Highlights of Major Progress on Single-Atom Catalysis in 2017
Abstract
:1. Introduction
2. The Preparation Strategies of Single-Atom Catalysts (SACs)
2.1. SACs with Ordered Structure
2.2. Single-Atom Alloy (SAA)
2.3. Support Effect
2.3.1. Functional Groups on The Support
2.3.2. Surface Defects of Support
2.4. External Forces Induced SAC Synthesis
2.4.1. Iced Photochemical Reduction
2.4.2. Electrodeposition
3. Novel Applications of SACs
3.1. CH4 Conversion
3.2. Methanol and Formic Acid Reforming
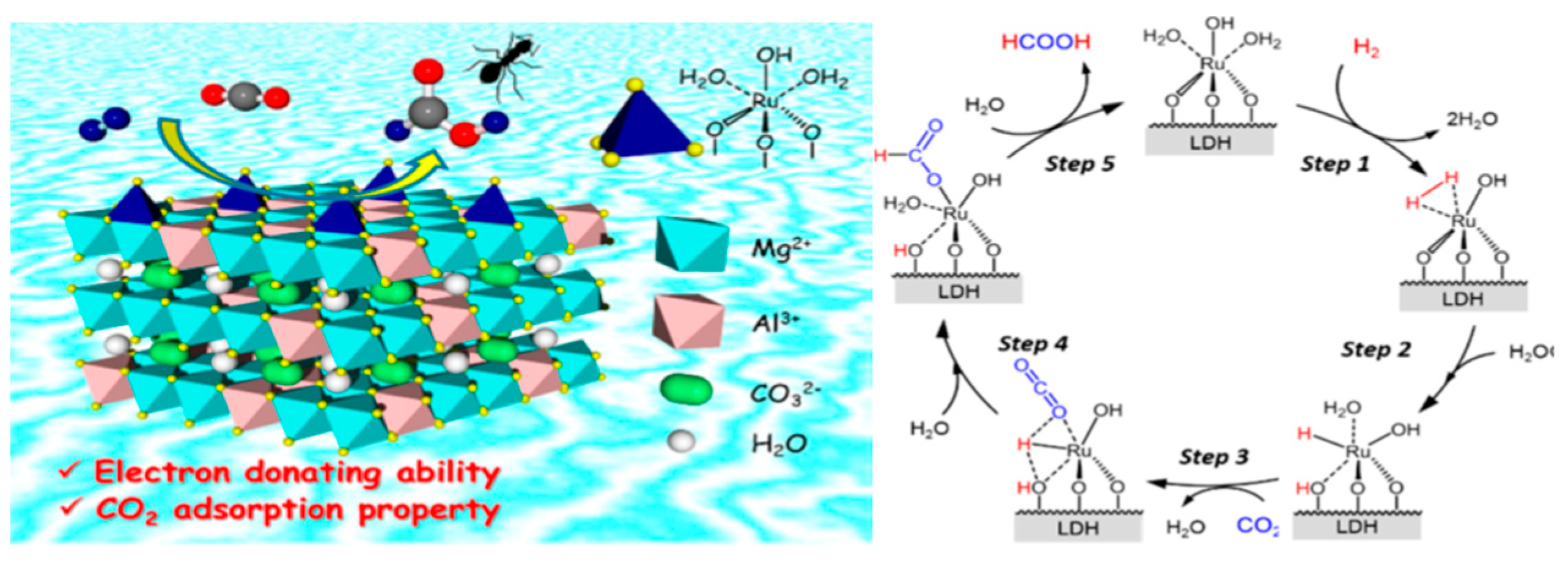
3.3. CO2 Conversion
3.4. CO Conversion
3.5. Photoelectrocatalytic Reactions
3.6. Heterogenization of Homogeneously Catalyzed Processes
4. Strong Metal-Support Interaction of SACs
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Catalysis by Supported Single Metal Atoms. ACS Catal. 2017, 7, 34–59. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhang, T. Preface to the Special Issue of the International Symposium on Single-Atom Catalysis (ISSAC-2016). Chin. J. Catal. 2017, 38, 1431. [Google Scholar] [CrossRef]
- Schwarz, H. Ménage-à-trois: Single-atom catalysis, mass spectrometry, and computational chemistry. Catal. Sci. Technol. 2017, 7, 4302–4314. [Google Scholar] [CrossRef]
- Yang, X.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2012, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Flytzani-Stephanopoulos, M.; Gates, B.C. Atomically dispersed supported metal catalysts. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 545–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Deng, D.; Bao, X. Two-dimensional materials confining single atoms for catalysis. Chin. J. Catal. 2017, 38, 1443–1453. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Wang, A.; Miao, S.; Liu, X.; Pan, X.; Su, Y.; Li, L.; Tan, Y.; Zhang, T. Pd/ZnO catalysts with different origins for high chemoselectivity in acetylene semi-hydrogenation. Chin. J. Catal. 2016, 37, 692–699. [Google Scholar] [CrossRef]
- Qiao, B.; Liang, J.-X.; Wang, A.; Liu, J.; Zhang, T. Single atom gold catalysts for low-temperature CO oxidation. Chin. J. Catal. 2016, 37, 1580–1587. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Pfisterer, J.H.K.; Liang, Y.; Schneider, O.; Bandarenka, A.S. Direct instrumental identification of catalytically active surface sites. Nature 2017, 549, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lang, R.; Qiao, B.; Wang, A.; Zhang, T. Highlights of the major progress in single-atom catalysis in 2015 and 2016. Chin. J. Catal. 2017, 38, 1498–1507. [Google Scholar] [CrossRef]
- Qiao, B.; Liang, J.; Wang, A.; Xu, C.; Li, J.; Zhang, T.; Liu, J. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res. 2015, 8, 2913–2924. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.P.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Ganzler, A.M.; Casapu, M.; Vernoux, P.; Loridant, S.; Cadete Santos Aires, F.J.; Epicier, T.; Betz, B.; Hoyer, R.; Grunwaldt, J.D. Tuning the Structure of Platinum Particles on Ceria in Situ for Enhancing the Catalytic Performance of Exhaust Gas Catalysts. Angew. Chem. Int. Ed. Engl. 2017, 56, 13078–13082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.G.; Chen, W.; Dong, J.; Zheng, L.; Luo, J.; Wan, J.; Tian, S.; Cheong, W.C.; Wang, D.; et al. Metal (Hydr)oxides@Polymer Core-Shell Strategy to Metal Single-Atom Materials. J. Am. Chem. Soc. 2017, 139, 10976–10979. [Google Scholar] [CrossRef]
