Improvement of the Photocatalytic Activity of ZnO/Burkeite Heterostructure Prepared by Combustion Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
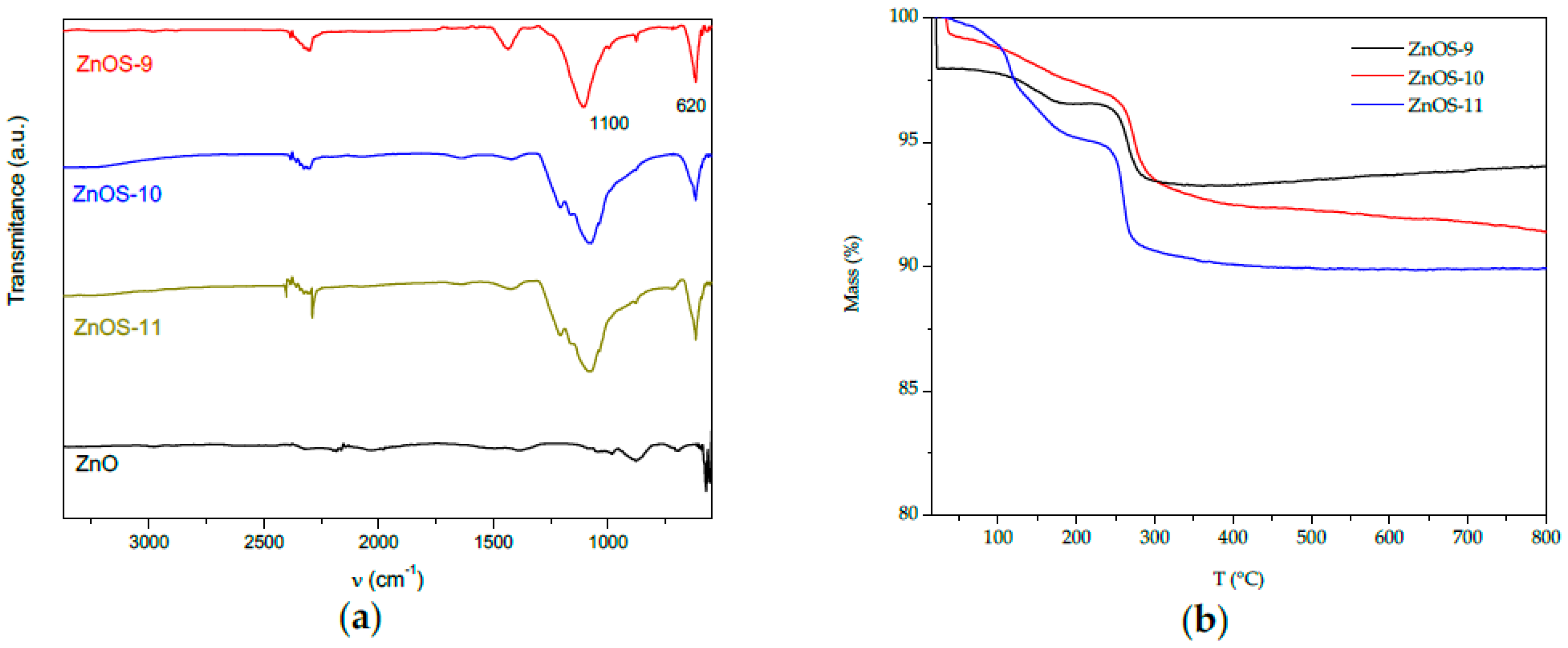
2.2. FTIR and Thermal Analysis
2.3. SEM Analysis
2.4. Surface Area Analysis
2.5. UV-Vis Analysis
2.6. Degradation of Rhodamine B
3. Materials and Methods
3.1. Synthesis of ZnO/Burkeite Heterostructure
3.2. Characterization and Analytical Techniques
3.3. Degradation tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hutton, F.G.; Feulner, G.; Lund, P.D.; Henson, S.; Røttingen, J.-A.; Hoffman, S.J.; Butler, D. Global Challenges: An innovative journal for tackling humanity’s major challenges. Glob. Chall. 2017, 1, 3–4. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, L.; Guo, R.-R.; Zeng, S.; Wang, H.; Lu, J.-X. Electroreduction of CO2 into Ethanol over an Active Catalyst: Copper Supported on Titania. Catalysts 2017, 7, 220. [Google Scholar] [CrossRef]
- Thakur, R.S.; Chaudhary, R.; Singh, C. Fundamentals and applications of the photocatalytic treatment for the removal of industrial organic pollutants and effects of operational parameters: A review. J. Renew. Sustain. Energy 2010, 2, 042701. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.G.; Koteswara Rao, S.R. Zinc oxide based photocatalysis: Tailoring surfacebulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Srikanth, B.; Goutham, R.; Badri Narayan, R.; Ramprasath, A.; Gopinath, K.P.; Sankaranarayanan, A.R. Recent advancements in supporting materials for immobilized photocatalytic applications in waste water treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Sorrell, C.C. Sand Supported Mixed-Phase TiO2 Photocatalysts for Water Decontamination Applications. Adv. Eng. Mater. 2014, 16, 248–254. [Google Scholar] [CrossRef]
- Korsakov, A.V.; Golovin, A.V.; De Gussem, K.; Sharygin, I.S.; Vandenabeele, P. First finding of burkeite in melt inclusions in olivine from sheared lherzolite xenoliths. Spectrochim. Acta Part A 2009, 73, 424–427. [Google Scholar] [CrossRef]
- Shi, B.; Frederick, W.J., Jr.; Rousseau, R.W. Effects of Calcium and Other Ionic Impurities on the Primary Nucleation of Burkeite. Ind. Eng. Chem. Res. 2003, 42, 2861–2869. [Google Scholar] [CrossRef]
- Bonakdar, T.; Ghadiri, M.; Ahmadiam, H.; de Juan, M.L.; Xu, D.; Tantawy, H.; Smith, D. Impact attrition of spray-dried burkeite particles. Powder Technol. 2016, 304, 2–7. [Google Scholar] [CrossRef]
