Catalytic Combustion of Dimethyl Disulfide on Bimetallic Supported Catalysts Prepared by the Wet-Impregnation Method
Abstract
1. Introduction
2. Results and Discussion
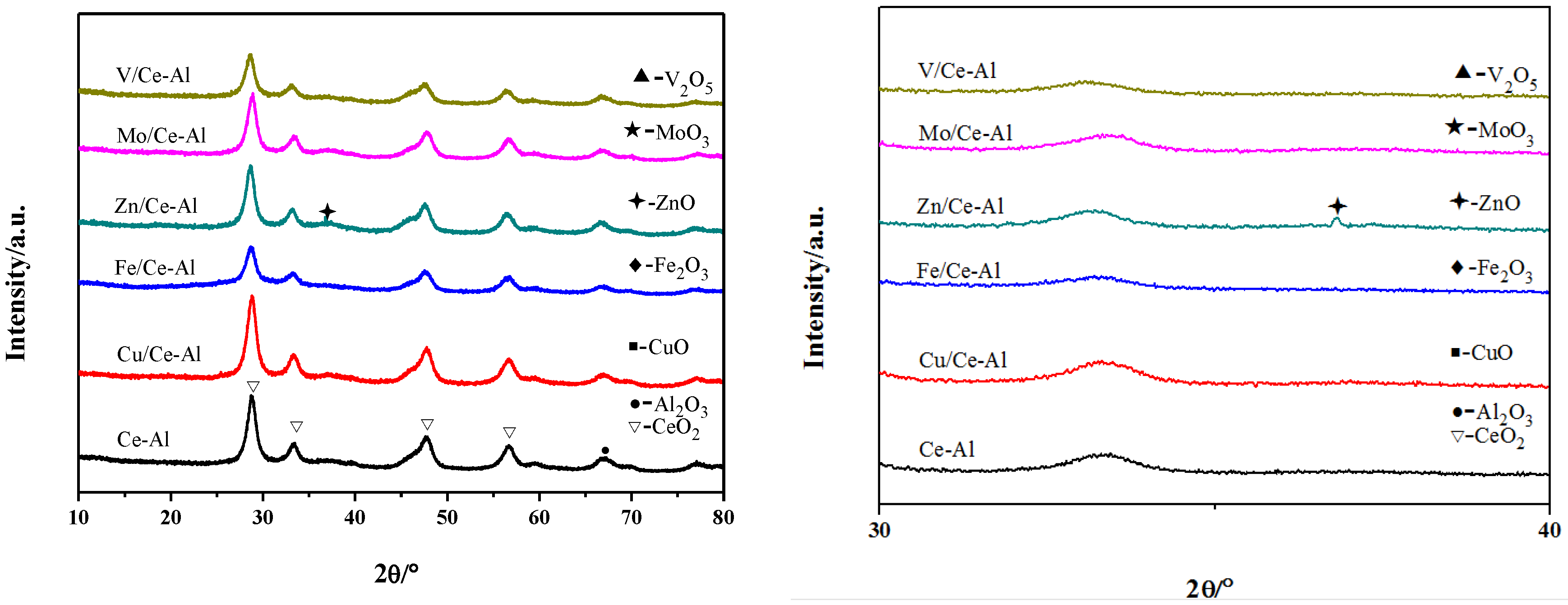
2.1. Effect of the Supports
2.2. Single-Metal Supported Catalyst
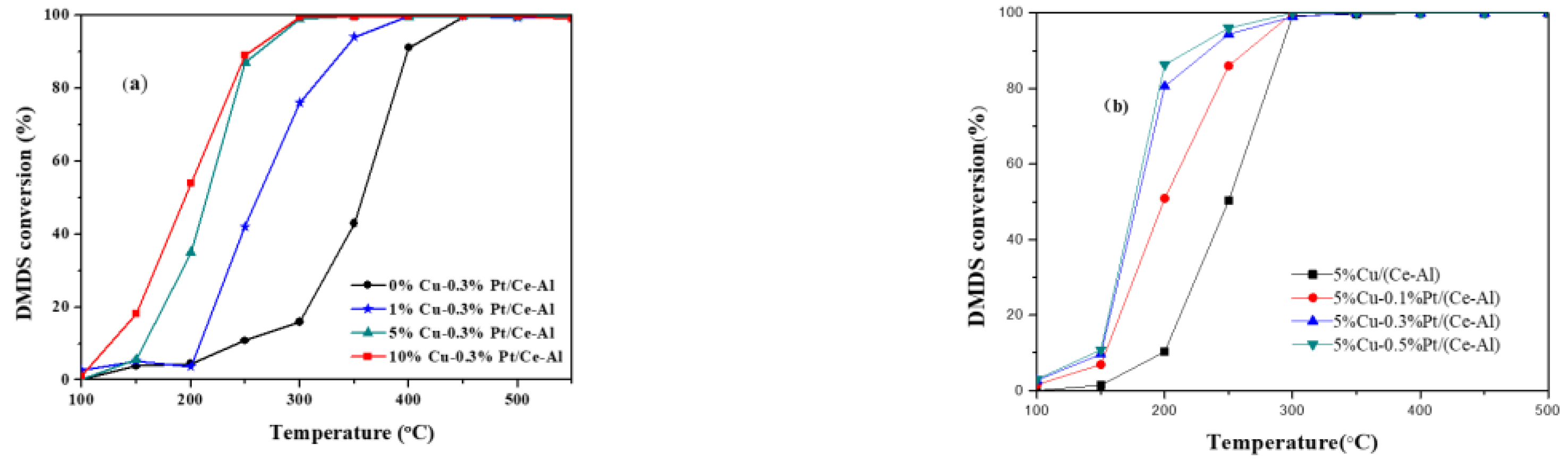
2.3. Bimetallic Catalyst
3. Experimental
3.1. Catalyst Preparation and Materials
3.2. Evaluation of the Catalysts
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aellach, B.; Ezzamarty, A.; Leglise, J.; Lamonier, C.; Lamonier, J.F. Calcium-Deficient and Stoichiometric Hydroxyapatites Promoted by Cobalt for the Catalytic Removal of Oxygenated Volatile Organic Compounds. Catal. Lett. 2010, 135, 197–206. [Google Scholar] [CrossRef]
- Faisal, I.; Khan, A.K. Removal of Volatile Organic Compounds from polluted air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar]
- Lalanne, F.; Malhautier, L.; Roux, J.C. Absorption of a mixture of volatile organic compounds (VOCs) in aqueous solutions of soluble cutting oil. Biol. Technol. 2008, 99, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Paloma, H.; Salvador, O.; Aurelio, V.; Fernando, V.D. Catalytic combustion of methane over commercial catalystsin presence of ammonia and hydrogen sulphide. Chemosphere 2004, 55, 681–689. [Google Scholar]
- Wyrwalski, F.; Giraudon, J.M.; Lamonier, J.F. Synergistic Coupling of the Redox Properties of Supports and Cobalt Oxide Co3O4 for the Complete Oxidation of Volatile Organic Compounds. Catal. Lett. 2010, 137, 141–149. [Google Scholar] [CrossRef]
- Hamad, A.; Fayed, M.E. Simulation-Aided Optimization of Volatile Organic Compounds Recovery Using Condensation. Chem. Eng. Res. Des. 2004, 82, 895–906. [Google Scholar] [CrossRef]
- Azalim, S.; Franco, M.; Brahmi, R.; Giraudon, J.M.; Lamonier, J.F.; Hazard, J. Removal of oxygenated volatile organic compounds by catalytic oxidation over Zr–Ce–Mn catalysts. J. Hazard. Mater. 2011, 188, 422–427. [Google Scholar] [CrossRef]
- Hongyan, P.; Mingyao, X.; Zhong, L.; Sisi, H.; Chun, H. Catalytic combustion of styrene over copper based catalyst: Inhibitory effect of water vapor. Chemosphere 2009, 76, 721–726. [Google Scholar]
- Spivey, J.J.; Butt, J.B. Literature review: Deactivation of catalysts in the oxidation of volatile organic compounds. Catal. Today 1992, 11, 465–500. [Google Scholar] [CrossRef]
