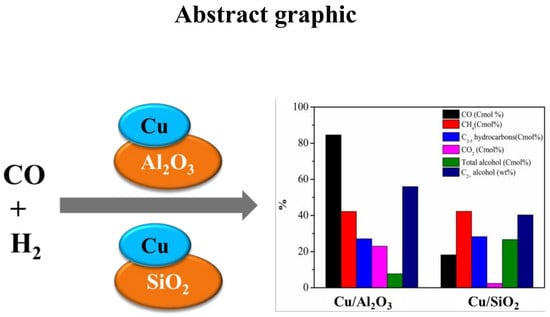
The Support Effects on the Direct Conversion of Syngas to Higher Alcohol Synthesis over Copper-Based Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Preparation of Cu/Al2O3, K-Cu/Al2O3 and Cu/SiO2, K-Cu/SiO2 Catalysts
2.3. Catalyst Characterization
2.4. Catalytic Performance Evaluation
3. Results and Discussion
3.1. Catalyst Characterization
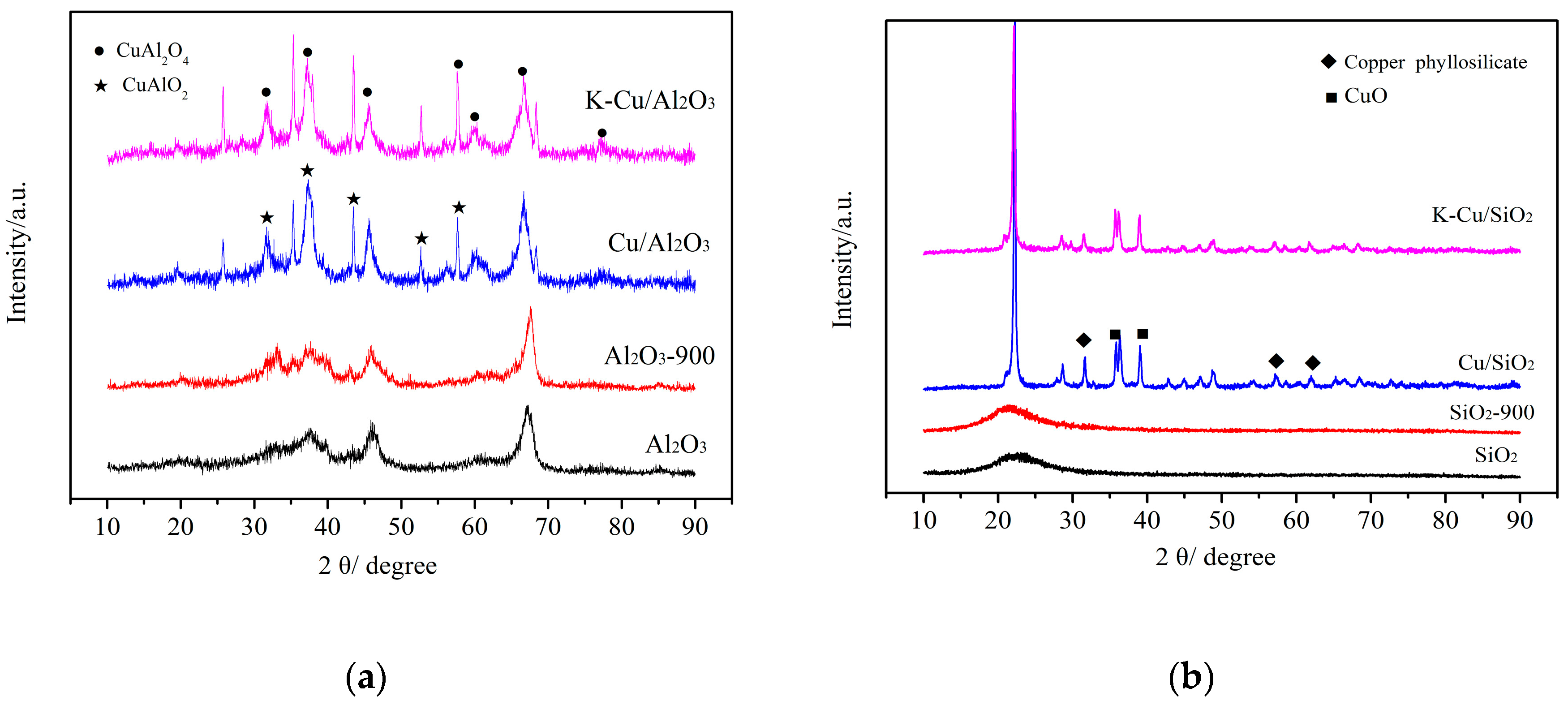
3.1.1. XRD
3.1.2. N2 Absorption-Desorption
3.1.3. H2-TPR
3.1.4. NH3-TPD
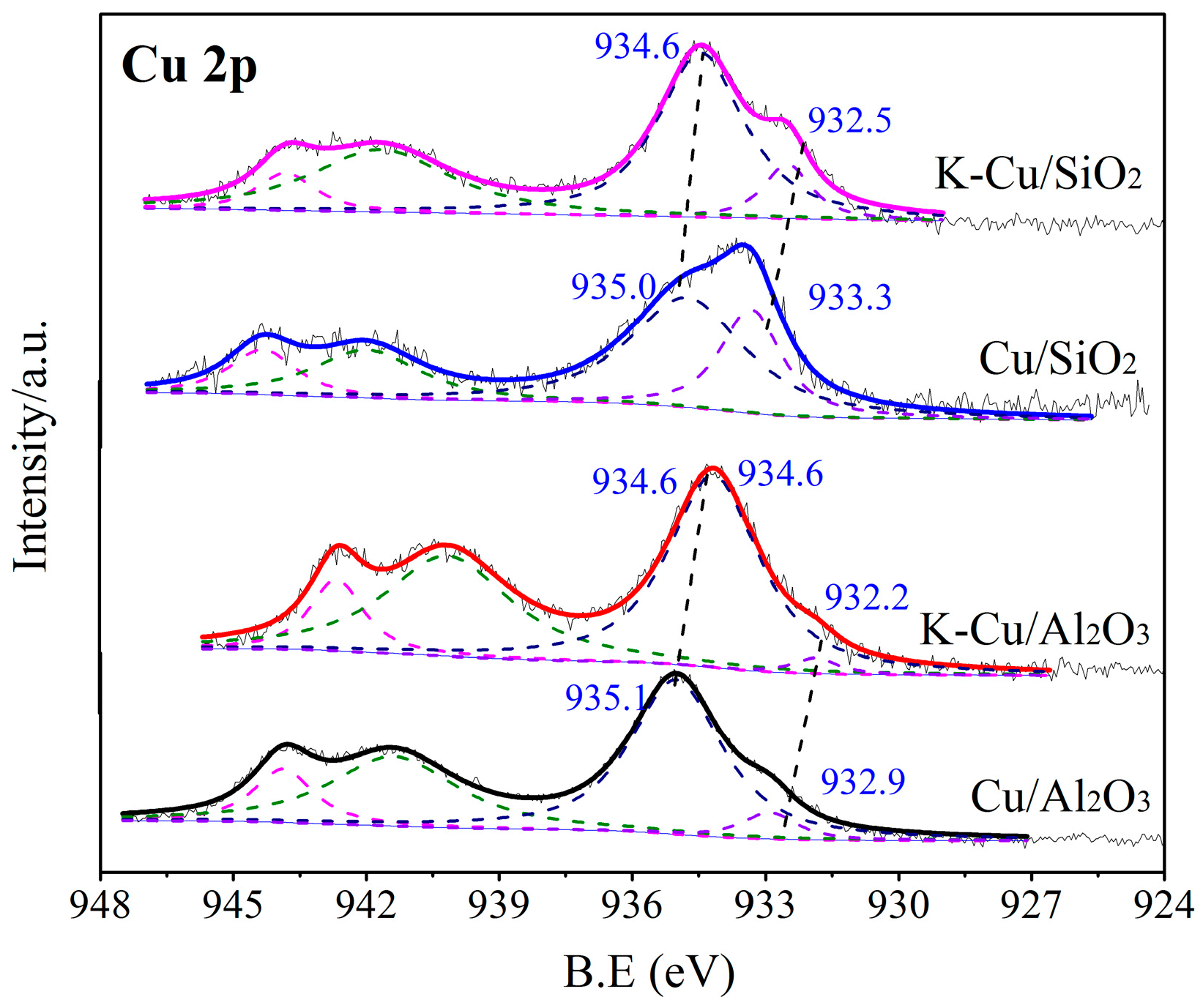
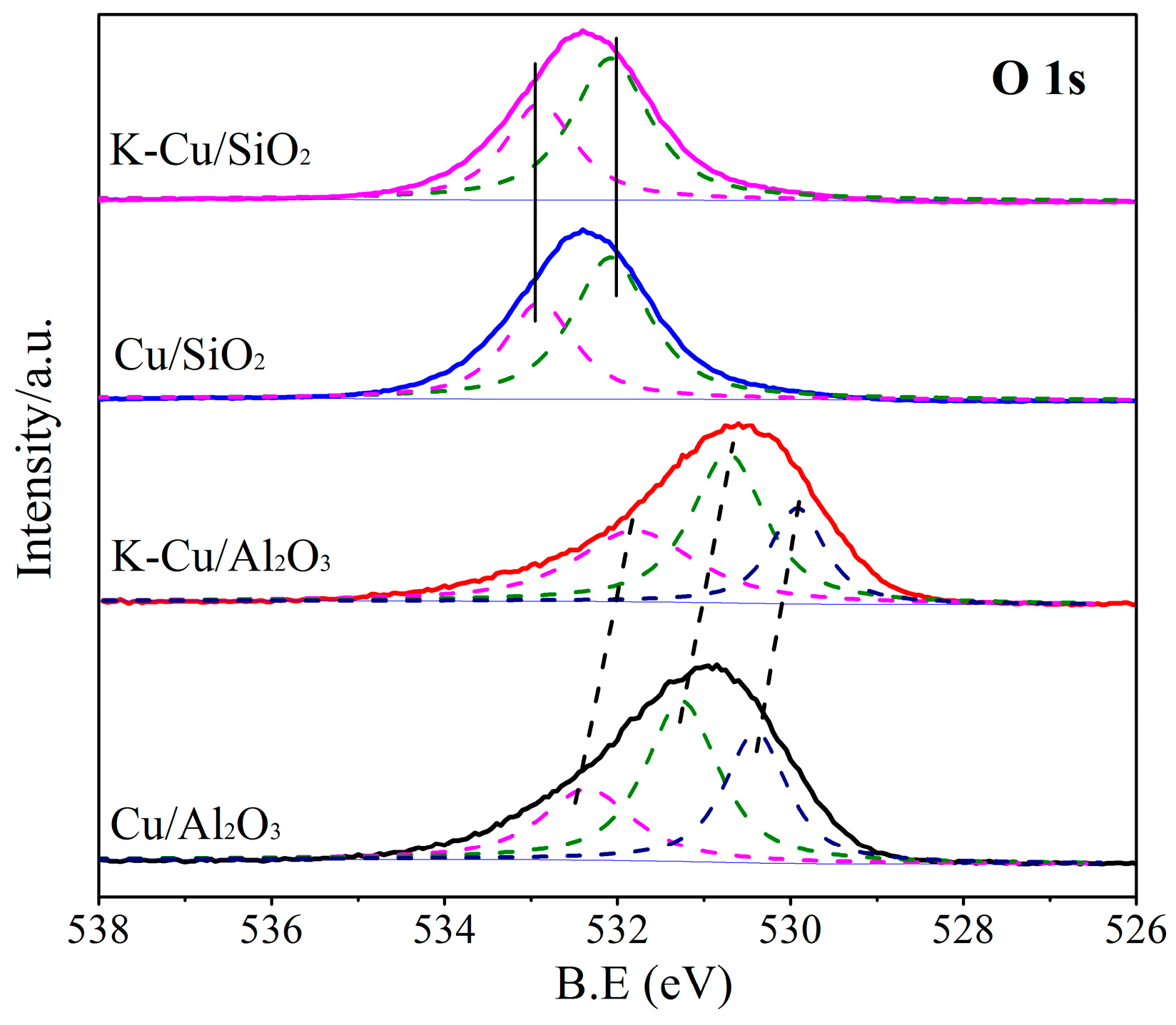
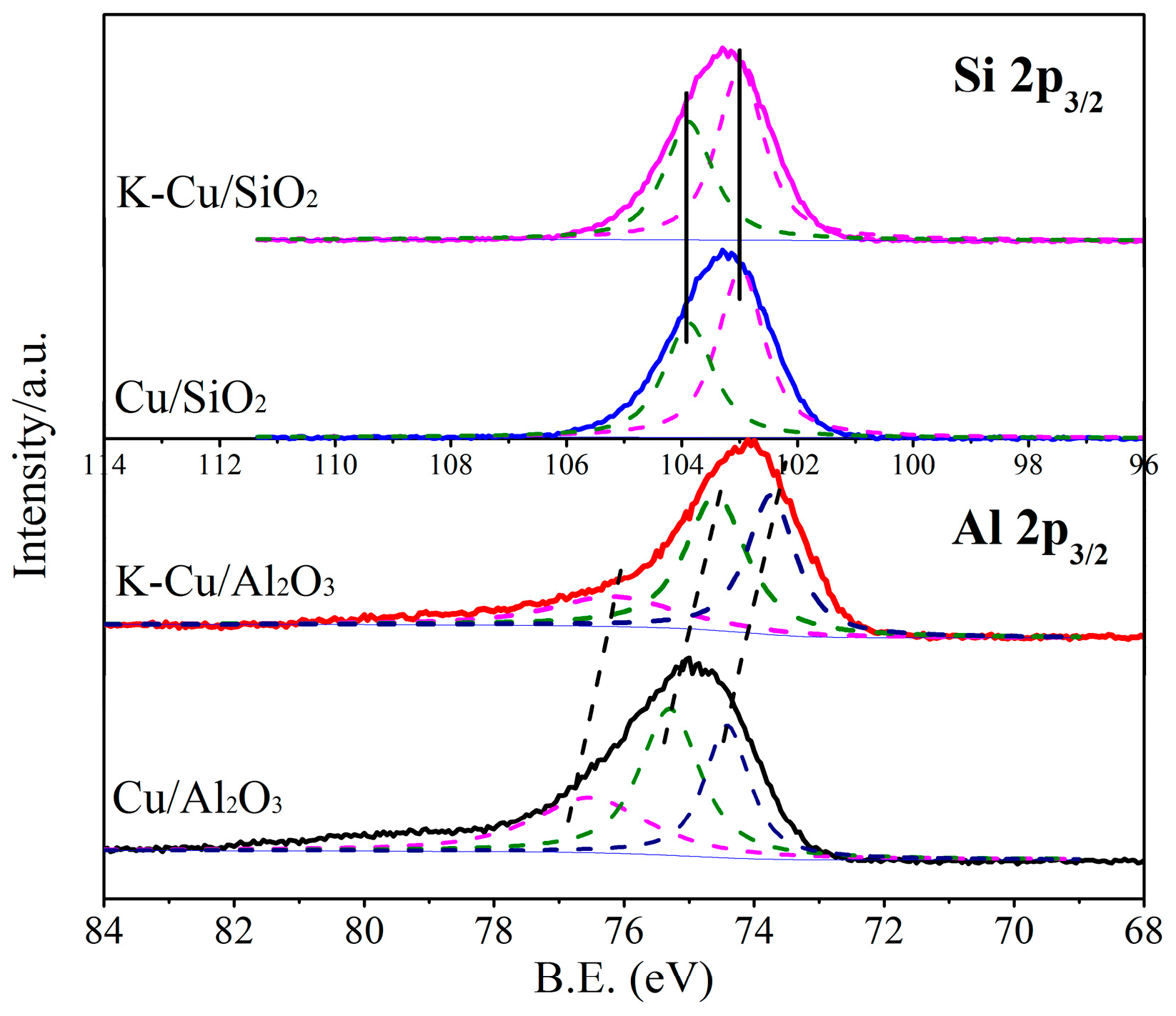
3.1.5. XPS
3.2. Catalyst Evaluation
3.3. In Situ FTIR
3.4. Discussion
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Qi, X.; Wang, X.; Lv, D.; Yu, F.; Zhong, L.; Wang, H.; Sun, Y.H. Deactivation study of CuCo catalyst for higher alcohol synthesis via syngas. Catal. Today 2016, 270, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Santos, V.P.; van der Linden, B.; Chojecki, A.; Budroni, G.; Corthals, S.; Shibata, H.; Meima, G.R.; Kapteijn, F.; Makkee, M.; Gascon, J. Mechanistic Insight into the Synthesis of Higher Alcohols from Syngas: The Role of K Promotion on MoS2 Catalysts. ACS Catal. 2013, 3, 1634–1637. [Google Scholar] [CrossRef]
- Goldemberg, J. Ethanol for a sustainable energy future. Science 2007, 315, 808–810. [Google Scholar] [CrossRef]
- Luk, H.T.; Mondelli, C.; Ferré, D.C.; Stewart, J.A.; Pérez-Ramírez, J. Status and prospects in higher alcohols synthesis from syngas. Chem. Soc. Rev. 2017, 46, 1358–1426. [Google Scholar] [CrossRef]
- Zhang, R.; Duan, T.; Wang, B.J.; Ling, L.X. Unraveling the role of support surface hydroxyls and its effect on the selectivity of C2 species over Rh/γ-Al2O3 catalyst in syngas conversion: A theoretical study. Appl. Surf. Sci. 2016, 379, 384–394. [Google Scholar] [CrossRef]
- Choi, Y.; Liu, P. Mechanism of ethanol synthesis from syngas on Rh (111). J. Am. Chem. Soc. 2009, 131, 13054–13061. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, Y.F.; Liu, J.M.; Huang, Q.W.; He, S.; Li, C.M.; Zhao, J.W.; Wei, M. Catalytic conversion of syngas to mixed alcohols over CuFe-based catalysts derived from layered double hydroxides. Catal. Sci. Technol. 2013, 3, 1324–1332. [Google Scholar] [CrossRef]
