CO2 Microwave Plasma—Catalytic Reactor for Efficient Reforming of Methane to Syngas
Abstract
:1. Introduction
2. Result and Discussion
2.1. Analysis of CO2 Dissociation in Torch
2.2. Temperature and Speciation Measurement of CO2 Microwave Plasma Torch Flame
2.3. Catalyst Preparation and Utilization
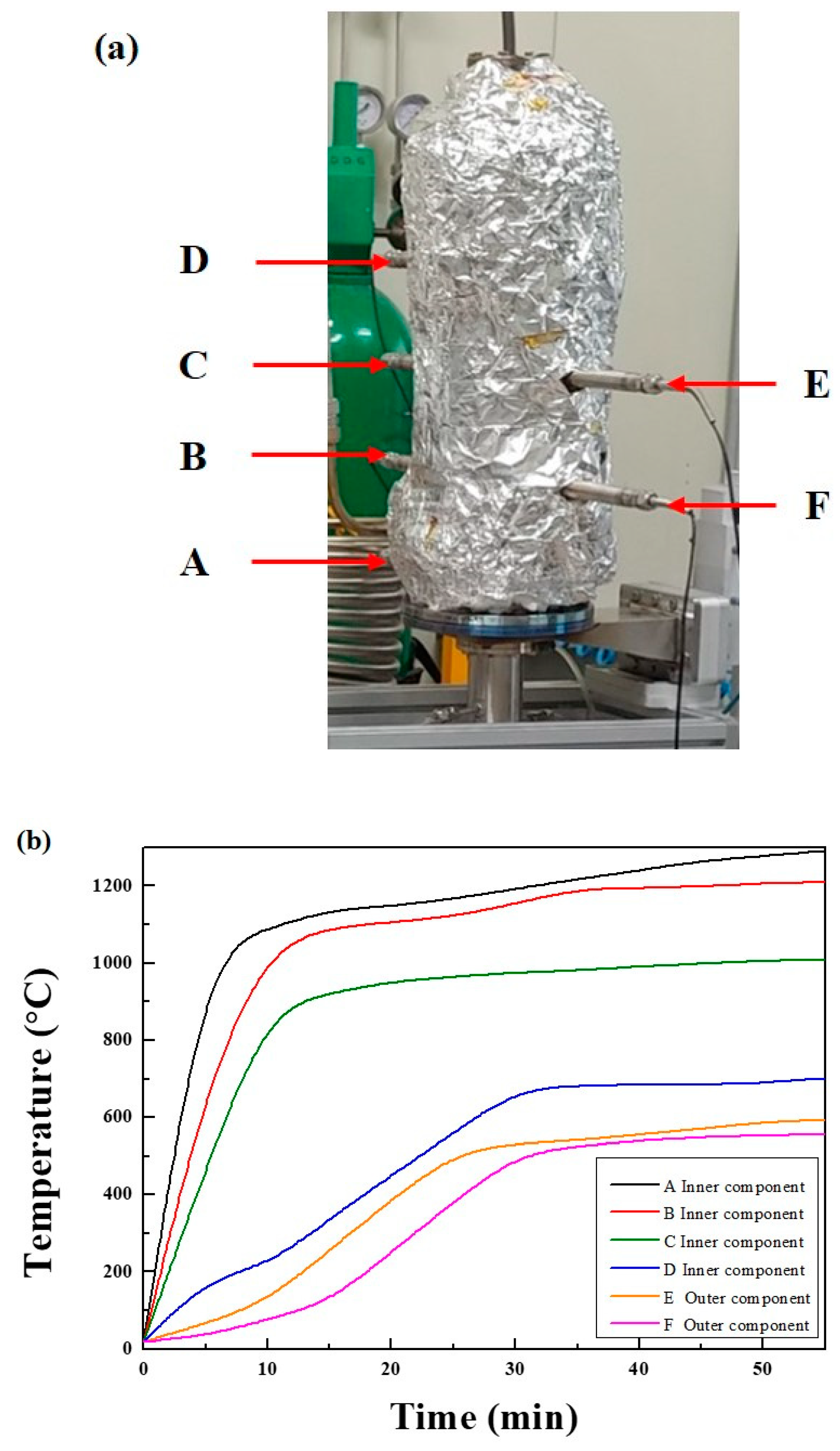
2.4. CO2 Microwave Plasma-Catalytic Reactor Performance Evaluation
3. Experimental Setup
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291–12295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, X.; Bai, M.; Li, X.; Long, H.; Shang, S.; Yin, Y.; Dai, X. CH4-CO2 reforming by plasma—Challenges and opportunities. Prog. Energy Combust. Sci. 2011, 37, 113–124. [Google Scholar] [CrossRef]
- Yu, K.M.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent Advances in CO2 Capture and Utilization. ChemSusChem 2008, 1, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.C.J.; Vannice, M.A. CO2 reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Uhm, H.S.; Kim, J.H.; Hong, Y.C. Microwave steam torch. Appl. Phys. Lett. 2007, 90, 211502. [Google Scholar] [CrossRef]
- Fridman, A.A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Kwak, H.S.; Uhm, H.S.; Hong, Y.C.; Choi, E.H. Disintegration of Carbon Dioxide Molecules in a Microwave Plasma Torch. Sci. Rep. 2015, 5, 18436. [Google Scholar] [CrossRef] [Green Version]
- Ogungbesan, B.O.; Kumar, R.; Sassi, M. Optical Characterization of a Microwave Plasma Torch for Hydrogen Production. IJIRSE 2012, 6, 2543–2549. [Google Scholar]
- Green, K.M.; Borras, M.C.; Woskov, P.P. Electron Excitation Temperature Profiles in an Air Microwave Plasma Torch. IEEE Trans. Plasma Sci. 2001, 29, 399–406. [Google Scholar] [CrossRef]
- Souza, M.M.V.M.; Schmal, M. Autothermal reforming of methane over Pt/ZrO2/Al2O3 catalysts. Appl. Catal. A 2005, 281, 19–24. [Google Scholar] [CrossRef]
- Majstorovie, G.L.; Sisovie, N.M.; Konjevie, N. Rotational and vibrational temperatures of molecular hydrogen in a hollow cathode glow discharge. Plasma Source Sci. Technol. 2007, 16, 750–756. [Google Scholar] [CrossRef]
- Biloiu, C.; Sun, X.; Harvey, Z.; Scime, E. Determination of rotational and vibrational temperatures of a nitrogen helicon plasma. Rev. Sci. Instrum. 2006, 77, 10F117. [Google Scholar] [CrossRef]
