1. Introduction
Biofuels will have a significant role in the energetic matrix of the low-carbon economy, helping to meet the goals established at Conference of the Parties (COP 21) [
1,
2]. Among biofuels, bioethanol production from lignocellulosic materials has been intensively studied once it was shown that these byproducts had high availability, had a low cost, and did not compete with the production of food [
3]. Lignocellulosic raw materials are mainly composed of cellulose and hemicellulose (up to 70%), which are polysaccharides that, after a hydrolysis step, generate fermentable sugars, mostly xylose from hemicellulose and glucose from cellulose [
4]. The use of these two polysaccharides is important for the economic feasibility of the biofuel production process.
Some microorganisms that naturally ferment pentoses to ethanol have been tested for industrial use, such as
Scheffersomyces stipitis and
Pachysolen tannophilus [
5,
6]. However, these microorganisms have a low tolerance to ethanol and slow fermentation rates and are inhibited by compounds generated during the biomass pretreatment step, such as furfural [
7].
Saccharomyces cerevisiae is the most common microorganism used for ethanol production from hexoses, due to its high rate of fermentation and superior ethanol yield. In addition, this yeast exhibits unbeatable tolerance to ethanol, to inhibitors, and to high concentrations of sugar [
8,
9]. However, in its wild form
S. cerevisiae is unable to efficiently metabolize D-xylose.
The genetic modification of
S. cerevisiae aimed at xylose fermentation has been extensively studied [
8,
10,
11,
12]. However, the low specific growth rate, high xylitol production, reduced yeast tolerance, and possible genetic instability are still hindrances for the application of recombinant strains on an industrial scale [
7].
In spite of the inability of
S. cerevisiae to metabolize xylose, it is capable of fermenting its isomer, xylulose, to ethanol. Hence, an alternative for the utilization of the hemicellulose fraction for bioethanol production would be to isomerize xylose to xylulose ex vivo, followed by fermentation by
S. cerevisiae [
7]. The enzyme xylose isomerase (XI) (EC 5.3.1.5) is widely used in the industry for the production of fructose syrup from corn starch and also catalyzes the reversible isomerization of xylose to xylulose [
13]. Although the xylose/xylulose chemical equilibrium is unfavorable (3.5:1 at 60 °C) [
14], the reaction can be displaced by the simultaneous isomerization and fermentation (SIF) process, where the continuous conversion of xylulose to ethanol might allow the complete depletion of the available xylose [
15].
The use of catalysts with immobilized enzymes may be crucial for the application of multi-enzymatic processes on an industrial scale. This approach allows the continuous operation of the reactor and facilitates the product recovery as well as the use of high loads of cells and enzymes [
16]. The production of an active and stable enzyme derivative using a non-expensive support is also an important issue in enzyme immobilization [
17]. The literature reports successful applications of immobilized enzymes on an industrial scale [
18,
19] and immobilized XI is one of the most successful and established examples [
13]. Silva et al. [
7] developed a biocatalyst containing chitosan-immobilized XI, co-immobilized with
S. cerevisiae in calcium alginate gel. Calcium alginate gel was chosen for being a natural polymer widely studied as a support for the immobilization of viable cells [
20]. However, this system showed to be susceptible to contamination by xylose-consuming bacteria. High concentrations of xylose in the medium disfavored the
S. cerevisiae population, due to its low uptake rates of xylulose.
An alternative to tackling the contamination problem is to use a cultivation medium containing non-readily fermentable substrates, such as xylo-oligomers obtained by the solubilization of hemicellulose under mild conditions [
21]. The hetero-polysaccharides that compose hemicellulose are polymers with about 100 units of monomers, mainly xylose, and their solubility depends on the number of monomeric units in the chain [
22]. Thus, the extraction of xylan in the form of large oligomers must be carried out under conditions that allow a sufficient number of glycosidic bonds to be broken, so that soluble polymers with lower molecular weight (xylo-oligomers) are released.