- Xiao, K.; Qi, X.Z.; Bao, Z.H.; Wang, X.X.; Zhong, L.S.; Fang, K.G.; Lin, M.G.; Sun, Y.H. CuFe, CuCo and CuNi nanoparticles as catalysts for higher alcohol synthesis from syngas: a comparative study. Catal. Sci. Technol. 2013, 3, 1591–1602. [Google Scholar] [CrossRef]
- Zaman, S.F.; Pasupulety, N.; Al-Zahrani, A.A.; Daous, M.A.; Al-Shahrani, S.; Driss, H.; Petrov, L.A. Carbon monoxide hydrogenation on potassium promoted Mo2N catalysts. Appl. Catal. A Gen. 2017, 532, 133–145. [Google Scholar] [CrossRef]
- Wang, P.; Bai, Y.X.; Xiao, H.; Tian, S.P.; Zhang, Z.Z.; Wu, Y.Q.; Xie, H.J.; Yang, G.H.; Han, Y.Z.; Tan, Y.S. Effect of the dimensions of carbon nanotube channels on copper-cobalt-cerium catalysts for higher alcohols synthesis. Catal. Commun. 2016, 75, 92–97. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.F.; Bai, Y.X.; Xiao, H.; Tian, S.P.; Xie, H.J.; Yang, G.H.; Tsubaki, N.; Han, Y.Z.; Tan, Y.S. Ternary copper-cobalt-cerium catalyst for the production of ethanol and higher alcohols through CO hydrogenation. Appl. Catal. A Gen. 2016, 514, 14–23. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.Y.; Bai, Y.X.; Gao, X.F.; Li, X.L.; Sun, K.; Xie, H.J.; Yang, G.H.; Han, Y.Z.; Tan, Y.S. Effect of the promoter and support on cobalt-based catalysts for higher alcohols synthesis through CO hydrogenation. Fuel 2017, 195, 69–81. [Google Scholar] [CrossRef]
- Sun, J.; Cai, Q.X.; Wan, Y.; Wan, S.L.; Wang, L.; Lin, J.D.; Mei, D.H.; Wang, Y. Promotional effects of cesium promoter on higher alcohol synthesis from syngas over cesium-promoted Cu/ZnO/Al2O3 catalysts. ACS Catal. 2016, 6, 5771–5785. [Google Scholar] [CrossRef]
- Liu, G.L.; Niu, T.; Cao, A.; Geng, Y.X.; Zhang, Y.; Liu, Y. The deactivation of Cu-Co alloy nanoparticles supported on ZrO2 for higher alcohols synthesis from syngas. Fuel 2016, 176, 1–10. [Google Scholar] [CrossRef]