- Cong, L.; Jialiang, Z.; Zhi, Y.; Xingwei, W.; Chenfei, Z.; Hongbin, D. Diagnostic of electron, vibrational and rotational temperatures in an Ar/N2 shock plasma jet produced by a low pressure DC cascade arc discharge. Plasma Sci. Technol. 2013, 15, 875. [Google Scholar]
- Woskov, P.P.; Hadidi, K.; Borras, M.C.; Thomas, P.; Green, K.; Flores, G.J. Spectroscopic diagnostics of an atmospheric microwave plasma for monitoring metals pollution. Rev. Sci. Instrum. 2014, 70, 489–492. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Shi, D.Q.; Xu, W.; Miao, C.Y.; Ma, C.Y.; Ren, C.S.; Zhang, C.; Yi, Z. Determination of vibrational and rotational temperatures in highly constricted nitrogen plasma by fitting the second positive system of N2 molecules. AIP Adv. 2015, 5, 057158. [Google Scholar] [CrossRef]
- Specair. Features. 2012. Available online: http://www.specair-radiation.net/features.php (accessed on 3 July 2013).
- Kim, J.H.; Hong, Y.C.; Shin, D.H.; Uhm, H.S. Characterization of carbon powder produced by a microwave-plasma torch at atmospheric pressure. IEEE Trans. Plasma Sci. 2006, 34, 2600–2605. [Google Scholar] [CrossRef]
- Chun, S.M.; Hong, Y.C.; Choi, D.H. Reforming of methane to syngas in a microwave plasma torch at atmospheric pressure. J. CO2 Util. 2017, 19, 221–229. [Google Scholar] [CrossRef]
- Petitpas, G.; Rollier, J.-D.; Darmon, A.; Gonzalez-Aguilar, J.; Metkemeijer, R.; Fulcheri, L. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrogen Energy 2007, 32, 2848–2867. [Google Scholar] [CrossRef]
- Burlica, R.; Shih, K.-Y.; Locke, B.R. Formation of H2 and H2O2 in a Water-Spray Gliding Arc Nonthermal Plasma Reactor. Ind. Eng. Chem. Res. 2010, 49, 6342–6349. [Google Scholar] [CrossRef]
- Henriques, J.; Bundaleska, N.; Tatarova, E.; Dias, F.M.; Ferreira, C.M. Microwave plasma torches driven by surface wave applied for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 345–354. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Tatarova, E.; Ferreira, C.M. Ethanol reforming into hydrogen-rich gas applying microwave ‘tornado’-type plasma. Int. J. Hydrogen Energy 2013, 38, 14512–14526. [Google Scholar] [CrossRef]
- Mizeraczyk, J.; Jasinski, M. Plasma processing methods for hydrogen production. Eur. Phys. J. Appl. Phys. 2016, 75, 24702. [Google Scholar] [CrossRef]
- Randolph, K. 2013 Annual Merit Review and Peer Evaluation Meeting. U.S. DOE. Available online: http://www.hydrogen.energy.gov/pdfs/review13/pd000_randolph_2013_o.pdf (accessed on 16 May 2013).