Xylanases (β-1,4-D-xylanase) are enzymes that catalyze the hydrolysis of the glycosidic bonds between xylose units. The enzymatic complex is commonly composed of endoxylanases, exoxylanases, β-D-xylosidases as well as accessory enzymes such as glucuronidase and arabinofuranosidase that act on the ramifications of the xylan chain [
23]. The addition of these enzymes to the biocatalyst proposed by Silva et al. [
7] would allow the feeding of xylo-oligomers to the bioreactor. This substrate might decrease the probability of contamination during the operation of the bioreactor for long periods, which are typical in industry. Preliminary results showed the technical viability of this process [
24].
The development of viable processes to increase ethanol yields from lignocellulosic materials is crucial, despite the challenges that still remain for the production of 2G ethanol from xylose. Considering the higher production cost of 2G ethanol (compared to 1G ethanol), the use of pentoses as raw material could make its production more profitable and might overcome the costs of 2G ethanol extra steps [
25]. The integration of several biocatalytic transformations in a multi-enzymatic cascade system is particularly appealing to the development of cleaner and more efficient biochemical processes. Multi-enzymatic cascade reactions offer advantages such as lower demand of time, reduced costs, easier recovery of products, completion of reversible reactions as well as concentrations of inhibitory compounds restrained to a minimum [
26].
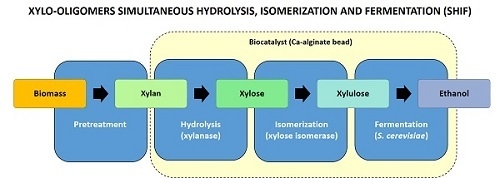
In this context, the simultaneous hydrolysis, isomerization, and fermentation (SHIF) process stands out for 2G ethanol production since unmodified
S. cerevisiae remains the preferred microorganism in industry, due to its robustness, high ethanol tolerance, and production rates. The use of wild strains to produce ethanol from xylose is an important issue in countries like Brazil, where biosafety regulations are strict [
15]. In addition, one advantage of this approach is that XI, along with amylases and proteases, is among the most widely and cheaply available commercial enzymes [
27]. The present work reports the results of using this new biocatalyst for the simultaneous hydrolysis, isomerization, and fermentation of xylan derived from the hemicellulose fraction of biomass, aimed at the production of ethanol (
Figure 1).
2. Results
2.1. Application of the New Biocatalyst in the SHIF Process
The biocatalyst is designed for the industrial production of second-generation ethanol in continuous, fixed-bed reactors through long-term operation, by applying simultaneous hydrolysis, isomerization, and fermentation (SHIF) of the hemicellulosic fraction of biomass. First, xylanase and xylose isomerase were covalently immobilized on chitosan. The obtained XI derivative presented an activity of 252.5 ± 1.6 IU/g (immobilization yield of 93% and recovered activity of 91%), whereas the Accellerase derivative exhibited 346.3 ± 9.2 IU/g (immobilization yield of 54% and recovered activity of 12%). Both derivatives were co-encapsulated with S. cerevisiae in Ca-alginate gel and this biocatalyst was used to produce ethanol from commercial birchwood xylan.
Birchwood xylan, which is a heteropolymer composed of long chains, was first hydrolyzed to smaller xylo-oligomers by the action of recombinant endoxylanase of
Bacillus subtilis (XynA) in order to increase the concentration of xylo-oligomers with smaller chains that may diffuse into the catalyst beads [
28]. This step was carried out to make xylan more similar to lignocellulosic hydrolysates obtained from the pretreatment of biomass (data not shown). The composition of the substrate after xylan pre-hydrolysis is shown in
Table 1.
As expected, there was no xylose production since XynA is a strict endoxylanase [
28]. The solubilized fraction corresponded to 67% (w/w) of the offered xylan. According to Gray et al. [
22], the solubility of the xylan oligomers depends on the degree of polymerization of each compound. Under the used conditions, 33% (w/w) of birchwood xylan is insoluble. Therefore, the substrate obtained for the SHIF process had 73 g/L of xylo-oligomers.
