | Si/Al | Na/Al | EIEM-Fe2+ | EIEM-Fe3+ | ||
| NaMOR | - | 48.9 | 7.5 | 9.7 | - | - | 6.5 | 1.29 | 1.29 | - |
| AgMOR | ambient | 42.8 | 6.8 | 2.4 | 4.9 | - | 6.3 | 0.35 | 1.07 | - |
| AgFeMOR | 41.7 | 6.3 | 1.0 | 3.8 | 0.9 | 6.6 | 0.16 | 1.05 | 1.19 | |
| FeAgMOR | 39.9 | 6.1 | 1.1 | 4.4 | 0.6 | 6.5 | 0.18 | 1.10 | 1.20 | |
| mAgFeMOR | 41.1 | 6.3 | 1.7 | 3.9 | 0.6 | 6.5 | 0.27 | 1.08 | 1.17 | |
| FeMOR | 44.2 | 6.9 | 4.0 | - | 1.1 | 6.4 | 0.58 | 0.90 | 1.06 | |
| AgMOR | 60 °C | 40.8 | 6.4 | 1.3 | 5.4 | - | 6.4 | 0.20 | 1.05 | - |
| AgFeMOR | 40.1 | 6.3 | 1.0 | 3.5 | 0.9 | 6.4 | 0.16 | 1.00 | 1.14 | |
| FeAgMOR | 41.9 | 6.4 | 1.5 | 4.4 | 0.7 | 6.5 | 0.23 | 1.14 | 1.25 | |
| mAgFeMOR | 41.7 | 6.4 | 1.6 | 4.0 | 0.7 | 6.5 | 0.25 | 1.09 | 1.20 | |
| FeMOR | 46.7 | 7.3 | 4.1 | - | 1.3 | 6.4 | 0.56 | 0.92 | 1.09 | |
| Sample | Ag 3d5/2 Binding Energy [eV] | |||
|---|---|---|---|---|
| Ag0 (%) 368.0–368.3 eV | Ag < 2 nm (%) ≥ 369.0 eV | Ag+ (%) 366.2 eV | Ag-Support (%) 367.4–368.0 eV | |
| AgMORTa | - | 369.0 (80) | 367.0 (20) | |
| AgFeMORTa | - | 369.7 (78) | - | 367.7 (22) |
| FeAgMORTa | 368.8 (80) | 366.8 (20) | ||
| mAgFeMORTa | - | 369.0 (80) | 367.0 (20) | |
| AgMORT60 | - | 369.3 (82) | - | 367.5 (18) |
| AgFeMORT60 | 368.8 (84) | 367.0 (16) | ||
| FeAgMORT60 | - | 369.0 (82) | - | 367.8 (18) |
| mAgFeMORT60 | - | 369.3 (74) | - | 367.3 (26) |
| Catalyst | Temperature of the Ion Exchange | Average Particle Diameter, nm |
|---|---|---|
| AgMOR | ambient | 4.4 |
| 60 °C | 6.2 | |
| AgFeMOR | ambient | 3.8 |
| 60 °C | 4.1 | |
| FeAgMOR | ambient | 2.8 |
| 60 °C | 3.4 | |
| mAgFeMOR | ambient | 6.8 |
| 60 °C | 3.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-López, P.; Kotolevich, Y.; Miridonov, S.; Chávez-Rivas, F.; Fuentes, S.; Petranovskii, V. Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO. Catalysts 2019, 9, 58. https://doi.org/10.3390/catal9010058
Sánchez-López P, Kotolevich Y, Miridonov S, Chávez-Rivas F, Fuentes S, Petranovskii V. Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO. Catalysts. 2019; 9(1):58. https://doi.org/10.3390/catal9010058
Chicago/Turabian StyleSánchez-López, Perla, Yulia Kotolevich, Serguei Miridonov, Fernando Chávez-Rivas, Sergio Fuentes, and Vitalii Petranovskii. 2019. "Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO" Catalysts 9, no. 1: 58. https://doi.org/10.3390/catal9010058
APA StyleSánchez-López, P., Kotolevich, Y., Miridonov, S., Chávez-Rivas, F., Fuentes, S., & Petranovskii, V. (2019). Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO. Catalysts, 9(1), 58. https://doi.org/10.3390/catal9010058