- Gupta, M.; Smith, M.L.; Spivey, J.J. Heterogeneous catalytic conversion of dry syngas to ethanol and higher alcohols on Cu-based catalysts. ACS Catal. 2011, 1, 641–656. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, Q.D.; Xie, H.J.; Gao, X.F.; Wu, Y.Q.; Yang, G.H.; Wang, P.; Tian, S.P.; Tan, Y.S. Facile preparation of Cu-Al oxide catalysts and their application in the direct synthesis of ethanol from syngas. Chem. Select 2017, 2, 10365–10370. [Google Scholar] [CrossRef]
- Li, X.L.; Xie, H.J.; Gao, X.F.; Wu, Y.Q.; Wang, P.; Tian, S.P.; Zhang, T.; Tan, Y.S. Effects of calcination temperature on structure-activity of K-ZrO2/Cu/Al2O3 catalysts for ethanol and isobutanol synthesis from CO hydrogenation. Fuel 2018, 227, 199–207. [Google Scholar] [CrossRef]
- Sun, K.; Gao, X.F.; Bai, Y.X.; Tan, M.H.; Yang, G.H.; Tan, Y.S. Synergetic catalysis of bimetallic copper-cobalt nanosheets for direct synthesis of ethanol and higher alcohols from syngas. Catal. Sci. Technol. 2018, 8, 3936–3947. [Google Scholar] [CrossRef]
- Liakakou, E.T.; Heracleous, E.; Triantafyllidis, K.S.; Lemonidou, A.A. K-promoted NiMo catalysts supported on activated carbon for the hydrogenation reaction of CO to higher alcohols: Effect of support and active metal. Appl. Catal. B Environ. 2015, 165, 296–305. [Google Scholar] [CrossRef]
- Wang, J.J.; Chernavskii, P.A.; Khodakov, A.Y. Influence of the support and promotion on the structure and catalytic performance of copper-cobalt catalysts for carbon monoxide hydrogenation. Fuel 2013, 103, 1111–1122. [Google Scholar] [CrossRef]
- Wang, J.J.; Chernavskii, P.A.; Khodakov, A.Y.; Wang, Y. Structure and catalytic performance of alumina-supported copper–cobalt catalysts for carbon monoxide hydrogenation. J. Catal. 2012, 286, 51–61. [Google Scholar] [CrossRef]
- Ding, J.; Popa, T.; Tang, J.; Gasem, K.A.M.; Fan, M.; Zhong, Q. Highly selective and stable Cu/SiO2 catalysts prepared with a green method for hydrogenation of diethyl oxalate into ethylene glycol. Appl. Catal. B Environ. 2017, 209, 530–542. [Google Scholar] [CrossRef]