- Wang, C.H.; Lin, S.S.; Liou, S.B.; Weng, H.S. The promoter effect and a rate expression of the catalytic incineration of (CH3)2S2 over an improved CuO–MoO3/γ-Al2O3 catalyst. Chemosphere 2002, 49, 389–394. [Google Scholar] [CrossRef]
- Després, J.; Elsener, M.; Koebel, M. Catalytic oxidation of nitrogen monoxide over Pt/SiO2. Appl. Catal. B 2004, 50, 73–82. [Google Scholar] [CrossRef]
- Cellier, C.; Gaigneaux, E.M.; Grange, P. Sulfur resistance and high activity of hydrated manganese sulfate in the catalytic oxidation of methanethiol. J. Catal. 2004, 1, 255–259. [Google Scholar] [CrossRef]
- Halevi, B.; Vohs, J.M. Reactions of CH3SH and (CH3)2S2 on the (0001) and (0001̄) Surfaces of ZnO. J. Phys. Chem. B 2005, 109, 23976–23982. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Barakat, T.; Idakiev, V.; Cousin, R.; Shao, G.S.; Yuan, Z.Y. Total oxidation of toluene over noble metal based Ce, Fe and Ni doped titanium oxides. Appl. Catal. B 2014, 146, 138–146. [Google Scholar] [CrossRef]
- Tidahy, L.; Siffert, S.; Wyrwalski, F.; Lamonier, J.F.; Aboukaïs, A. Catalytic activity of copper and palladium based catalysts for toluene total oxidation. Catal. Today 2007, 119, 317–320. [Google Scholar] [CrossRef]
- Yazawa, Y.; Takagi, N.; Yoshida, H. The support effect on propane combustion over platinum catalyst: Control of the oxidation-resistance of platinum by the acid strength of support materials. Appl. Catal. A 2002, 233, 103–112. [Google Scholar] [CrossRef]
- Lojewska, J.; Kolodziej, A.; Zak, J.; Stoch, J. Pd/Pt promoted Co3O4 catalysts for VOCs combustion: Preparation of active catalyst on metallic carrier. Catal. Today 2005, 105, 655–661. [Google Scholar] [CrossRef]
- Beck, B.; Harth, M.; Hamilton, N.G. Partial oxidation of ethanol on vanadia catalysts on supporting oxides with different redox properties compared to propane. J. Catal. 2012, 296, 120–131. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Zhang, Y. Catalytic decomposition of HCN on copper manganese oxide at low temperatures: Performance and mechanism. J. Chem. Eng. 2018, 346, 621–629. [Google Scholar] [CrossRef]
- Mishra, T.; Mahapatra, P.; Parida, K.M. Synthesis, characterisation and catalytic evaluation of iron–manganese mixed oxide pillared clay for VOC decomposition reaction. Appl. Catal. B 2008, 79, 279–285. [Google Scholar] [CrossRef]
