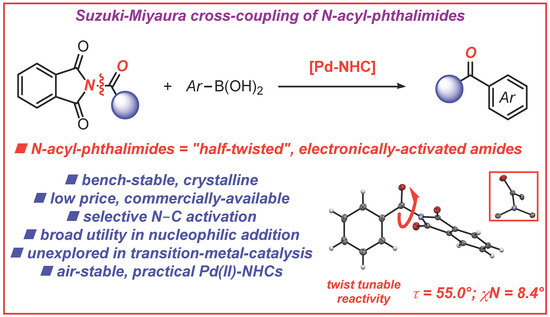
N-Acylphthalimides: Efficient Acyl Coupling Reagents in Suzuki–Miyaura Cross-Coupling by N–C Cleavage Catalyzed by Pd–PEPPSI Precatalysts
Abstract
:1. Introduction
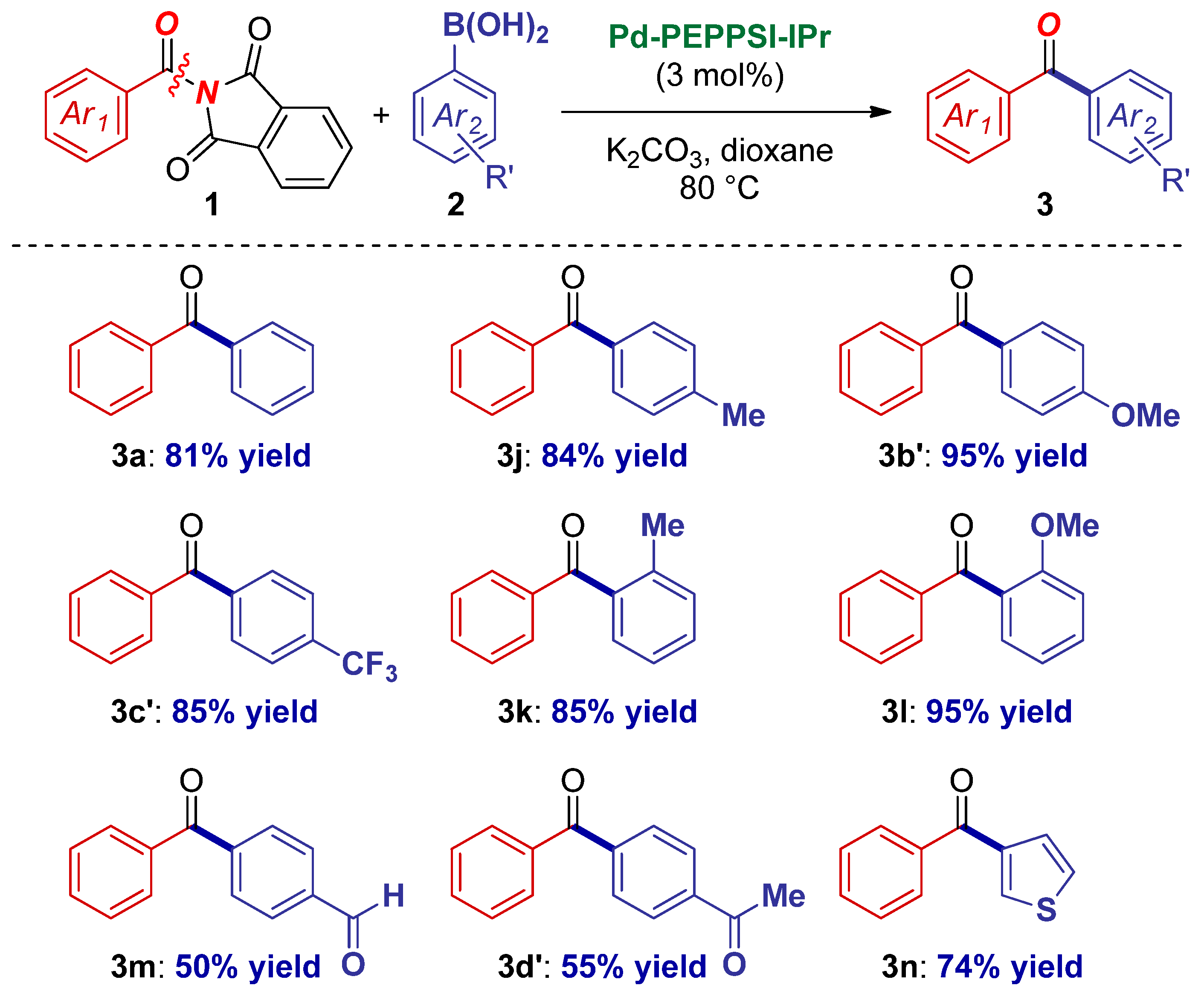
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Bauer, A.; Lemmerer, M.; Maulide, N. Amide Activation: An Emerging Tool for Chemoselective Synthesis. Chem. Soc. Rev. 2018, 47, 7899–7925. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Szostak, M. N-Acyl-Glutarimides: Privileged Scaffolds in Amide N–C Bond Cross-Coupling. Eur. J. Org. Chem. 2018, 20–21, 2352–2365. [Google Scholar] [CrossRef]
- Liu, C.; Szostak, M. Decarbonylative Cross-Coupling of Amides. Org. Biomol. Chem. 2018, 16, 7998–8010. [Google Scholar] [CrossRef] [PubMed]
- Takise, R.; Muto, K.; Yamaguchi, J. Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev. 2017, 46, 5864–5888. [Google Scholar] [CrossRef] [PubMed]
- Dander, J.E.; Garg, N.K. Breaking Amides using Nickel Catalysis. ACS Catal. 2017, 7, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Szostak, M. Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N–C Amide Bond Activation. Chem. Eur. J. 2017, 23, 7157–7173. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Szostak, M. Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. [Google Scholar]
- Greenberg, A.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Pattabiraman, V.R.; Bode, J.W. Rethinking Amide Bond Synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef]
- Szostak, R.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, M. Ground-State Distortion in N-Acyl-tert-butyl-carbamates (Boc) and N-Acyl-tosylamides (Ts): Twisted Amides of Relevance to Amide N–C Cross-Coupling. J. Org. Chem. 2016, 81, 8091–8094. [Google Scholar] [CrossRef]
- Szostak, R.; Meng, G.; Szostak, M. Resonance Destabilization in N-Acylanilines (Anilides): Electronically-Activated Planar Amides of Relevance in N–C(O) Cross-Coupling. J. Org. Chem. 2017, 82, 6373–6378. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Szostak, M. Sterically Controlled Pd-Catalyzed Chemoselective Ketone Synthesis via N-C Cleavage in Twisted Amides. Org. Lett. 2015, 17, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Hie, L.; Nathel, N.F.F.; Shah, T.K.; Baker, E.L.; Hong, X.; Yang, Y.F.; Liu, P.; Houk, K.N.; Garg, N.K. Conversion of Amides to Esters by the Nickel-Catalysed Activation of Amide C–N Bonds. Nature 2015, 524, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Weires, N.A.; Baker, E.L.; Garg, N.K. Nickel-catalysed Suzuki−Miyaura coupling of Amides. Nat. Chem. 2016, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, G. Acylative Suzuki coupling of amides: Acyl-nitrogen activation via synergy of independently modifiable activating groups. Chem. Commun. 