- Lee, J.H.; Reddy, K.H.; Jung, J.S.; Yang, E.; Moon, D.J. Role of support on higher alcohol synthesis from syngas. Appl. Catal. A Gen. 2014, 480, 128–133. [Google Scholar] [CrossRef]
- Schumann, J.; Eichelbaum, M.; Lunkenbein, T.; Thomas, N.; Álvarez Galván, M.C.; Schlögl, R.; Behrens, M. Promoting strong metal support interaction: Doping ZnO for enhanced activity of Cu/ZnO:M (M = Al, Ga, Mg) catalysts. ACS Catal. 2015, 5, 3260–3270. [Google Scholar] [CrossRef]
- Schneider, J.; Struve, M.; Trommler, U.; Schlüter, M.; Seidel, L.; Dietrich, S.; Rönsch, S. Performance of supported and unsupported Fe and Co catalysts for the direct synthesis of light alkenes from synthesis gas. Fuel Process. Technol. 2018, 170, 64–78. [Google Scholar] [CrossRef]
- Kim, T.W.; Kleitz, F.; Jun, J.W.; Chae, H.J.; Kim, C.U. Catalytic conversion of syngas to higher alcohols over mesoporous perovskite catalysts. J. Ind. And Eng. Chem. 2017, 51, 196–205. [Google Scholar] [CrossRef]
- Liu, Y.J.; Deng, X.; Han, P.D.; Huang, W. CO hydrogenation to higher alcohols over CuZnAl catalysts without promoters: Effect of pH value in catalyst preparation. Fuel Process. Technol. 2017, 167, 575–581. [Google Scholar] [CrossRef]
- París, R.S.; Montes, V.; Boutonnet, M.; Järås, S. Higher alcohol synthesis over nickel-modified alkali-doped molybdenum sulfide catalysts prepared by conventional coprecipitation and coprecipitation in microemulsions. Catal. Today 2015, 258, 294–303. [Google Scholar] [CrossRef]
- Song, Z.Y.; Shi, X.P.; Ning, H.Y.; Liu, G.L.; Zhong, H.X.; Liu, Y. Loading clusters composed of nanoparticles on ZrO2 support via a perovskite-type oxide of La0.95Ce0.05Co0.7Cu0.3O3 for ethanol synthesis from syngas and its structure variation with reaction time. Appl. Surf. Sci. 2017, 405, 1–12. [Google Scholar] [CrossRef]
- Choi, S.M.; Kang, Y.J.; Kim, S.W. Effect of γ-alumina nanorods on CO hydrogenation to higher alcohols over lithium-promoted CuZn-based catalysts. Appl. Catal. A Gen. 2018, 549, 188–196. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, H.Y.; Gao, P.; Li, X.P.; Zhang, J.M.; Liu, H.J.; Wang, H.; Wei, W.; Sun, Y.H. Slurry methanol synthesis from CO2 hydrogenation over micro-spherical SiO2 support Cu/ZnO catalysts. J. CO2 Util. 2018, 26, 642–651. [Google Scholar] [CrossRef]