- Bundaleska, N.; Tsyganov, D.; Saavedra, R.; Tatarova, E.; Dias, F.M.; Ferreira, C.M. Hydrogen production from methanol reforming in microwave “tornado”-type plasma. Int. J. Hydrogen Energy 2013, 38, 9145–9157. [Google Scholar] [CrossRef]
- Kappes, T.; Hammer, T.; Ulrich, A. Methane reforming with low energy electron beams. In Proceedings of the 16th International Symposium Plasma Chemistry, Taormina, Italy, 22–27 June 2003. [Google Scholar]
- Dors, M.; Izdebski, T.; Berendt, A.; Miceraczyk, J. Hydrogen Production via Biomethane Reforming in DBD Reactor. IJPEST 2012, 6, 93–97. [Google Scholar]
- Sarmiento, B.; Brey, J.J.; Viera, I.G.; Gonzalez-Elipe, A.R.; Cotrino, J.; Rico, V.J. Hydrogen production by reforming of hydrocarbons and alcohols in a dielectric barrier discharge. J. Power Sources 2007, 169, 140–143. [Google Scholar] [CrossRef]
- Jasinski, M.; Czylkowski, D.; Hrycak, B.; Dors, M.; Mizeraczyk, J. Hydrogen production via methane conversion in an atmospheric pressure microwave (2.14 GHz) plasma. In Proceedings of the 5th World Hydrogen Technologies Convention, Shanghai, China, 25–28 September 2013; pp. 206–207. [Google Scholar]
- Kim, J.H.; Hong, Y.C.; Kim, H.S.; Uhm, H.S. Simple Microwave Plasma Source at Atmospheric Pressure. J. Korean Phys. Soc. 2003, 42, S876–S879. [Google Scholar]
- Uhm, H.S.; Hong, Y.C.; Shin, D.H. A microwave plasma torch and its applications. Plasma Sources Sci. Technol. 2006, 15, S26–S34. [Google Scholar] [CrossRef]











| Production Method | Initial Composition | Production Rate g(H2)/h | Energy Yield g(H2)/kWh | References |
|---|---|---|---|---|
| Liquid (vaporized) fuels | ||||
| Water electrolysis | H2O | - | 20–40 | [25] |
| Gliding arc (water spray) | H2O + Ar | 0.004 | 13 | [21] |
| Dielectric barrier discharge | CH3OH + CO2/H2O CH3CH2OH + CO2 | - | 3.3 6.7 | [22] |
| Microwave (2.45 GHz) plasma | CH3OH + Ar C2H5OH + H2O + Ar | 0.6 0.3 | 1.4 0.5 | [22] |
| Microwave (2.45 GHz) plasma | C2H5OH + Ar | - | 0.55 | [23] |
| Microwave (2.45 GHz) plasma | CH3OH + Ar C2H5OH + H2O + Ar | - | 0.29 0.41 | [26] |
| Gaseous fuels | ||||
| Conventional steam reforming of methane (catalyst) | CH4 + H2O + Air | - | 60 | [25] |
| Electron beam radiolysis | CH4 + H2O | - | 3.6 | [27] |
| Dielectric barrier discharge | CH4 + CO2 | 0.25 | 5.2 | [28] |
| Dielectric barrier discharge | CH4 + CO2/H2O | - | 0.5 | [29] |
| Metal-cylinder-based microwave plasma | CH4 + CO2/H2O | 180 | 42.9 | [30] |
| Microwave (2.45 GHz) plasma | CH4 + CO2 | 240 | 41.4 | [19] |
| This work | CH4 + CO2 | 177 | 59.1 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, S.M.; Shin, D.H.; Ma, S.H.; Yang, G.W.; Hong, Y.C. CO2 Microwave Plasma—Catalytic Reactor for Efficient Reforming of Methane to Syngas. Catalysts 2019, 9, 292. https://doi.org/10.3390/catal9030292
Chun SM, Shin DH, Ma SH, Yang GW, Hong YC. CO2 Microwave Plasma—Catalytic Reactor for Efficient Reforming of Methane to Syngas. Catalysts. 2019; 9(3):292. https://doi.org/10.3390/catal9030292
Chicago/Turabian StyleChun, Se Min, Dong Hun Shin, Suk Hwal Ma, Geon Woo Yang, and Yong Cheol Hong. 2019. "CO2 Microwave Plasma—Catalytic Reactor for Efficient Reforming of Methane to Syngas" Catalysts 9, no. 3: 292. https://doi.org/10.3390/catal9030292