For the SHIF assays, the offered enzyme activity in the reactor was 1.7 × 10
4 IU/L
reactor for xylanase (Accellerase XY) and 3.7 × 10
4 IU/L
reactor of xylose isomerase. Accellerase XY was used due to the presence of β-xylosidase, which is necessary to xylose formation. Commercial baker’s yeast (Itaiquara
®) concentration was 50 g
dry mass/L
reactor at the beginning of the SHIF assays. Results in
Figure 2 show the production of ethanol through SHIF using the developed biocatalyst. Ethanol production was higher compared to xylitol, presenting a selectivity of 2.61 (2.2 g/L ethanol and 0.84 g/L xylitol). Ethanol productivity of 0.092 g/L/h and yield (Y
P/S) of 0.160 g
ethanol/g
potentialxylose (32% of theoretical, calculated on the basis of potential xylose in the xylan) were achieved at the end of the SHIF run.
Figure 2 shows that ethanol is produced from xylo-oligomers. The slower velocity of SHIF compared to the control experiment (where the substrate is xylose, resulting in a simultaneous isomerization, and fermentation (SIF) process) was expected since xylose concentration in SHIF depends on the velocity of hydrolysis of the xylo-oligomers. However, a decrease in the xylose consumption was observed after eight hours of SHIF (
Figure 2a), which indicates that the xylose isomerization was impaired. There are two possible reasons for this occurrence: XI is not catalyzing isomerization of xylose in the required velocity; or the yeast is not consuming the generated xylulose, which would be accumulating and consequently stopping the isomerization due to the xylose:xylulose equilibrium ratio. Since the yeast remained viable (initial and final cell viability unchanged: 96%) and there was no accumulation of xylulose in the medium, the accumulation of xylose seemed to be related to the isomerization step.
In the control experiment, using only xylose as substrate, XI catalyzed the isomerization reaction effectively, almost until depletion of the pentose (60 g/L of xylose were consumed in 12 h, a productivity of 1.2 g/L/h, with Y
P/S of 0.303 g/g and selectivity of 2.3 with respect to xylitol). Thus, an increase of the isomerization velocity must be sought. Some of the possible causes for the hindrance of the isomerization step were then investigated. High calcium concentrations are known to inhibit XI action [
29], and the hydrolysis reaction could be demanding a higher release of the calcium ion (increased hydrolysis of CaCO
3 to control the pH). However, even with a higher release of calcium, the results shown in
Figure 2 indicate that the hydrolysis step led to a drop of pH to approximately 5.1. XI shows maximum activity at pH 8, being highly sensitive to a drop of pH to this range [
15]. On the other hand, it is known that the magnesium ion is an important cofactor for XI, as an activator of this enzyme [
30]. The influence of pH and Ca
2+ and Mg
2+ ions in the isomerization step was then investigated.
2.2. Influence of pH, Ca2+, and Mg2+ on XI Activity
In order to investigate the influence of calcium and magnesium ions on XI, the activity of this enzyme to catalyze fructose–glucose isomerization was measured at different pHs (5.0 to 8.0) in the presence of different concentrations of Ca
2+ and Mg
2+ ions. The standard medium for assessing activity was 2 M fructose, pH 8.0 (50 mM tris-maleate buffer) supplemented with 50 mM of MgSO
4 and 2.5 mM of CoCl
2 at 60 °C. Both Co
2+ and Mg
2+ are essential for the activity of XI, however they play differentiated roles. Mg
2+ is superior to Co
2+ as an activator, while the latter is responsible for the stabilization of the enzyme and maintenance of its conformation, especially the quaternary structure [
31].
Table 2 shows the measured activities of XI in each condition studied, referred to the test performed with the standard medium at pH 8.0 as 100%.
The Ca2+ ion proved to be an inhibitor of this enzyme, since a significant decrease in the XI activity occurred when the Ca2+ concentration in the medium increased. The Mg2+ ion, in turn, was able to activate the enzyme, increasing its activity in 16.2% at pH 8.0 and 163% at pH 5.0, both in calcium-free medium. This cofactor was still able to bypass the inhibition caused by calcium, since it reactivated the enzyme in the presence of this ion, increasing its catalytic activity in all studied pHs.