- Su, J.J.; Zhang, Z.P.; Fu, D.L.; Liu, D.; Xu, X.C.; Shi, B.F.; Wang, X.; Si, R.; Jiang, Z.; Xu, J.; Han, Y.F. Higher alcohols synthesis from syngas over CoCu/SiO2 catalysts: Dynamic structure and the role of Cu. J. Catal. 2016, 336, 94–106. [Google Scholar] [CrossRef]
- Huang, X.M.; Ma, M.; Miao, S.; Zheng, Y.P.; Chen, M.S.; Shen, W.J. Hydrogenation of methyl acetate to ethanol over a highly stable Cu/SiO2 catalyst: Reaction mechanism and structural evolution. Appl. Catal. A Gen. 2017, 531, 79–88. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Yang, J.X.; Sun, M.L.; Li, F.; Li, B.; Yao, Y.G. A new low-cost and effective method for enhancing the catalytic performance of Cu-SiO2 catalysts for the synthesis of ethylene glycol via the vapor-phase hydrogenation of dimethyl oxalate by coating the catalysts with dextrin. J. Catal. 2017, 350, 122–132. [Google Scholar] [CrossRef]
- Li, X.L.; Yang, G.H.; Zhang, M.; Gao, X.F.; Xie, H.J.; Bai, Y.X.; Wu, Y.Q.; Pan, J.X.; Tan, Y.S. Insight into the correlation between Cu species evolution and ethanol selectivity in the direct ethanol synthesis from CO hydrogenation. ChemCatChem 2018, 11, 1123–1130. [Google Scholar] [CrossRef]
- Cosultchi, A.; Pérez-Luna, M.; Morales-Serna, J.A.; Salmón, M. Characterization of modified Fischer-Tropsch catalysts promoted with alkaline metals for higher alcohol synthesis. Catal. Lett. 2012, 142, 368–377. [Google Scholar] [CrossRef]
- Tan, L.; Yang, G.H.; Yoneyama, Y.; Kou, Y.L.; Tan, Y.S.; Vitidsant, T. Iso-butanol direct synthesis from syngas over the alkali metals modified Cr/ZnO catalysts. Appl. Catal. A Gen. 2015, 505, 141–149. [Google Scholar] [CrossRef]
- Sun, J.; Wan, S.; Wang, F.; Lin, J.D.; Wang, Y. Selective synthesis of methanol and higher alcohols over Cs/Cu/ZnO/Al2O3 catalysts. Ind. Eng. Chem. Res. 2015, 54, 7841–7851. [Google Scholar] [CrossRef]