- Dai, X.; Chen, Z.; Yao, T.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Wei, S.; et al. Single Ni sites distributed on N-doped carbon for selective hydrogenation of acetylene. Chem. Commun. 2017, 53, 11568–11571. [Google Scholar] [CrossRef]
- Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D.; et al. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Zhang, L.; Yao, T.; Liu, W.; Lin, Y.; Ju, H.; Dong, J.; Zheng, L.; Yan, W.; et al. Uncoordinated Amine Groups of Metal-Organic Frameworks to Anchor Single Ru Sites as Chemoselective Catalysts toward the Hydrogenation of Quinoline. J. Am. Chem. Soc. 2017, 139, 9419–9422. [Google Scholar] [CrossRef]
- Cao, W.; Luo, W.; Ge, H.; Su, Y.; Wang, A.; Zhang, T. UiO-66 derived Ru/ZrO2@C as a highly stable catalyst for hydrogenation of levulinic acid to γ-valerolactone. Green Chem. 2017, 19, 2201–2211. [Google Scholar] [CrossRef]
- Boucher, M.B.; Zugic, B.; Cladaras, G.; Kammert, J.; Marcinkowski, M.D.; Lawton, T.J.; Sykes, E.C.H.; Flytzani-Stephanopoulos, M. Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions. Phys. Chem. Chem. Phys. 2013, 15, 12187–12196. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.X.; Liu, X.Y.; Yang, X.; Zhang, L.; Wang, A.; Li, L.; Wang, H.; Wang, X.; Zhang, T. Performance of Cu-Alloyed Pd Single-Atom Catalyst for Semihydrogenation of Acetylene under Simulated Front-End Conditions. ACS Catal. 2017, 7, 1491–1500. [Google Scholar] [CrossRef]
- Pei, G.; Liu, X.; Chai, M.; Wang, A.; Zhang, T. Isolation of Pd atoms by Cu for semi-hydrogenation of acetylene: Effects of Cu loading. Chin. J. Catal. 2017, 38, 1540–1548. [Google Scholar] [CrossRef]
- Kruppe, C.M.; Krooswyk, J.D.; Trenary, M. Polarization-Dependent Infrared Spectroscopy of Adsorbed Carbon Monoxide To Probe the Surface of a Pd/Cu(111) Single-Atom Alloy. J. Phys. Chem. C 2017, 121, 9361–9369. [Google Scholar] [CrossRef]
- Li, Z.; He, T.; Matsumura, D.; Miao, S.; Wu, A.; Liu, L.; Wu, G.; Chen, P. Atomically Dispersed Pt on the Surface of Ni Particles: Synthesis and Catalytic Function in Hydrogen Generation from Aqueous Ammonia–Borane. ACS Catal. 2017, 6762–6769. [Google Scholar] [CrossRef]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 2017, 358, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tak, Y.J.; Kim, J.; Soon, A.; Lee, H. Support Effects in Single-Atom Platinum Catalysts for Electrochemical Oxygen Reduction. ACS Catal. 2017, 7, 1301–1307. [Google Scholar] [CrossRef]
- Mahmoodinia, M.; Astrand, P.O.; Chen, D. Tuning the Electronic Properties of Single-Atom Pt Catalysts by Functionalization of the Carbon Support Material. J. Phys. Chem. C 2017, 121, 20802–20812. [Google Scholar] [CrossRef]
- Hasegawa, S.; Kunisada, Y.; Sakaguchi, N. Diffusion of a Single Platinum Atom on Light-Element-Doped Graphene. J. Phys. Chem. C 2017, 121, 17787–17795. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.; Yang, X.; Hu, J.; Peng, L.; Bai, L.; Huo, Q.; Guan, J. Highly efficient single atom cobalt catalyst for selective oxidation of alcohols. Appl. Catal. A Gen. 2017, 543, 61–66. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Lu, L.; Barkholtz, H.M.; Li, Y.; Wang, Y.; Jiang, L.; Wu, Z.; Liu, D.J.; Zhuang, L.; et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 15938. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Gracia-Espino, E.; Ma, J.; Zang, K.; Luo, J.; Wang, L.; Gao, S.; Mamat, X.; Hu, G.; Wagberg, T.; et al. Synergistic Effects between Atomically Dispersed Fe-N-C and C-S-C for the Oxygen Reduction Reaction in Acidic Media. Angew. Chem. Int. Ed. Engl. 2017, 56, 13800–13804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Z. Single Mo Atom Supported on Defective Boron Nitride Monolayer as an Efficient Electrocatalyst for Nitrogen Fixation: A Computational Study. J. Am. Chem. Soc. 2017, 139, 12480–12487. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Robertson, A.W.; Li, M.M.; Kuo, W.C.H.; Darby, M.T.; Muhieddine, M.H.; Lin, Y.C.; Suenaga, K.; Stamatakis, M.; Warner, J.H.; et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 2017, 9, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Huang, K.; Wang, D.; Zhang, R.; Ge, B.; Ma, J.; Wen, B.; Zhang, S.; Li, Q.; Lei, M.; et al. Iced photochemical reduction to synthesize atomically dispersed metals by suppressing nanocrystal growth. Nat. Commun. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Dick, J.E.; Bard, A.J. Electrodeposition of Isolated Platinum Atoms and Clusters on Bismuth-Characterization and Electrocatalysis. J. Am. Chem. Soc. 2017, 139, 17677–17682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, L.; Liu, H.; Liu, X.; Luo, J. Potential-Cycling Synthesis of Single Platinum Atoms for Efficient Hydrogen Evolution in Neutral Media. Angew. Chem. Int. Ed. 2017, 56, 13694–13698. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Li, T.; Matsumura, D.; Miao, S.; Ren, Y.; Cui, Y.T.; Tan, Y.; Qiao, B.; Li, L.; Wang, A.; et al. Hydroformylation of Olefins by a Rhodium Single-Atom Catalyst with Activity Comparable to RhCl(PPh3)3. Angew. Chem. Int. Ed. 2016, 55, 16054–16058. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.; Wang, S.; Gao, Z.; Luo, Z.; Wang, X.; Zeng, R.; Li, A.; Li, H.; Wang, M.; et al. Atomic-level insights in optimizing reaction paths for hydroformylation reaction over Rh/CoO single-atom catalyst. Nat. Commun. 2016, 7, 14036. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.; Schlögl, R. Methane Activation by Heterogeneous Catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef]
- Shan, J.; Li, M.; Allard, L.F.; Lee, S.; Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 2017, 551, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, T.Y.; Kwon, G.; Yi, J.; Lee, H. Selective Activation of Methane on Single-Atom Catalyst of Rhodium Dispersed on Zirconia for Direct Conversion. J. Am. Chem. Soc. 2017, 139, 17694–17699. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowski, M.D.; Liu, J.; Murphy, C.J.; Liriano, M.L.; Wasio, N.A.; Lucci, F.R.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Selective Formic Acid Dehydrogenation on Pt-Cu Single-Atom Alloys. ACS Catal. 2017, 7, 413–420. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; et al. Low-temperature hydrogen production from water and methanol using Pt/alpha-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Frazier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M.; et al. The projected timing of climate departure from recent variability. Nature 2013, 502, 183. [Google Scholar] [CrossRef]