Xylose isomerization catalyzed by XI is initiated by opening the sugar ring, followed by isomerization through the exchange of hydride and finally stabilization of the product by ring closure [
32]. Although there is no relationship between the presence of magnesium and the ring opening step, this cation is essential for the isomerization [
33]. According to Kasumi et al. [
34], the reaction mechanism demands the formation of a binary divalent enzyme–cation complex, since the substrate will bind only to the active site of this complex. Xylose isomerase has two active sites, each containing two divalent cations [
33]. Thus, the presence of higher concentrations of magnesium in the reaction medium, improving the probability of the presence of this ion in the active sites of the enzyme, would increase the rates of the isomerization reaction.
Although calcium is a divalent cation and belongs to the same family as magnesium (same configuration in the valence layer) in the periodic table, Ca2+ has a larger ionic radius than Mg2+. This fact could be the reason why Mg2+ is an activator of the enzyme while Ca2+ inhibits XI, that is, the difference in their atomic radii would cause a different interaction with the active site of the enzyme.
In addition to the significant influence of Ca
2+ and Mg
2+, XI showed great sensitivity to pH, losing activity significantly at pH 5.0. The sensitivity of XI to pH was previously observed by Milessi et al. [
15], who emphasized the importance of pH control during the simultaneous isomerization and fermentation (SIF) of xylose. However, the activation provided by Mg
2+ is potentiated at lower pHs. When Ca
2+ (4 g/L) was added, the magnesium ion was able to recover XI activity more effectively at pH 5.0 than at pH 8.0 (
Table 2, media 4 and 7).
Data presented in
Table 2 also prove that XI activity was greatly reduced at the SHIF pH range (5.0 to 6.0). The quaternary structure of this enzyme is composed of four subunits that are delicately folded and associated with noncovalent links and without interchain disulfide bonds [
31,
32]. At pH 8.0, the enzyme structure is composed of all four subunits combined, resulting in the maximum catalytic activity. However, as the pH of the reaction medium lowers, the enzyme is more likely to suffer structure distortions, unfolding and dissociating its tetrameric structure. The presence of Ca
2+ ions at the low pH of the reaction medium results in a combined effect, acting both on the 3D structure and on the active site. Therefore, the performance of the SHIF process will certainly benefit from a pH control system (pH 5.6). Unfortunately, the substitution of calcium chloride by magnesium chloride in the coagulation solution during sodium alginate gelation was not possible since the resulting beads were not stable. For this reason, SHIF experiments with pH control and excess of magnesium were run, in order to minimize the inhibition of XI caused by the hydrolysis reactions, which release acids from the structures of the xylo-oligomers [
35].
2.3. Xylanase Selection
Xylan hydrolysis has to occur efficiently to enable the cascade SHIF process. Hydrolysis cannot be the rate-determining step of these reactions in series: due to the unfavorable equilibrium of xylose to xylulose isomerization, the supply of xylose must not control the reaction [
7]. Considering that the composition of each xylanase complex influences the hydrolysis efficiency, different xylanases were evaluated with the purpose of selecting the most efficient for the SHIF process.
The xylanase family is strongly related to the profile of products generated in the process [
36]. A xylanase capable of depolymerizing xylan into xylose efficiently is required to ensure that the SHIF process will proceed as expected. Thus, besides Accellerase XY A03304, used in previous SHIF tests, two additional xylanases were evaluated: recombinant
B. subtilis endoxylanase (XynA) and Multifect CX XL A03139.Hydrolysis profiles and xylooligosaccharide (XOS) composition are reported in
Table 3 and
Figure 3, respectively.
Figure 3 shows that Multifect stands out, with a xylan conversion of 78.7%. Moreover, the higher xylose concentration achieved with this enzyme at the end of the experiments indicates that this xylanase complex has a more stable β-xylosidase enzyme, responsible for catalyzing the hydrolysis of xylobiose, the essential final step for the complete xylan hydrolysis. Hence, this xylanase seems to be the most suitable for the production of xylose in the SHIF process, among the studied enzymes.