2015, 51, 5089–5092. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Meng, G.; Shi, S. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling of Amides via Site-Selective N−C Bond Cleavage by Cooperative Catalysis. ACS Catal. 2016, 6, 7335–7339. [Google Scholar]
- Liu, C.; Meng, G.; Liu, Y.; Liu, R.; Lalancette, R.; Szostak, R.; Szostak, M. N-Acylsaccharins: Stable Electrophilic Amide-Based Acyl Transfer Reagents in Pd-Catalyzed Suzuki−Miyaura Coupling via N−C Cleavage. Org. Lett. 2016, 18, 4194–4197. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Cui, M.; Jian, J.; Zeng, Z. Suzuki Coupling of Amides via Palladium-Catalyzed C-N Cleavage of N-Acylsaccharins. Adv. Synth. Catal. 2016, 358, 3876–3880. [Google Scholar] [CrossRef]
- Szostak, M.; Liu, C.; Liu, Y.; Liu, R.; Lalancette, R.; Szostak, R. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling of N-Mesylamides by N−C Cleavage: Electronic Effect of the Mesyl Group. Org. Lett. 2017, 19, 1434–1437. [Google Scholar]
- Meng, G.; Szostak, R.; Szostak, M. Suzuki−Miyaura Cross-Coupling of N-Acylpyrroles and Pyrazoles: Planar, Electronically Activated Amides in Catalytic N-C Cleavage. Org. Lett. 2017, 19, 3596–3599. [Google Scholar] [CrossRef]
- Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. N-Methylamino Pyrimidyl Amides (MAPA): Highly Reactive, Electronically-Activated Amides in Catalytic N-C(O) Cleavage. Org. Lett. 2017, 19, 4656–4659. [Google Scholar] [CrossRef] [PubMed]
- Osumi, Y.; Liu, C.; Szostak, M. N-Acylsuccinimides: Twist-controlled, acyl-transfer reagents in Suzuki–Miyaura cross-coupling by N–C amide bond activation. Org. Biomol. Chem. 2017, 15, 8867–8871. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Chen, Z.; Liu, T.; Wang, H.; Zeng, Z. N-Acylsuccinimides: Efficient acylative coupling reagents in palladium-catalyzed Suzuki coupling via C-N cleavage. Tetrahedron Lett. 2017, 58, 3819–3822. [Google Scholar] [CrossRef]