- Back, S.; Lim, J.; Kim, N.-Y.; Kim, Y.-H.; Jung, Y. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 2017, 8, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Jung, Y. TiC- and TiN-Supported Single-Atom Catalysts for Dramatic Improvements in CO2 Electrochemical Reduction to CH4. ACS Energy Lett. 2017, 2, 969–975. [Google Scholar] [CrossRef]
- Long, R.; Li, Y.; Liu, Y.; Chen, S.; Zheng, X.; Gao, C.; He, C.; Chen, N.; Qi, Z.; Song, L.; et al. Isolation of Cu Atoms in Pd Lattice: Forming Highly Selective Sites for Photocatalytic Conversion of CO2 to CH4. J. Am. Chem. Soc. 2017, 139, 4486–4492. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, X.; Yao, T.; Chen, W.; Wang, X.; Wang, J.; Yang, J.; Wei, S.; Wu, Y.; Li, Y. Ionic Exchange of Metal-Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar] [CrossRef]
- Mori, K.; Taga, T.; Yamashita, H. Isolated Single-Atomic Ru Catalyst Bound on a Layered Double Hydroxide for Hydrogenation of CO2 to Formic Acid. ACS Catal. 2017, 3147–3151. [Google Scholar] [CrossRef]
- Flytzani-Stephanopoulos, M. Gold Atoms Stabilized on Various Supports Catalyze the Water-Gas Shift Reaction. Acc Chem. Res. 2013, 47, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Zou, X.-P.; He, S.G. Metal-mediated catalysis in the gas phase: A review. Chin. J. Catal. 2017, 38, 1515–1527. [Google Scholar] [CrossRef]
- Gu, X.-K.; Huang, C.-Q.; Li, W.-X. First-principles study of single transition metal atoms on ZnO for the water gas shift reaction. Catal. Sci. Technol. 2017, 7, 4294–4301. [Google Scholar] [CrossRef]
- Guan, H.; Lin, J.; Qiao, B.; Miao, S.; Wang, A.-Q.; Wang, X.; Zhang, T. Enhanced performance of Rh1/TiO2 catalyst without methanation in water-gas shift reaction. AIChE J. 2017, 63, 2081–2088. [Google Scholar] [CrossRef]
- Sun, X.C.; Lin, J.; Zhou, Y.; Li, L.; Su, Y.; Wang, X.D.; Zhang, T. FeOx Supported Single-Atom Pd Bifunctional Catalyst for Water Gas Shift Reaction. AIChE J. 2017, 63, 4022–4031. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wu, D.; Zhao, X.; Zhou, Z. K1–xMo3P2O14 as Support for Single-Atom Catalysts. J. Phys. Chem. C 2017, 121, 22895–22900. [Google Scholar] [CrossRef]
- Wang, C.; Gu, X.-K.; Yan, H.; Lin, Y.; Li, J.; Liu, D.; Li, W.-X.; Lu, J. Water-Mediated Mars–Van Krevelen Mechanism for CO Oxidation on Ceria-Supported Single-Atom Pt1 Catalyst. ACS Catal. 2017, 7, 887–891. [Google Scholar] [CrossRef]
- Spezzati, G.; Su, Y.; Hofmann, J.P.; Benavidez, A.D.; DeLaRiva, A.T.; McCabe, J.; Datye, A.K.; Hensen, E.J.M. Atomically Dispersed Pd-O Species on CeO2(111) as Highly Active Sites for Low-Temperature CO Oxidation. ACS Catal. 2017, 7, 6887–6891. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.-G.; Li, J. Theoretical Investigations of Pt1@CeO2 Single-Atom Catalyst for CO Oxidation. J. Phys. Chem. C 2017, 121, 11281–11289. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, J. A highly active Pt-Fe/gamma-Al2O3 catalyst for preferential oxidation of CO in excess of H2 with a wide operation temperature window. Chem. Commun. 2017, 53, 9020–9023. [Google Scholar] [CrossRef]