Accellerase has the highest enzymatic activity under standard conditions. However, in long-term reaction it was able to convert only 58.8% of the available xylan. Several factors may have contributed to Accellerase’s inferior performance, such as the amount of each enzyme in the complex, thermal inactivation, substrate affinity, and inhibitory effects.
XynA was already known to have a strictly endoxylanase action, lacking β-xylosidase activity and consequently not producing xylose when hydrolyzing xylan [
28]. Accordingly, it presented the lowest conversion (44.8%), probably due to the absence of debranching enzymes.
None of the tested xylanases reached 100% of xylan conversion. Indeed, the incapacity of xylanases to completely hydrolyze xylan has been previously reported. Akpinar et al. [
37] observed a yield of 13.8% for tobacco xylan using
Aspergillus niger xylanase (200 IU/g) at 50 °C after 24 h. Aragon et al. [
38] achieved 13% of conversion in the hydrolysis of birch xylan (18 g/L) using
Aspergillus versicolor endoxylanase immobilized on agarose-glyoxyl at 25 °C and pH 5.0. In fact, since xylan is not a linear polymer of pure xylose, its complete depolymerization requires the use of a varied pool of enzymes [
21,
23,
39]. In this context, the xylanase Multifect CX XL A03139 was selected to be co-immobilized with XI and
S. cerevisiae in the SHIF process.
2.4. SHIF Assay with pH Control and Excess of Mg2+
In order to overcome the possible inhibition of Ca
2+ in XI activity, a SHIF assay was performed with pH control and excess of magnesium. Beads without CaCO
3 in its composition were prepared, since this salt is only necessary to sustain the pH at the desired range. Moreover, an isomerization free of CaCO
3 would contribute to reduce the undesired Ca
2+ effects. After all these modifications, the obtained derivative of xylanase Multifect presented 330.2 ± 8.1 IU/g (immobilization yield of 96% and recovered activity of 77%). For the SHIF experiment, the medium supplemented with 100 mM MgSO
4 (24.6 g/L) and 4 g/L of CaCl
2 (to maintain the integrity of the beads) was added together with the beads to the pH-stat stirred reactor. It is important to note that the moderate agitation used during the process did not affect the integrity of the beads. According to Rahim et al. [
40], damages to Ca-alginate immobilized biocatalysts due to stirring are usually observed above 200 rpm. In fact, Carvalho et al. [
41], in experiments carried out at 300 rpm, noticed a 30% reduction in the size of Ca-alginate beads during experiments with immobilized
Candida guilliermondii. Accordingly, in the present work, an agitation of 150 rpm was employed and the structural characteristics of the biocatalyst beads were preserved.
The obtained results, showed in
Figure 4, indicated a higher ethanol production in SHIF using pH control and excess of Mg
2+ (3.1 g/L of ethanol) compared to the value of 2.2 g/L, which was achieved under the original SHIF conditions (
Figure 2). Ethanol yield (0.39 g/g, 76% of the theoretical), selectivity (3.12), and productivity (0.26 g/L/h) were also improved.
To the best of our knowledge, these are the highest yield and productivity reported in the literature for ethanol production from xylan through the simultaneous hydrolysis, isomerization, and fermentation (SHIF) process. Only a few works have studied ethanol production from pentoses using ex vivo isomerization and native
S. cerevisiae, due to the differences in optimal pH and temperature ranges for each step (and none of them with co-immobilized enzymes/cells). The inclusion of the xylan hydrolysis step should not be a problem in relation to the temperature and pH of the process. Xylanases have the highest catalytic pH and temperature at approximately 5.5 and 50 °C, respectively, whereas XI optimal conditions are pH 7.0–8.0 and 70 °C [
7]. Alcoholic fermentation, on the other hand, operates at pH 5.0 and 30 °C. Due to these facts, the process integration for ethanol production is still a challenge. Nakata et al. [
42] studied ethanol production of hot-compressed water pretreated Japanese beech using soluble β-xylosidase, XI, and
S. cerevisiae. It should be stressed that in this work the absence of exo- and endoxylanases would be a restraint to the saccharification step. The best results reported (0.62 g/L of ethanol, corresponding to 13% of theoretical yield) were achieved at pH 5.0, 30 °C after 100 h. Hence, the immobilized biocatalyst containing enzymes and yeast co-encapsulated reported in the present work was significantly more efficient than using enzymes and microorganisms in their soluble form, leading to a better yield and productivity.