- Ferrandon, M.; Carno, J.; Jaras, S.; Bjornbom, E. Total oxidation catalysts based on manganese or copper oxides and platinum or palladium I: Characterisation. Appl. Catal. A 1999, 180, 141–151. [Google Scholar] [CrossRef]
- Nevanperä, T.K.; Ojala, S.; Laitinen, T.; Pitkäaho, S.; Saukko, S.; Keiski, R.L. Catalytic Oxidation of Dimethyl Disulfide over Bimetallic Cu–Au and Pt–Au Catalysts Supported on γ-Al2O3, CeO2, and CeO2–Al2O3. Catalysts 2019, 9, 603. [Google Scholar] [CrossRef]
- Zhang, J.; Cullen, D.A.; Forest, R.V.; Wittkopf, J.A.; Zhuang, Z. Platinum–Ruthenium Nanotubes and Platinum–Ruthenium Coated Copper Nanowires as Efficient Catalysts for Electro-Oxidation of Methanol. ACS Catal. 2015, 5, 1468–1474. [Google Scholar] [CrossRef]
- Wang, C.H.; Weng, H.S. Promoting effect of molybdenum on CuO/γ-Al2O3 catalyst for the oxidative decomposition of (CH3)2S. Appl. Catal. A 1998, 170, 73–80. [Google Scholar] [CrossRef]
- Pitkäaho, S.; Nevanperä, T.; Matejova, L.; Ojala, S.; Keiski, R.L. Oxidation of dichloromethane over Pt, Pd, Rh, and V2O5 catalysts supported on Al2O3, Al2O3–TiO2 and Al2O3–CeO2. Appl. Catal. A 2013, 138-139, 33–42. [Google Scholar] [CrossRef]
- Nevanperä, T.K.; Ojala, S.; Bion, N. Catalytic oxidation of dimethyl disulfide (CH3SSCH3) over monometallic Au, Pt and Cu catalysts supported on γ-Al2O3, CeO2 and CeO2-Al2O3. Appl. Catal. B 2016, 182, 611–625. [Google Scholar] [CrossRef]
- Wang, C.H.; Weng, H.S. Al2O3-Supported Mixed-Metal Oxides for Destructive Oxidation of(CH3)2S2. Ind. Eng. Chem. Res. 1997, 36, 2537–2542. [Google Scholar] [CrossRef]
- Wang, C.H.; Lee, C.N.; Weng, H.S. Effect of Acid Treatment on the Performance of the CuO-MoO3/Al2O3 Catalyst for the Destructive Oxidation of (CH3)2S2. Ind. Eng. Chem. Res. 1998, 37, 1774–1780. [Google Scholar] [CrossRef]
- Darif, B.; Ojala, S.; Pirault-Roy, L.; Bensitel, M.; Brahmi, R.; Keiski, R.L. Study on the catalytic oxidation of DMDS over Pt-Cu catalysts supported on Al2O3, AlSi20 and SiO2. Appl. Catal. B 2016, 181, 24–33. [Google Scholar] [CrossRef]
- Darif, B.; Ojala, S.; Kärkkäinen, M.; Pronier, S.; Maunula, T.; Brahmi, R.; Keiski, R.L. Study on sulfur deactivation of catalysts for DMDS oxidation. Appl. Catal. B 2017, 206, 653–665. [Google Scholar] [CrossRef]
