Electrochemical Oxidation of Urea on NiCu Alloy Nanoparticles Decorated Carbon Nanofibers
Abstract
:1. Introduction
2. Results and Discussion
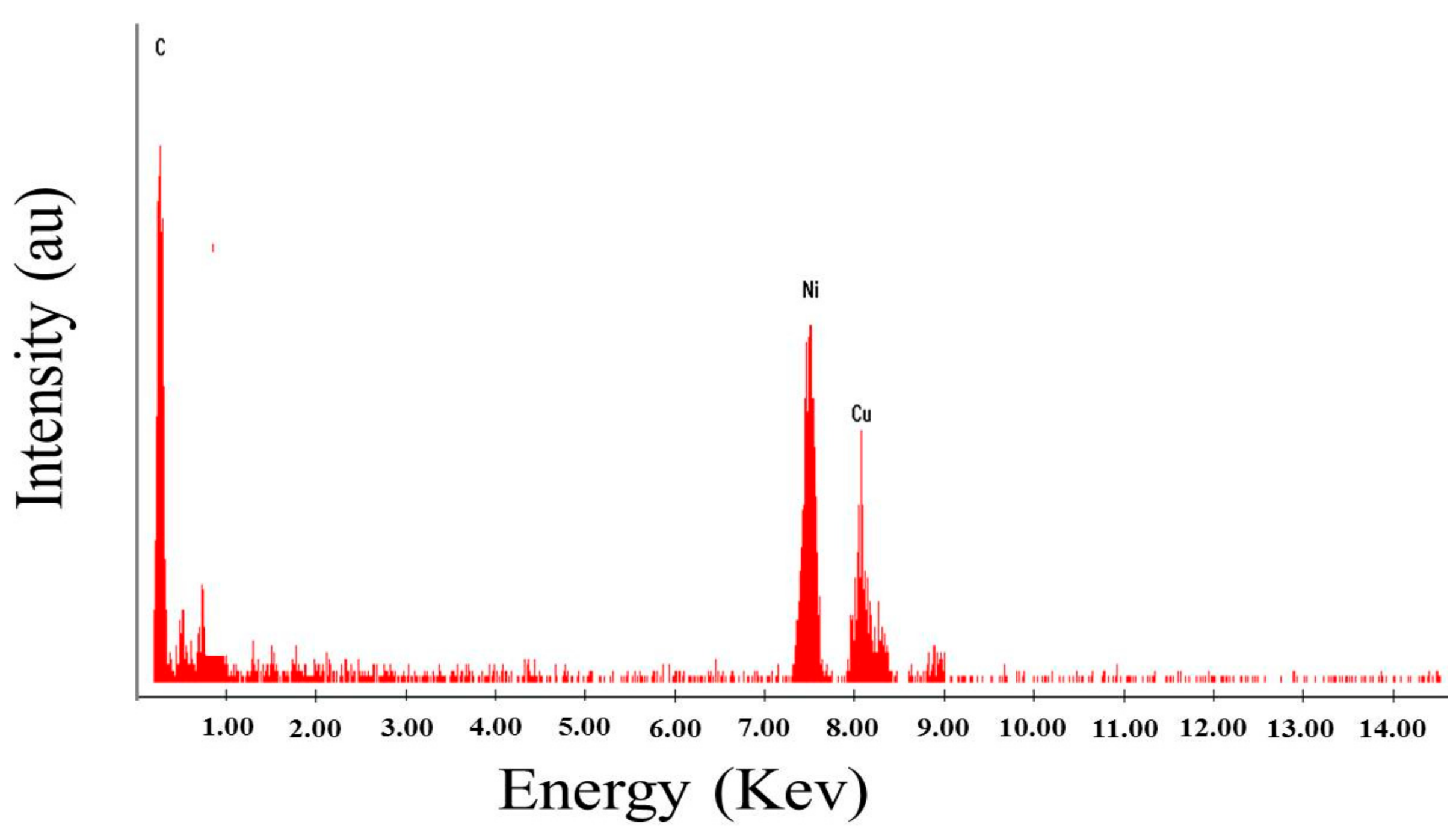