- Anton, J.; Nebel, J.; Song, H.Q.; Froese, C.; Weide, P.; Ruland, H.; Muhler, M.; Kaluza, S. The effect of sodium on the structure-activity relationships of cobalt-modified Cu/ZnO/Al2O3 catalysts applied in the hydrogenation of carbon monoxide to higher alcohols. J. Catal. 2016, 335, 175–186. [Google Scholar] [CrossRef]
- Tien-Thao, N.; Zahedi-Niaki, M.H.; Alamdari, H.; Kaliaguine, S. Effect of alkali additives over nanocrystalline Co-Cu-based perovskites as catalysts for higher-alcohol synthesis. J. Catal. 2007, 245, 348–357. [Google Scholar] [CrossRef]
- Lopez, L.; Montes, V.; Kusar, H.; Cabrera, S.; Boutonnet, M.; Järås, S. Syngas conversion to ethanol over a mesoporous Cu/MCM-41 catalyst: Effect of K and Fe promoters. Appl. Catal. A Gen. 2016, 526, 77–83. [Google Scholar] [CrossRef]
- Yang, Q. Synthesis of γ-Al2O3 nanowires through a boehmite precursor route. Bull. Mater. Sci. 2011, 34, 239. [Google Scholar] [CrossRef]
- Kato, S.; Fujimaki, R.; Ogasawara, M.; Wakabayashi, T.; Nakahara, Y.; Nakata, S. Oxygen storage capacity of CuMO2 (M = Al, Fe, Mn, Ga) with a delafossite-type structure. Appl. Catal. B Environ. 2009, 89, 183–188. [Google Scholar] [CrossRef]
- Tada, S.H.; Watanabe, F.; Kiyota, K.; Shimoda, N.; Hayashi, R.; Takahashi, M.; Nariyuki, A.; Igarashi, A.; Satokawa, S. Ag addition to CuO-ZrO2 catalysts promotes methanol synthesis via CO2 hydrogenation. J. Catal. 2017, 351, 107–118. [Google Scholar] [CrossRef]
- Li, M.M.J.; Zeng, Z.Y.; Liao, F.L.; Hong, X.L.; Tsang, S.C.E. Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts. J. Catal. 2016, 343, 157–167. [Google Scholar] [CrossRef]
- Wang, B.; Cui, Y.Y.; Wen, C.; Chen, X.; Dong, Y.; Dai, W.L. Role of copper content and calcination temperature in the structural evolution and catalytic performance of Cu/P25 catalysts in the selective hydrogenation of dimethyl oxalate. Appl. Catal. A Gen. 2016, 509, 66–74. [Google Scholar] [CrossRef]
- Soled, S. Silica-supported catalysts get a new breath of life. Science 2015, 350, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Ding, J.; Bi, J.C.; Sun, Y.P.; Zhang, J.; Liu, K.F.; Kong, F.H.; Xiao, H.C.; Chen, J.G. Effect of Cu-doping on the structure and performance of molybdenum carbide catalyst for low-temperature hydrogenation of dimethyl oxalate to ethanol. Appl. Catal. A Gen. 2017, 529, 143–155. [Google Scholar] [CrossRef]