- Lu, C.; Liu, T.; Shi, Q.; Li, Q.; Xin, Y.; Zheng, L.; Zhang, Z. Plausibility of potassium ion-exchanged ZSM-5 as soot combustion catalysts. Sci. Rep. 2017, 7, 3300. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wu, X.; Weng, D. Effect of barium loading on CuOx–CeO2 catalysts, NOx storage capacity, NO oxidation ability and soot oxidation activity. Catal. Today 2011, 175, 124–132. [Google Scholar] [CrossRef]
- Lin, Y.H.; Tseng, T.K.; Chu, H. Photo-catalytic degradation of dimethyl disulfide on S and metal-ions co-doped TiO2 under visible-light irradiation. Appl. Catal. A 2014, 469, 221–228. [Google Scholar] [CrossRef]
- Leyva, C.; Rana, M.S.; Ancheyta, J. Surface characterization of Al2O3–SiO2 supported NiMo catalysts: An effect of support composition. Catal. Today 2008, 130, 345–353. [Google Scholar] [CrossRef]
- Gatti, G.; Costenaro, D.; Vittoni, C. CO2 adsorption on different organo-modified SBA-15 silicas: A multidisciplinary study on the effects of basic surface groups. Phys. Chem. Chem. Phys. 2017, 19, 14114. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Lin, Y.; Li, M.; Weng, D. Active oxygen-assisted NO-NO2 recycling and decomposition of surface oxygenated species on diesel soot with Pt/Ce0.6Zr0.4O2 catalyst. Chin. J. Catal. 2014, 35, 407–415. [Google Scholar] [CrossRef]
- Yang, J.; Lukashuk, L.; Akbarzadeh, J.; Stöger-Pollach, M.; Peterlik, H.; Föttinger, K.; Rupprechter, G.; Schubert, U. Different synthesis protocols for Co3O4-CeO2 catalysts-Part 1, influence on the morphology onthe nanoscale. Chem. Eur. J. 2015, 21, 885–892. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, X. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Volatile organic compounds abatement over copper-based catalysts, effect of support. Inorg. Chim. Acta 2017, 455, 473–482. [Google Scholar] [CrossRef]
- Guan, B.; Lin, H.; Zhan, R.; Huang, Z. The catalysts of three-dimensionally ordered macroporous Ce1-xZrxO2-supported gold nanoparticles for soot combustion, The metal–support interaction. J. Catal. 2012, 287, 13–29. [Google Scholar]
- Guan, B.; Lin, H.; Zhan, R.; Huang, Z. Catalytic combustion of soot over Cu, Mn substitution CeZrO2-δ, nanocomposites catalysts prepared by self-propagating high-temperature synthesis method. Chem. Eng. Sci. 2018, 189, 320–339. [Google Scholar] [CrossRef]