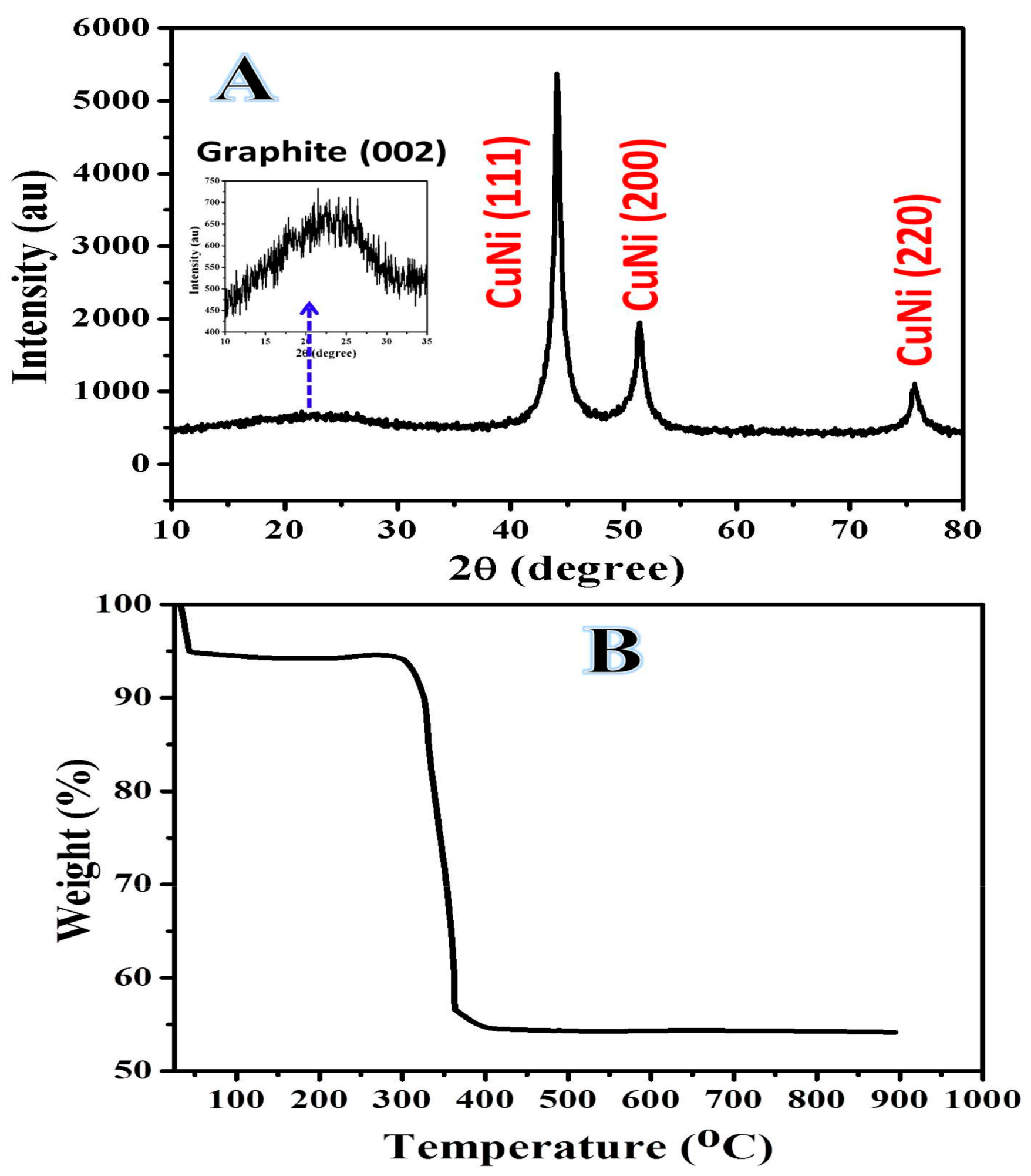
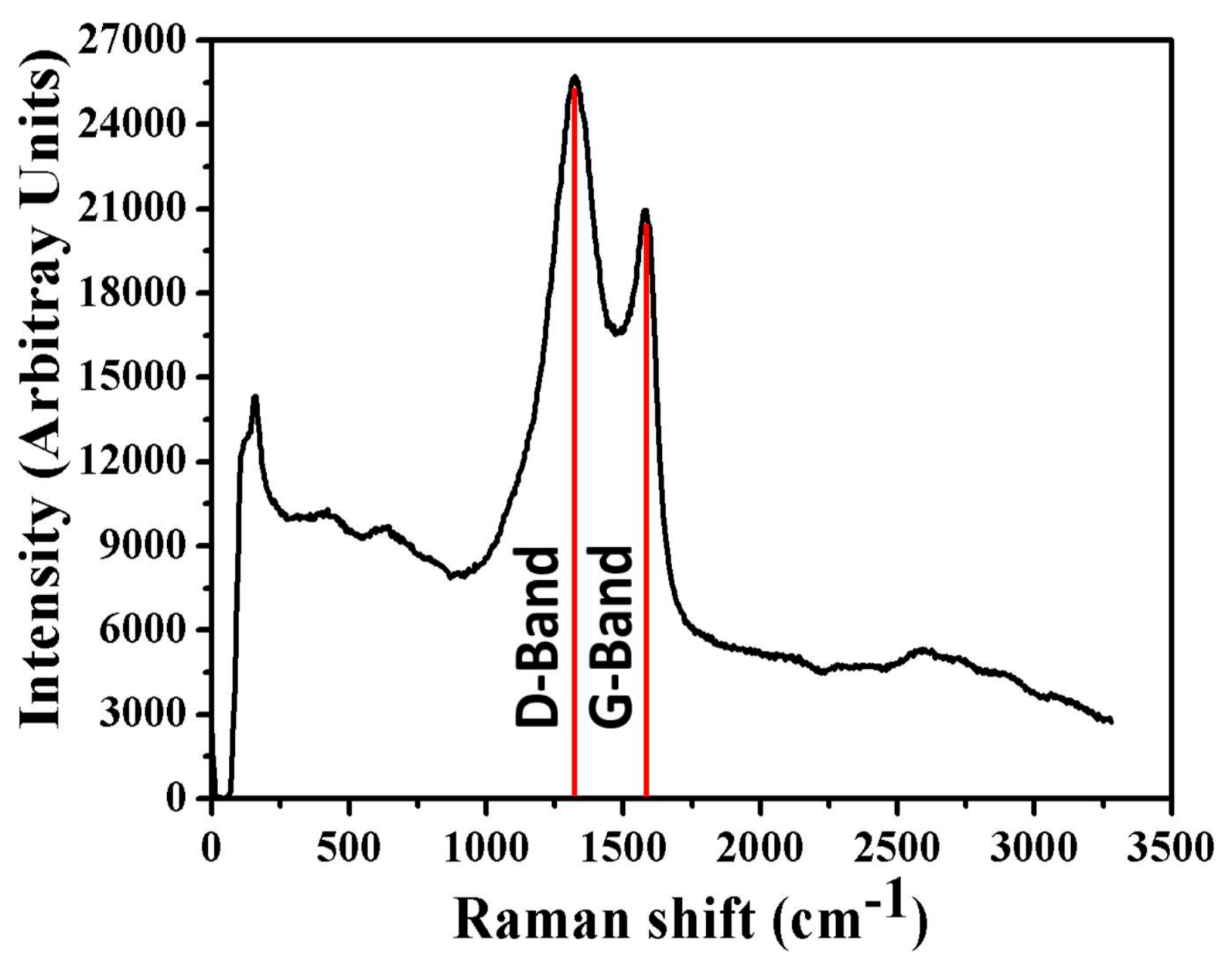