- Wang, T.; Guo, J.; Wang, H.; Guo, H.; Jia, D.; Zhang, W.; Liu, L. N-heterocyclic carbene palladium(II)-catalyzed Suzuki-Miyaura cross coupling of N-acylsuccinimides by C-N cleavage. J. Organomet. Chem. 2018, 877, 80–84. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. Acyl and Decarbonylative Suzuki Coupling of N-Acetyl Amides: Electronic Tuning of Twisted, Acyclic Amides in Catalytic Carbon−Nitrogen Bond Cleavage. ACS Catal. 2018, 8, 9131–9139. [Google Scholar] [CrossRef]
- Evans, T.W.; Dehn, W.M. The Reaction of Phthalyl Chloride with Amides. J. Am. Chem. Soc. 1929, 51, 3651–3652. [Google Scholar] [CrossRef]
- Rabjohn, N.; Drumm, M.F.; Elliott, R.L. Some Reactions of N-Acetylphthalimides. J. Am. Chem. Soc. 1956, 78, 1631–1634. [Google Scholar] [CrossRef]
- Chiriac, C.I. Amides from N-Acylphthalimides and Aliphatic Amines. Rev. Roum. Chim. 1986, 31, 525–527. [Google Scholar]
- Gabriel, S. Ueber eine Darstellungsweise primärer Amine aus den entsprechenden Halogenverbindungen. Ber. Deutsch. Chem. Ges. 1887, 29, 2224–2226. [Google Scholar] [CrossRef]
- Ariffin, A.; Khan, M.N.; Lan, L.C.; May, F.Y.; Yun, C.S. Suggested Improved Method for Ing-Manske and Related Reactions for the Second Step of Gabriel Synthesis of Primary Amines. Synth. Commun. 2004, 34, 4439–4445. [Google Scholar] [CrossRef]
- Rao, S.N.; Mohan, D.C.; Adimurthy, S. L-Proline: An Efficient Catalyst for Transamidation of Carboxamides with Amines. Org. Lett. 2013, 15, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Pace, V.; Holzer, W.; Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Structures of Highly Twisted Amides Relevant to Amide N–C Cross-Coupling: Evidence for Ground-State Amide Destabilization. Chem. Eur. J. 2016, 22, 14494–14498. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R.; Szostak, M. N-Acyl-Glutarimides: Resonance and Proton Affinities of Rotationally-Inverted Twisted Amides Relevant to N−C(O) Cross-Coupling. Org. Lett. 2018, 20, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Meng, G.; Szostak, M. General Method for the Suzuki-Miyaura Cross-Coupling of Amides Using Commercially Available, Air- and Moisture-Stable Palladium/NHC (NHC = N-Heterocyclic Carbene) Complexes. ACS Catal. 2017, 7, 1960–1965. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Ling, Y.; An, J.; Szostak, M. Pd-PEPPSI: Pd-NHC Precatalyst for Suzuki-Miyaura Cross-Coupling Reactions of Amides. J. Org. Chem. 2017, 82, 6638–6646. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P.; Cazin, C.S.J. (Eds.) Science of Synthesis: N-Heterocyclic Carbenes in Catalytic Organic Synthesis; Thieme: Stuttgart, Germany, 2017. [Google Scholar]