- Gatti, G.; Vittoni, C.; Costenaro, D. The influence of particle size of amino-functionalized MCM-41 silicas on CO2 adsorption. Phys. Chem. Chem. Phys. 2017, 19, 29449–29460. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—Areview. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today. 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Demoulin, O.; Clef, B.L.; Navez, M.; Ruiz, P. Combustion of methane, ethane and propane and of mixtures of methane with ethane or propane on Pd/γ-Al2O3 catalysts. Appl. Catal. A 2008, 344, 1–9. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Avila, M.S.; Vignatti, C.I.; Apesteguía, C.R.; Garetto, T.F. Effect of support on the deep oxidation of propane and propylene on Pt-based catalysts. Chem. Eng. J. 2014, 241, 52–59. [Google Scholar] [CrossRef]
- Sanz, O.; Banús, E.D.; Goya, A.; Larumbe, H.; Delgado, J.J.; Monzón, A.; Montes, M. Stacked wire-mesh monoliths for VOCs combustion: Effect of the mesh-opening in the catalytic performance. Catal. Today 2017, 296, 76–83. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Schmid, S.; Jecklin, M.C.; Zenobi, R. Degradation of volatile organic compounds in a non-thermal plasma air purifier. Chemosphere 2010, 79, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Chiang, H.M.; Huang, G.Y. Adsorption characteristics of acetone, chloroform and acetonitrile on sludge-derived adsorbent, commercial granular activated carbon and activated carbon fibers. J. Hazard. Mater. 2008, 154, 1183–1191. [Google Scholar] [CrossRef] [PubMed]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Gao, S.; Wei, J.; Zhao, H.; Zhang, J. Catalytic Combustion of Dimethyl Disulfide on Bimetallic Supported Catalysts Prepared by the Wet-Impregnation Method. Catalysts 2019, 9, 994. https://doi.org/10.3390/catal9120994
Gao J, Gao S, Wei J, Zhao H, Zhang J. Catalytic Combustion of Dimethyl Disulfide on Bimetallic Supported Catalysts Prepared by the Wet-Impregnation Method. Catalysts. 2019; 9(12):994. https://doi.org/10.3390/catal9120994
Chicago/Turabian StyleGao, Junan, Song Gao, Jun Wei, Hong Zhao, and Jie Zhang. 2019. "Catalytic Combustion of Dimethyl Disulfide on Bimetallic Supported Catalysts Prepared by the Wet-Impregnation Method" Catalysts 9, no. 12: 994. https://doi.org/10.3390/catal9120994
APA StyleGao, J., Gao, S., Wei, J., Zhao, H., & Zhang, J. (2019). Catalytic Combustion of Dimethyl Disulfide on Bimetallic Supported Catalysts Prepared by the Wet-Impregnation Method. Catalysts, 9(12), 994. https://doi.org/10.3390/catal9120994