Although there are only a few studies addressing the SHIF of xylan, the simultaneous isomerization and fermentation (SIF) of xylose has been more frequently reported. Rao et al. [
27] studied the xylose SIF in the presence of 0.05 M borax to shift equilibrium concentration of xylulose/xylose and improve the isomerization step. However, although the isomerization was enhanced, only half of the available xylose was consumed. Lastick et al. [
43] observed an ethanol titer of 2.1% (w/v) from the SIF of 6% xylose using XI and
Schizosaccharomyces pombe (Y-164). Silva et al. [
7] studied the SIF of 65 g/L of xylose at 30 °C, using a biocatalyst containing 32.5 x 10
3 IU/L of xylose isomerase and 20 g/L of yeast co-immobilized in Ca-alginate gel, and reported an ethanol productivity of 0.25 g/L/h. However, the isomerization step became a limiting factor, due to the decrease of the pH from 5.3 (initial) to 4.8 (final). Milessi et al. [
15] incorporated CaCO
3 into SIF beads to control the pH of the process. The biocatalysts were prepared with 20% chitosan-immobilized XI and 10% fresh yeast. An ethanol yield of 0.35 g/g (70% of the theoretical yield) and 2 g/L/h productivity was observed. However, the long time needed by
S. cerevisiae to ferment xylulose makes the SIF process susceptible to contamination by bacteria capable of metabolizing the xylose.
In this context, the proposed SHIF process appears as a promising approach for 2G ethanol production from hemicellulose. Process conditions and enzyme loads in the biocatalyst can be optimized to achieve higher yields and productivity as well as to overcome the difference between optimal pH ranges for each step of the process. Despite the improvement achieved after pH control and supplementation with excess of Mg2+, a small accumulation of xylose was still observed, which suggests that the isomerization step may be still limiting the process. There are other factors that might be affecting the isomerization and/or the fermentation steps, such as the presence of xylooligosaccharides (2–8 xylose units) or other intermediate products released during the xylan hydrolysis, which are not present in the SIF process. The understanding of the influence of these compounds as well as the optimization of the biocatalyst composition regarding the balance of the enzyme pool are important issues to be addressed in order to improve ethanol production rates.
In general, the SHIF process using co-immobilized enzymes and cells stands out for 2G ethanol production. Besides presenting the advantages of multi-enzymatic cascade reactions, it also enables an easy recovery of the biocatalyst, which could be applied in continuous or repeated batch ethanol production runs using a medium that inhibits contamination. In addition, it builds on the advantage of using the same native yeast, already employed in 1G ethanol industry, which simplifies the operation of the fermentation unit. The low genetic stability of recombinant microorganisms together with the strict Brazilian biosafety regulations for genetically modified organisms (GMO) application in the industrial environment make the 2G ethanol production process based on a native yeast an attractive alternative.
4. Materials and Methods
4.1. Materials
GENSWEET® SGI (3400 IU/mL, 127 mgprotein/mL, DuPont™ Genencor®, Palo Alto, CA, USA), an enzymatic extract of commercial xylose isomerase (EC 5.3.1.5) from Streptomyces rubiginosus, and the commercial enzyme preparations Accellerase XY (3670 IU/mL, 9.8 mgprotein/mL) and Multifect CX XL A03139 (785 IU/mL, 35 mgprotein/mL) were kindly donated by DuPont™ Genencor® (Palo Alto, CA, USA). The Bacillus subtilis recombinant endoxylanase (502 IU/mL, 9.2 mgprotein/mL) was donated by Verdartis (Ribeirão Preto, SP, Brazil). Powdered chitosan (85.2% deacetylation degree) was supplied by Polymar Ind. Ltda (Fortaleza, CE, Brazil) and 25% glutaraldehyde solution was purchased from Vetec (Duque de Caxias, RJ, Brazil). Birchwood and beechwood xylans were from Sigma-Aldrich (St. Louis, MO, USA). A Saccharomyces cerevisiae industrial strain (purchased from Itaiquara®, Tapiratiba, SP, Brazil) was used in all SHIF experiments. All other reagents were of analytical grade.