- Nolan, S.P. (Ed.) N-Heterocyclic Carbenes; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Cazin, C.S.J. (Ed.) N-Heterocyclic Carbenes in Transition Metal Catalysis; Springer: New York, NY, USA, 2011. [Google Scholar]
- Shi, S.; Szostak, M. Nickel-Catalyzed Negishi Cross-Coupling of N-Acylsuccinimides: Stable, Amide-Based, Twist-Controlled Acyl-Transfer Reagents via N–C Activation. Synthesis 2017, 49, 3602–3608. [Google Scholar]
- Kantchev, E.A.B.; O’Brien, C.J.O.; Organ, M.G. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross-Coupling Reactions: A Synthetic Chemist’s Perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813. [Google Scholar] [CrossRef]
- Valente, C.; Calimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium/NHC Complexes for the Most Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Lombardi, C.; Pompeo, M.; Rucker, R.P.; Organ, M.G. Designing Pd-N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [Google Scholar] [CrossRef]
- Li, G.; Lei, P.; Szostak, M.; Casals, E.; Poater, A.; Cavallo, L.; Nolan, S.P. Mechanistic Study of Suzuki-Miyaura Cross-Coupling Reactions of Amides Mediated by [Pd(NHC)(allyl)Cl] Precatalysts. ChemCatChem 2018, 10, 3096–3106. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air- and Moisture-Stable Pd-NHC (NHC = N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalysts for the Suzuki-Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef] [PubMed]
- Chartoire, A.; Frogneux, X.; Boreux, A.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*)(3-Cl-pyridinylCl2]: A Novel and Efficient PEPPSI Precatalyst. Organometallics 2012, 31, 6947–6951. [Google Scholar] [CrossRef]
- Viciu, M.S.; Navarro, O.; Germaneau, R.F.; Kelly, R.A., III; Sommer, W.; Marion, N.; Stevens, E.D.; Cavallo, L.; Nolan, S.P. Synthetic and Structural Studies of (NHC)Pd(allyl)Cl Complexes (NHC = N-heterocyclic carbene). Organometallics 2004, 23, 1629–1635. [Google Scholar] [CrossRef]
- Nahm, S.; Weinreb, S.M. N-Methoxy-N-methylamides as Effective Acylating Agents. Tetrahedron Lett. 1981, 22, 3815–3818. [Google Scholar] [CrossRef]
- Buchspies, J.; Pyle, D.J.; He, H.; Szostak, M. Pd-Catalyzed Suzuki-Miyaura Cross-Coupling of Pentafluorophenyl Esters. Molecules 2018, 23, 3134. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Nolan, S.P. Well-defined N-heterocyclic carbenes-palladium(II) precatalysts for cross-coupling reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Marion, N.; Navarro, O.; Mei, J.; Stevens, E.D.; Scott, N.M.; Nolan, S.P. Modified (NHC)Pd(allyl)Cl (NHC = N-heterocyclic carbene) complexes for room-temperature Suzuki-Miyaura and Buchwald-Hartwig reactions. J. Am. Chem. Soc. 2006, 128, 4101–4111. [Google Scholar] [CrossRef]