- Yang, T.; Fukuda, R.; Hosokawa, S.; Tanaka, T.; Sakaki, S.; Ehara, M. A Theoretical Investigation on CO Oxidation by Single-Atom Catalysts M1/γ-Al2O3 (M=Pd, Fe, Co and Ni). ChemCatChem 2017, 9, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, C.-Q.; Cheng, D.; Li, J. Identification of activity trends for CO oxidation on supported transition-metal single-atom catalysts. Catal. Sci. Technol. 2017, 7, 5860–5871. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, J. CO Oxidation on Metal Oxide Supported Single Pt atoms: The Role of the Support. Ind. Chem. Res. 2017, 56, 6916–6925. [Google Scholar] [CrossRef]
- Liu, J.; Duan, S.; Wang, R. The Stability of High Metal-Loading Pt1/Fe2O3 Single-Atom Catalyst Under Different Gas Environment. Microsc. Microanal. 2017, 23, 1898–1899. [Google Scholar] [CrossRef]
- DeRita, L.; Dai, S.; Lopez-Zepeda, K.; Pham, N.; Graham, G.W.; Pan, X.; Christopher, P. Catalyst Architecture for Stable Single Atom Dispersion Enables Site-Specific Spectroscopic and Reactivity Measurements of CO Adsorbed to Pt Atoms, Oxidized Pt Clusters, and Metallic Pt Clusters on TiO2. J. Am. Chem. Soc. 2017, 139, 14150–14165. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Zhou, Y.; Li, L.; Qiao, B.; Wang, A.; Liu, J.; Wang, X.; Zhang, T. More active Ir subnanometer clusters than single-atoms for catalytic oxidation of CO at low temperature. AIChE J. 2017, 63, 4003–4012. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Wang, Y.; Dong, J.; Chen, W.; Li, Z.; Shen, R.; Zheng, L.; Zhuang, Z.; Wang, D.; et al. Isolated Single Iron Atoms Anchored on N-Doped Porous Carbon as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2017, 56, 6937–6941. [Google Scholar] [CrossRef]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, P.; Yan, Y.; Li, X.; Xing, Y.; Li, H.; Peng, Z.; Yang, J.; Zeng, J. Gold atom-decorated CoSe2 nanobelts with engineered active sites for enhanced oxygen evolution. J. Mater. Chem. A 2017, 5, 20202–20207. [Google Scholar] [CrossRef]
- Chao, T.; Luo, X.; Chen, W.; Jiang, B.; Ge, J.; Lin, Y.; Wu, G.; Wang, X.; Hu, Y.; Zhuang, Z.; et al. Atomically Dispersed Copper-Platinum Dual Sites Alloyed with Palladium Nanorings Catalyze the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2017, 56, 16047–16051. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Shi, Q.; Du, D.; Lin, Y. Single-Atom Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 13944–13960. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.; Feng, Y.; Zeng, Y.; Xie, Z.; Zhang, Q.; Su, Y.; Chen, P.; Liu, Y.; Yao, K.; et al. Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C3N4 for broad spectrum photocatalytic degradation of naproxen. Appl. Catal. B Environ. 2018, 221, 510–520. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Cao, D.; Wang, Y.; Zhu, Y. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Appl. Catal. B Environ. 2017, 211, 79–88. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Zhou, M.; Ma, Z.; Chen, J.; Tang, X. Single Silver Adatoms on Nanostructured Manganese Oxide Surfaces: Boosting Oxygen Activation for Benzene Abatement. Environ. Sci. Technol. 2017, 51, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, J.; Huang, Z.; Zhou, M.; Chen, J.; Li, C.; Ma, Z.; Chen, J.; Tang, X. Sodium Rivals Silver as Single-Atom Active Centers for Catalyzing Abatement of Formaldehyde. Environ. Sci. Technol. 2017, 51, 7084–7090. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Junge, K.; Dai, X.; Kreyenschulte, C.; Pohl, M.M.; Wohlrab, S.; Shi, F.; Bruckner, A.; Beller, M. Synthesis of Single Atom Based Heterogeneous Platinum Catalysts: High Selectivity and Activity for Hydrosilylation Reactions. ACS Cent. Sci. 2017, 3, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Liu, X.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Wang, A.; Zhang, T. Discriminating Catalytically Active FeNx Species of Atomically Dispersed Fe-N-C Catalyst for Selective Oxidation of the C-H Bond. J. Am. Chem. Soc. 2017, 139, 10790–10798. [Google Scholar] [CrossRef]