4.2. Biocatalyst Production
4.2.1. Preparation of Chitosan-Glutaraldehyde Beads
Chitosan gel (2% or 4%, w/v) was prepared as described by Budriene et al. [
44], using the coagulation of the chitosan-acetic acid solution in 0.5 M KOH. Activation of the support was carried out by the addition of glutaraldehyde (5%, v/v) in a suspension of chitosan at pH 7.0 (100 mM phosphate buffer, 1:10 m
support/v
suspension). After 60 min stirring at 25 °C, the support was filtered under vacuum, washed first with distilled water until neutrality, and then with ultrapure water.
4.2.2. Enzyme Immobilization
Xylose isomerase immobilization was carried out onto 2% (w/v) chitosan-glutaraldahyde according to Silva et al. [
7]. The enzyme solution was prepared in 50 mM Tris-maleate buffer (pH 7.0) containing 5 mM MgSO
4.7H
2O and 2.5 mM CoCl
2.6H
2O, in order to provide 50 mg
protein/g
support. The support was added to the enzyme solution at a ratio of 1:10 (w/v). After 20 h of immobilization at 25 °C under 150 rpm stirring, sodium borohydride was added (1 mg/mL) and the suspension was kept under gentle agitation for 30 min in an ice bath [
45]. The derivatives were filtered and washed under vacuum, first with 200 mM Tris-maleate buffer (pH 7.0), then with ultrapure water, and finally with 50 mM Tris-maleate buffer (pH 7.0), in order to remove borohydride and adsorbed enzyme.
Xylanase complexes were immobilized onto 4% (w/v) chitosan-glutaraldehyde according to Milessi et al. [
28]. The immobilizations were performed at pH 7.0 (100 mM phosphate buffer), 25 °C, and under constant stirring. A load of 20 mg
protein/g
support was offered, maintaining 1:10 (w/v) ratio of mass of support to volume of enzymatic solution. After the completion of immobilization, sodium borohydride was added (1 mg/mL) and the reduction reaction proceeded for 30 min at 4 °C. The derivatives were extensively washed with 50 mM citrate buffer pH 4.8 and stored until use.
4.2.3. Biocatalyst Co-immobilization
The biocatalyst preparation was carried out through Ca-alginate gel entrapment according to the methodology described in Silva et al. [
7]. The industrial strain, supplied as freshly compressed yeast cells, was used as purchased, without previous propagation or activation [
46]. A solution of sodium alginate (1% w/v), immobilized XI (15% w/v), immobilized xylanase (5% w/v), and fresh yeast (10% w/v) was gently dropped into a 0.25 M CaCl
2/0.25 M MgCl
2 solution. Spherical particles (Ø = 1–1.5 mm) were produced using a pneumatic extruder [
47]. The procedure was carried out in a laminar flow chamber (Airstream, ESCO, Horsham, PA, USA) and the sodium alginate and coagulation solutions were previously sterilized at 121 °C for 20 min. After immobilization, the beads were cured in a refrigerator for 12–16 h in cure solution (4 g/L of MgSO
4, 10 g/L of KH
2PO
4, 3 g/L of urea, 0.2 g/L of CoCl
2, and 4 g/L of CaCl
2.2H
2O).