- Melvin, P.R.; Nova, A.; Balcells, D.; Dai, W.; Hazari, N.; Hruszkewycz, D.P.; Shah, H.P.; Tudge, M.T. Design of a Versatile and Improved Precatalyst Scaffold for Palladium-Catalyzed Cross-Coupling: (η3-1-t-Bu-indenyl)2(μ-Cl)2Pd2. ACS Catal. 2015, 5, 5596–5606. [Google Scholar] [CrossRef]
- Meng, G.; Szostak, M. Palladium/NHC (NHC = N-Heterocyclic Carbene)-Catalyzed B-Alkyl Suzuki Cross-Coupling of Amides by Selective N−C Bond Cleavage. Org. Lett. 2018, 20, 6789–6793. [Google Scholar] [CrossRef]
- Shi, W.; Zou, G. Palladium-Catalyzed Room Temperature Acylative Cross-Coupling of Activated Amides with Trialkylboranes. Molecules 2018, 23, 2412. [Google Scholar] [CrossRef] [PubMed]





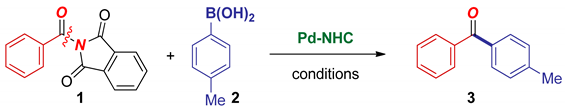
| Entry | Catalyst | Ar-B(OH)2 (equiv) | K2CO3 (equiv) | Solvent | T (°C) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | Pd–PEPPSI-IPr | 2.0 | 3.0 | dioxane | 60 | 27 |
| 2 | Pd–PEPPSI-IPr | 2.0 | 3.0 | dioxane | 110 | 80 |
| 3 2 | Pd–PEPPSI-IPr | 2.0 | 3.0 | dioxane | 110 | 80 |
| 4 | Pd–PEPPSI-IPr | 3.0 | 4.5 | dioxane | 110 | 50 |
| 5 | Pd–PEPPSI-IPr | 3.0 | 4.5 | dioxane | 80 | 90 |
| 6 | Pd–PEPPSI-IPr | 2.0 | 3.0 | dioxane | 80 | 89 |
| 7 | Pd–PEPPSI-IPr | 2.0 | 3.0 | THF | 80 | 27 |
| 8 3 | Pd–PEPPSI-IPr | 2.0 | 3.0 | THF | 80 | 32 |
| 9 3 | Pd–PEPPSI-IPr | 2.0 | 3.0 | dioxane | 80 | <10 |
| 10 | Pd–PEPPSI-IMes | 2.0 | 3.0 | dioxane | 80 | 30 |
| 11 | Pd–PEPPSI-IPr * | 2.0 | 3.0 | dioxane | 80 | 24 |
| 12 | Pd–PEPPSI-IBut | 2.0 | 3.0 | dioxane | 80 | <5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Buchspies, J.; Szostak, M. N-Acylphthalimides: Efficient Acyl Coupling Reagents in Suzuki–Miyaura Cross-Coupling by N–C Cleavage Catalyzed by Pd–PEPPSI Precatalysts. Catalysts 2019, 9, 129. https://doi.org/10.3390/catal9020129
Rahman MM, Buchspies J, Szostak M. N-Acylphthalimides: Efficient Acyl Coupling Reagents in Suzuki–Miyaura Cross-Coupling by N–C Cleavage Catalyzed by Pd–PEPPSI Precatalysts. Catalysts. 2019; 9(2):129. https://doi.org/10.3390/catal9020129
Chicago/Turabian StyleRahman, Md. Mahbubur, Jonathan Buchspies, and Michal Szostak. 2019. "N-Acylphthalimides: Efficient Acyl Coupling Reagents in Suzuki–Miyaura Cross-Coupling by N–C Cleavage Catalyzed by Pd–PEPPSI Precatalysts" Catalysts 9, no. 2: 129. https://doi.org/10.3390/catal9020129