- Kwak, G.; Woo, M.H.; Kang, S.C.; Park, H.G.; Lee, Y.J.; Jun, K.W.; Ha, K.S. In situ monitoring during the transition of cobalt carbide to metal state and its application as Fischer-Tropsch catalyst in slurry phase. J. Catal. 2013, 307, 27–36. [Google Scholar] [CrossRef]
- Claure, M.T.; Chai, S.H.; Dai, S.; Unocic, K.A.; Alamgir, F.M.; Agrawal, P.K.; Jones, C.W. Tuning of higher alcohol selectivity and productivity in CO hydrogenation reactions over K/MoS2 domains supported on mesoporous activated carbon and mixed MgAl oxide. J. Catal. 2015, 324, 88–97. [Google Scholar] [CrossRef]
- Tian, S.P.; Wang, S.C.; Wu, Y.Q.; Gao, J.W.; Wang, P.; Xie, H.J.; Yang, G.H.; Han, Y.Z.; Tan, Y.S. The role of potassium promoter in isobutanol synthesis over Zn-Cr based catalysts. Catal. Sci. Technol. 2016, 6, 4105–4115. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, X.; Wang, B. Insight into the Preference Mechanism of CHx (x = 1-3) and C-C Chain Formation Involved in C2 Oxygenate Formation from Syngas on the Cu (110) Surface. J. Phys. Chem. C 2013, 117, 6594–6606. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, G.; Wang, B. Insights into the mechanism of ethanol formation from syngas on Cu and an expanded prediction of improved Cu-based catalyst. J. Catal. 2013, 305, 238–255. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, M.; Wang, X.X.; Zhang, Q.D.; Song, F.E.; Tan, Y.S.; Han, Y.Z. Direct synthesis of isobutyraldehyde from methanol and ethanol on Cu-Mg/Ti-SBA-15 catalysts: the role of Ti. New J. Chem. 2017, 41, 9639–9648. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wu, Y.Q.; Li, L.; Wang, X.X.; Zhang, Q.D.; Zhang, T.; Tan, Y.S.; Han, Y.Z. Ti-SBA-15 supported Cu-MgO catalyst for synthesis of isobutyraldehyde from methanol and ethanol. RSC Adv. 2016, 6, 85940–85950. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Xie, H.J.; Tian, S.P.; Tsubaki, N.; Han, Y.Z.; Tan, Y.S. Isobutanol synthesis from syngas over K-Cu/ZrO2-La2O3(x) catalysts: effect of La-loading. J. Mol. Catal. A Chem. 2015, 396, 254–260. [Google Scholar] [CrossRef]