- Vignola, E.; Steinmann, S.N.; Al Farra, A.; Vandegehuchte, B.D.; Curulla, D.; Sautet, P. Evaluating the Risk of C–C Bond Formation during Selective Hydrogenation of Acetylene on Palladium. ACS Catal. 2018, 8, 1662–1671. [Google Scholar] [CrossRef]
- Lee, E.-K.; Park, S.-A.; Woo, H.; Hyun Park, K.; Kang, D.W.; Lim, H.; Kim, Y.-T. Platinum single atoms dispersed on carbon nanotubes as reusable catalyst for Suzuki coupling reaction. J. Catal. 2017, 352, 388–393. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, X.; Zhang, L.; Lang, R.; Qiao, B. Single-atom catalysis: Bridging the homo- and heterogeneous catalysis. Chin. J. Catal. 2018, 39, 893–898. [Google Scholar] [CrossRef]
- Malta, G.; Kondrat, S.A.; Freakley, S.J.; Davies, C.J.; Lu, L.; Dawson, S.; Thetford, A.; Gibson, E.K.; Morgan, D.J.; Jones, W.; et al. Identification of single-site gold catalysis in acetylene hydrochlorination. Science 2017, 355, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Ammal, S.C.; Heyden, A. Water-Gas Shift Activity of Atomically Dispersed Cationic Platinum versus Metallic Platinum Clusters on Titania Supports. ACS Catal. 2017, 7, 301–309. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Nguyen, L.; Zhao, Y.-F.; Wu, Z.; Goh, T.-W.; Liu, J.J.; Li, Y.; Zhu, T.; Huang, W.; et al. Catalysis on Singly Dispersed Rh Atoms Anchored on an Inert Support. ACS Catal. 2017, 8, 110–121. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Zhang, W.; Zhao, X.; Qiu, J.; Li, A.; Zheng, X.; Hu, Z.; Si, R.; Zeng, J. Supported Rhodium Catalysts for Ammonia-Borane Hydrolysis: Dependence of the Catalytic Activity on the Highest Occupied State of the Single Rhodium Atoms. Angew. Chem. Int. Ed. 2017, 56, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Yang, L.; Zou, L.; Zou, Z.; Chen, C.; Hu, Z.; Yang, H. Single Cobalt Atom and N Codoped Carbon Nanofibers as Highly Durable Electrocatalyst for Oxygen Reduction Reaction. ACS Catal. 2017, 7, 6864–6871. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, S.; Li, T.; Liu, Q.; Jiang, T.; Guo, Y.; Luo, J.-L. Atomically dispersed Pt on specific TiO2 facets for photocatalytic H2 evolution. J. Catal. 2017, 353, 250–255. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Asakura, H.; Zhang, B.; Zhang, J.; Zhou, M.; Han, Y.; Tanaka, T.; Wang, A.; Zhang, T.; et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 2017, 8, 16100. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Lang, R.; Qiao, B. Highlights of Major Progress on Single-Atom Catalysis in 2017. Catalysts 2019, 9, 135. https://doi.org/10.3390/catal9020135
Guo Y, Lang R, Qiao B. Highlights of Major Progress on Single-Atom Catalysis in 2017. Catalysts. 2019; 9(2):135. https://doi.org/10.3390/catal9020135
Chicago/Turabian StyleGuo, Yalin, Rui Lang, and Botao Qiao. 2019. "Highlights of Major Progress on Single-Atom Catalysis in 2017" Catalysts 9, no. 2: 135. https://doi.org/10.3390/catal9020135