- Alias, S.S.; Ismail, A.B.; Mohamad, A.A. Effect of pH on ZnO nanoparticle properties synthesized by sol–gel centrifugation. J. Alloy. Compd. 2010, 499, 231–237. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Yin, J.; Su, H.; Liao, C.; Yan, C. Control of ZnO Morphology via a Simple Solution Route. Chem. Mater. 2002, 14, 4172–4177. [Google Scholar] [CrossRef]
- Becker, J.; Raghupathi, K.R.; St. Pierre, J.; Zhao, D.; Koodali, R.T. Tuning of the Crystallite and Particle Sizes of ZnO Nanocrystalline Materials in Solvothermal Synthesis and Their Photocatalytic Activity for Dye Degradation. J. Phys. Chem. C 2011, 115, 13844–13850. [Google Scholar] [CrossRef]
- Cho, S.; Jung, S.-H.; Lee, K.-H. Morphology-Controlled Growth of ZnO Nanostructures Using Microwave Irradiation: From Basic to Complex Structures. J. Phys. Chem. C 2008, 112, 12769–12776. [Google Scholar] [CrossRef]
- Huang, W.; Jia, J.; Zhou, X.; Lin, Y. Morphology controllable synthesis of ZnO crystals—pH-dependent growth. Mater. Chem. Phys. 2010, 123, 104–108. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, Precursor Concentration, Growth Time, and Temperature on the Morphology of ZnO Nanostructures Grown by the Hydrothermal Method. J. Nanomater. 2011, 2011, 269692. [Google Scholar] [CrossRef]
- Li, F.-T.; Ran, J.; Jaroniec, M.; Qiao, S.Z. Solution combustion synthesis of metal oxide nanomaterials for energy storage and conversion. Nanoscale 2015, 7, 17590–17610. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.J.; Kokila, M.K.; Nagabhushana, H.; Rao, J.L.; Shivakumara, C.; Nagabhushana, B.M.; Chakradhar, R.S. Combustion synthesis, characterization and Raman studies of ZnO nanopowders. Spectrochim. Acta Part A 2011, 81, 53–58. [Google Scholar] [CrossRef]
- Nagaraja, R.; Kottam, N.; Girija, C.; Nagabhushana, B.M. Photocatalytic degradation of Rhodamine B dye under UV/solar light using ZnO nanopowder synthesized by solution combustion route. Powder Technol. 2012, 215, 91–97. [Google Scholar] [CrossRef]
- Cai, Y.; Fan, H.; Xu, M.; Li, Q. Rapid photocatalytic activity and honeycomb Ag/ZnO heterostructures via solution combustion synthesis. Colloids Surf. A 2013, 436, 787–795. [Google Scholar] [CrossRef]
- Schuyten, S.; Dinka, P.; Mukasyan, A.S.; Wolf, E. A Novel Combustion Synthesis Preparation of CuO/ZnO/ZrO2/Pd for Oxidative Hydrogen Production from Methanol. Catal. Lett. 2008, 121, 189–198. [Google Scholar] [CrossRef]
- Srinatha, N.; Kumar, V.D.; Nair, K.G.; Angadi, B. The effect of fuel and fuel-oxidizer combinations on ZnO nanoparticles synthesized by solution combustion technique. Adv. Powder Technol. 2015, 26, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.; Bulbulian, S. Synthesis of Li4SiO4 by a Modified Combustion Method. J. Am. Ceram. Soc. 2005, 88, 1720–1724. [Google Scholar] [CrossRef]