- Heracleous, E.; Liakakou, E.T.; Lappas, A.A.; Lemonidou, A.A. Investigation of K-promoted Cu-Zn-Al, Cu-X-Al and Cu-Zn-X (X= Cr, Mn) catalysts for carbon monoxide hydrogenation to higher alcohols. Appl. Catal. A Gen. 2013, 455, 145–154. [Google Scholar] [CrossRef]






| Catalyst | SBET (m2/g) | VPore (cm3/g) | dPore (nm) |
|---|---|---|---|
| Al2O3 | 157 | 0.43 | 10.8 |
| Al2O3-900 | 87.0 | 0.39 | 18.1 |
| Cu/Al2O3 | 41.6 | 0.20 | 19.1 |
| K-Cu/Al2O3 | 40.7 | 0.18 | 17.8 |
| SiO2 | 160 | 0.54 | 13.7 |
| SiO2-900 | 25.8 | 0.07 | 10.6 |
| Cu/SiO2 | 6.69 | 0.008 | 5.26 |
| K-Cu/SiO2 | 4.63 | 0.006 | 5.66 |
| Samples | CO Conversion (%) | STY (mg/mlcath) | Carbon Selectivity (%) | Alcohol Distribution (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2–5 | CO2 | ROH | MeOH | EtOH | PrOH | BuOH | C5+OH | |||
| Cu/Al2O3 | 84.6 | 93.7 | 42.2 | 27.1 | 23.0 | 7.7 | 44.0 | 42.5 | 8.0 | 4.6 | 0.9 |
| K-Cu/Al2O3 | 48.5 | 141.4 | 23.2 | 19.5 | 32.5 | 24.1 | 34.9 | 38.3 | 16.2 | 8.5 | 2.0 |
| Cu/SiO2 | 18.2 | 49.5 | 42.3 | 28.3 | 2.4 | 26.7 | 59.8 | 34.6 | 4.5 | 1.1 | 0.1 |
| K-Cu/SiO2 | 16.8 | 55.0 | 27.4 | 27.7 | 16.1 | 28.8 | 51.8 | 32.6 | 9.8 | 4.4 | 1.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, J.; Zhang, M.; Zhang, W.; Zhang, M.; Xie, H.; Wu, Y.; Tan, Y. The Support Effects on the Direct Conversion of Syngas to Higher Alcohol Synthesis over Copper-Based Catalysts. Catalysts 2019, 9, 199. https://doi.org/10.3390/catal9020199
Li X, Zhang J, Zhang M, Zhang W, Zhang M, Xie H, Wu Y, Tan Y. The Support Effects on the Direct Conversion of Syngas to Higher Alcohol Synthesis over Copper-Based Catalysts. Catalysts. 2019; 9(2):199. https://doi.org/10.3390/catal9020199
Chicago/Turabian StyleLi, Xiaoli, Junfeng Zhang, Min Zhang, Wei Zhang, Meng Zhang, Hongjuan Xie, Yingquan Wu, and Yisheng Tan. 2019. "The Support Effects on the Direct Conversion of Syngas to Higher Alcohol Synthesis over Copper-Based Catalysts" Catalysts 9, no. 2: 199. https://doi.org/10.3390/catal9020199