4.3. Simultaneous Hydrolysis, Isomerization, and Fermentation (SHIF) of Birchwood Xylan
First, the birchwood xylan substrate was hydrolyzed in smaller xylo-oligomers by the action of recombinant endoxylanase of
B. subtilis (XynA) in order to make xylan more similar to lignocellulosic hydrolysates, since after a pretreatment step the xylo-oligomers present in the medium are smaller than those in commercial xylan [
28]. Xylan pre-hydrolysis was carried out by XynA (150 IU/g
xylan, 3.8 IU/mL) immobilized on chitosan-glutaraldehyde (35.0 ± 0.8 IU/g) for 24 h and 50 °C under 150 rpm stirring. At the end, the immobilized enzyme was recovered by filtration and the dried xylan mass retained on the filter was quantified (xylan insoluble fraction). The pH of the xylan solubilized fraction (SHIF substrate) was adjusted to 5.6 with HCl or NaOH 1M and the medium was sterilized by filtration through a 0.22 µm membrane. SHIF experiments were carried out in a shaker incubator (32 °C and 150 rpm), using sealed tubes with a total reaction volume of 2.4 mL (bead ratio of 1:1, 1.2 g of beads and 1.2 mL of medium, bead density was 1 g/cm
3). The composition of SHIF medium was 108 g/L of birchwood xylan supplemented with MgSO
4 (4 g/L), KH
2PO
4 (10 g/L), urea (3 g/L), CoCl
2.6H
2O (0.2 g/L), and CaCl
2.2H
2O (4 g/L). Samples were collected at regular intervals for determination of pH, substrate consumption, and product formation.
4.4. Influence of pH, Ca2+, and Mg2+ on XI Activity
In order to study the influence of Ca2+ and Mg2+ ions on the catalytic activity of XI, the enzyme activity was measured at different pHs (5.0 to 8.0) and different ion concentrations. Nine medium compositions were tested for each studied pH. The standard medium was constituted of 2 M fructose at pH 8.0 (50 mM Tris-maleate buffer), 50 mM MgSO4, and 2.5 mM CoCl2.
4.5. Enzymatic Xylan Hydrolysis for Xylanase Selection
Xylan hydrolysis was carried out using the soluble fraction of the birchwood xylan obtained by adding 8 g of commercial birchwood xylan in 100 mL of 50 mM citrate buffer, pH 5.5 at 50 °C. After 1 h at 150 rpm stirring, the solution was centrifuged for 20 min at 9500× g and 5 °C. The supernatant was then recovered for further use at the concentration of 25 g/L of xylan. It was offered 150 IU/gxylan (3.8 IU/mLreactor). The reaction was conducted at 50 °C under mechanical stirring for 24 h.
4.6. Analytical Methods
4.6.1. Xylanase Activity
Xylanase activity was determined according to International Union of Pure and Applied Chemistry (IUPAC) [
48] by calculating the initial velocity of xylan hydrolysis catalyzed by a known amount of enzyme. The standard substrate was birchwood xylan (1% w/v) in 50 mM citrate buffer pH 5.5. Enzyme was added to the reaction medium and incubated at 50 °C for 10 min under 250 rpm stirring. Aliquots were withdrawn at 2 min intervals, and the released reducing sugars were quantified by the dinitrosalicylic (DNS) acid method [
49]. One unit of activity (IU) was defined as the amount of enzyme required to release 1 µmol of xylose per minute under the assayed conditions.
4.6.2. Xylose Isomerase Activity
Xylose isomerase activity was determined according to Giordano et al. [
13], by measuring the initial velocity of fructose isomerization to glucose, under the following conditions: 2 M fructose solution prepared in 50 mM Tris-maleate buffer containing 50 mM MgSO
4.7H
2O and 2.5 mM CoC1
2.6H
2O, at pH 7.0 and 60 °C. The glucose concentration was determined colorimetrically using the commercial enzyme kit containing glucose oxidase and peroxidase (GOD-PAP
®, Bioclin, Belo Horizonte, Mg, Brazil). One international unit (IU) of xylose isomerase was defined as the amount of enzyme that released 1 μmol of glucose per minute under the assayed conditions.
4.6.3. Substrate and Product Quantification
The concentrations of XOs, xylose, xylulose, xylitol, and ethanol were determined by high performance liquid chromatography (HPLC), equipped with a Waters Sugar-Pak I column (Milford, MA, USA) (300 × 6.5 mm) coupled to a refractive index detector (W410 Waters) (Milford, MA, USA). Ultrapure water was used as eluent at a flow rate of 0.5 mL/min. The column temperature was 80 °C, the detector was set at 40 °C, and the injected volume was 20 µL. Before the analysis, the samples were filtered using a 0.22 µm filter.