- Søndergaard, M.; Bøjesen, E.D.; Christensen, M.; Iversen, B.B. Size and Morphology Dependence of ZnO Nanoparticles Synthesized by a Fast Continuous Flow Hydrothermal Method. Cryst. Growth Des. 2011, 11, 4027–4033. [Google Scholar] [CrossRef]
- Bayuadri, C.; Verril, C.L.; Rousseau, R.W. Stability of Sodium Sulfate Dicarbonate (∼2Na2CO3·Na2SO4) Crystals Obtained from Evaporation of Aqueous Solutions of Na2CO3 and Na2SO4. Ind. Eng. Chem. Res. 2006, 45, 7144–7150. [Google Scholar] [CrossRef]
- Wang, L.; Liu, G.; Zou, L.; Xue, D. Phase evolution from rod-like ZnO to plate-like zinc hydroxysulfate during electrochemical deposition. J. Alloy. Compd. 2010, 493, 471–475. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Tokunaga, M.; Okamoto, H.; Senthilkumar, O.; Fujita, Y. Hydrogen related defect complexes in ZnO nanoparticles. Appl. Phys. Lett. 2010, 97, 091907. [Google Scholar] [CrossRef]
- De la Rosa, E.; Sepúlveda-Guzman, S.; Reeja-Jayan, B.; Torres, A.; Salas, P.; Elizondo, N.; Jose Yacaman, M. Controlling the Growth and Luminescense Properties of Well-Faceted ZnO Nanorods. J. Phys. Chem. C 2007, 111, 8489–8495. [Google Scholar] [CrossRef]
- Shi, B.; Rousseau, R.W. Structure of Burkeite and a New Crystalline Species Obtained from Solutions of Sodium Carbonate and Sodium Sulfate. J. Phys. Chem. B 2003, 107, 6932–6937. [Google Scholar] [CrossRef]
- Ramachandran, S.; Sivasamy, A. Synthesis and characterization of nanocrystalline N-doped semiconductor metal oxide and its visible photocatalytic activity in the degradation of an organic dye. J. Environ. Chem. Eng. 2018, 6, 3770–3779. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed]
- Pica, M.; Calzuola, S.; Donnadio, A.; Gentili, P.L.; Nocchetti, M.; Casciola, M. De-Ethylation and Cleavage of Rhodamine B by a Zirconium Phosphate/Silver Bromide Composite Photocatalyst. Catalysts 2019, 9, 3. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of Rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Zhang, Y.; Yu, H.; Lu, G.; Yeb, J.; Bi, Y. Concave trisoctahedral Ag3PO4 microcrystals with high-index facets and enhanced photocatalytic properties. Chem. Commun. 2013, 49, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.A.; Fernández-Cervantes, I.; Agustín-Serrano, R.; Ruíz-Salgado, S.; Sampedro, M.P.; Varela-Caselis, J.L.; Portillo, R.; Rubio, E. Ag3PO4 microcrystals with complex polyhedral morphologies diversity obtained by microwave-hydrothermal synthesis for MB degradation under sunlight. Results Phys. 2019, 12, 1344–1356. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, H.; Ouyang, S.; Jiao, Z.; Lu, G.; Ye, J. Selective growth of metallic Ag nanocrystals on Ag3PO4 submicro-cubes for photocatalytic applications. Chem. Eur. J. 2012, 18, 14272–14275. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, P.; Niu, F.; Huang, C.; Li, Y.; Yao, W. A novel molecular sieve supporting material for enhancing activity and stability of Ag3PO4 photocatalyst. Appl. Surf. Sci. 2016, 378, 552–563. [Google Scholar] [CrossRef]
- Kim, S.; Wang, Y.; Zhu, M.; Fujitsuka, M.; Majima, T. Facet Effects of Ag3PO4 on Charge-Carrier Dynamics: Trade-Off between Photocatalytic Activity and Charge-Carrier Lifetime. Chem. Eur. J. 2018, 24, 14928–14932. [Google Scholar] [CrossRef]
- Luna-Flores, A.; Valenzuela, M.A.; Luna-López, J.A.; Hernández de la Luz, A.D.; Muñoz-Arenas, L.C.; Méndez-Hernández, M.; Sosa-Sánchez, J.L. Synergetic Enhancement of the Photocatalytic Activity of TiO2 with Visible Light by Sensitization Using a Novel Push-Pull Zinc Phthalocyanine. Int. J. Photoenergy 2017, 2017, 1604753. [Google Scholar] [CrossRef]





| Sample | Eg (eV) | BET (m2/g) | Pore Size (Å) | RhB Abs. (%) | % Degradation of RhB | Kinetic Constant k (seg−1) | EDS (wt%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | C | S | Zn | O | I | |||||||
| ZnOS-9 | 3.24 | 3.54 | 42.39 | 21.7 | 82.5 | 0.01988 | 23.77 | 5.07 | 10.01 | 23.52 | 37.49 | 0.14 |
| ZnOS-10 | 3.27 | 48.7 | 20.5 | 6.3 | 7.3 | 0.000997 | 25.01 | 5.82 | 8.55 | 20.77 | 39.43 | 0.42 |
| ZnOS-11 | 3.25 | 2.7 | 42.19 | 8.2 | 92.4 | 0.027769 | 24.24 | 8.05 | 6.81 | 22.46 | 38.44 | 0.0 |
| ZnO | 3.11 | 21.8 a | 17.1 a | 4.3 | 63.8 | 0.009795 | - | - | - | 83.28 | 16.72 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Flores, A.; Morales, M.A.; Agustín-Serrano, R.; Portillo, R.; Luna-López, J.A.; Pérez-Sánchez, G.F.; Luz, A.D.H.-d.l.; Tepale, N. Improvement of the Photocatalytic Activity of ZnO/Burkeite Heterostructure Prepared by Combustion Method. Catalysts 2019, 9, 817. https://doi.org/10.3390/catal9100817
Luna-Flores A, Morales MA, Agustín-Serrano R, Portillo R, Luna-López JA, Pérez-Sánchez GF, Luz ADH-dl, Tepale N. Improvement of the Photocatalytic Activity of ZnO/Burkeite Heterostructure Prepared by Combustion Method. Catalysts. 2019; 9(10):817. https://doi.org/10.3390/catal9100817
Chicago/Turabian StyleLuna-Flores, A., M.A. Morales, R. Agustín-Serrano, R. Portillo, J.A. Luna-López, G.F. Pérez-Sánchez, A.D. Hernández-de la Luz, and N. Tepale. 2019. "Improvement of the Photocatalytic Activity of ZnO/Burkeite Heterostructure Prepared by Combustion Method" Catalysts 9, no. 10: 817. https://doi.org/10.3390/catal9100817