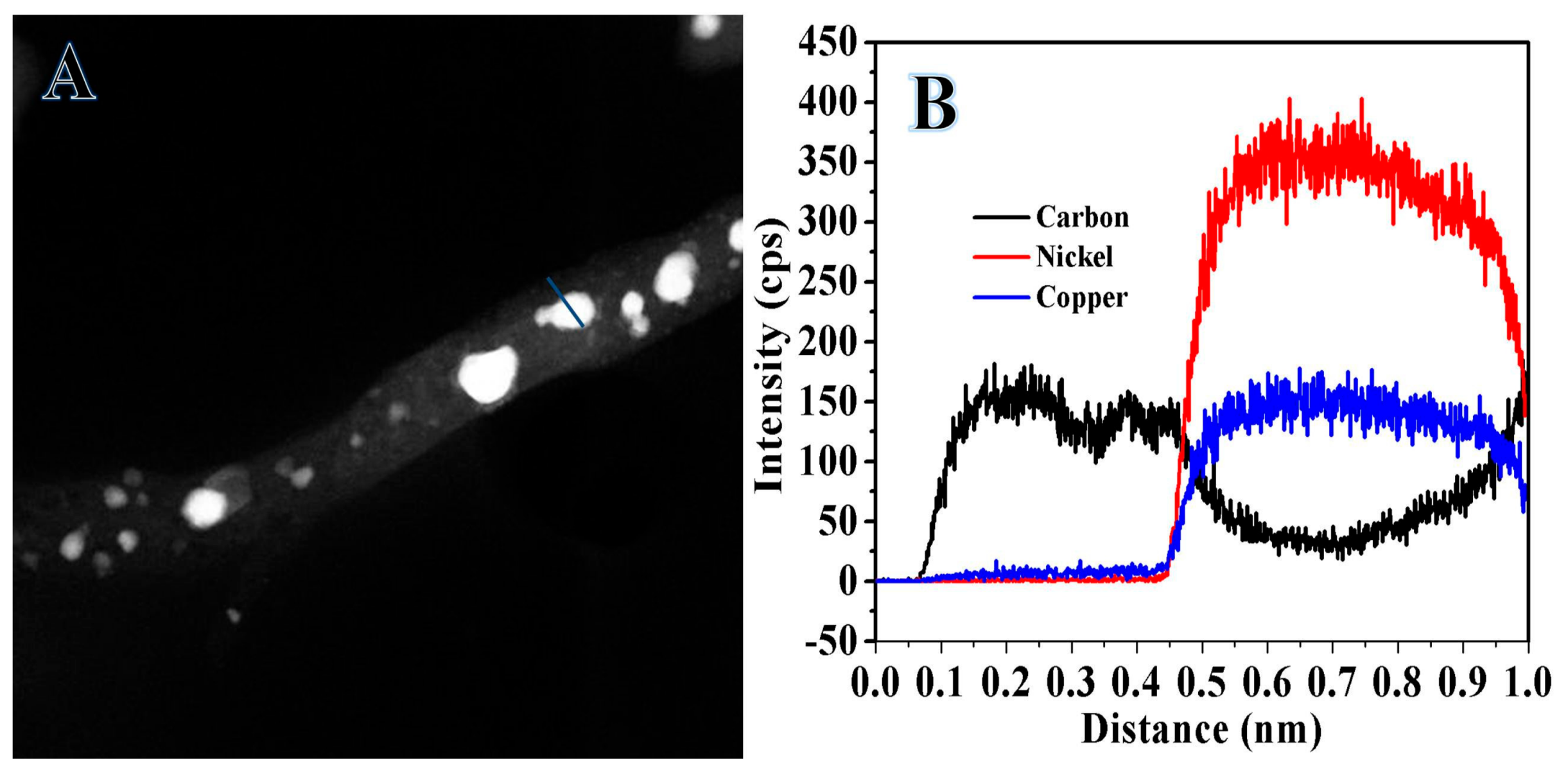
2.1. Characterization
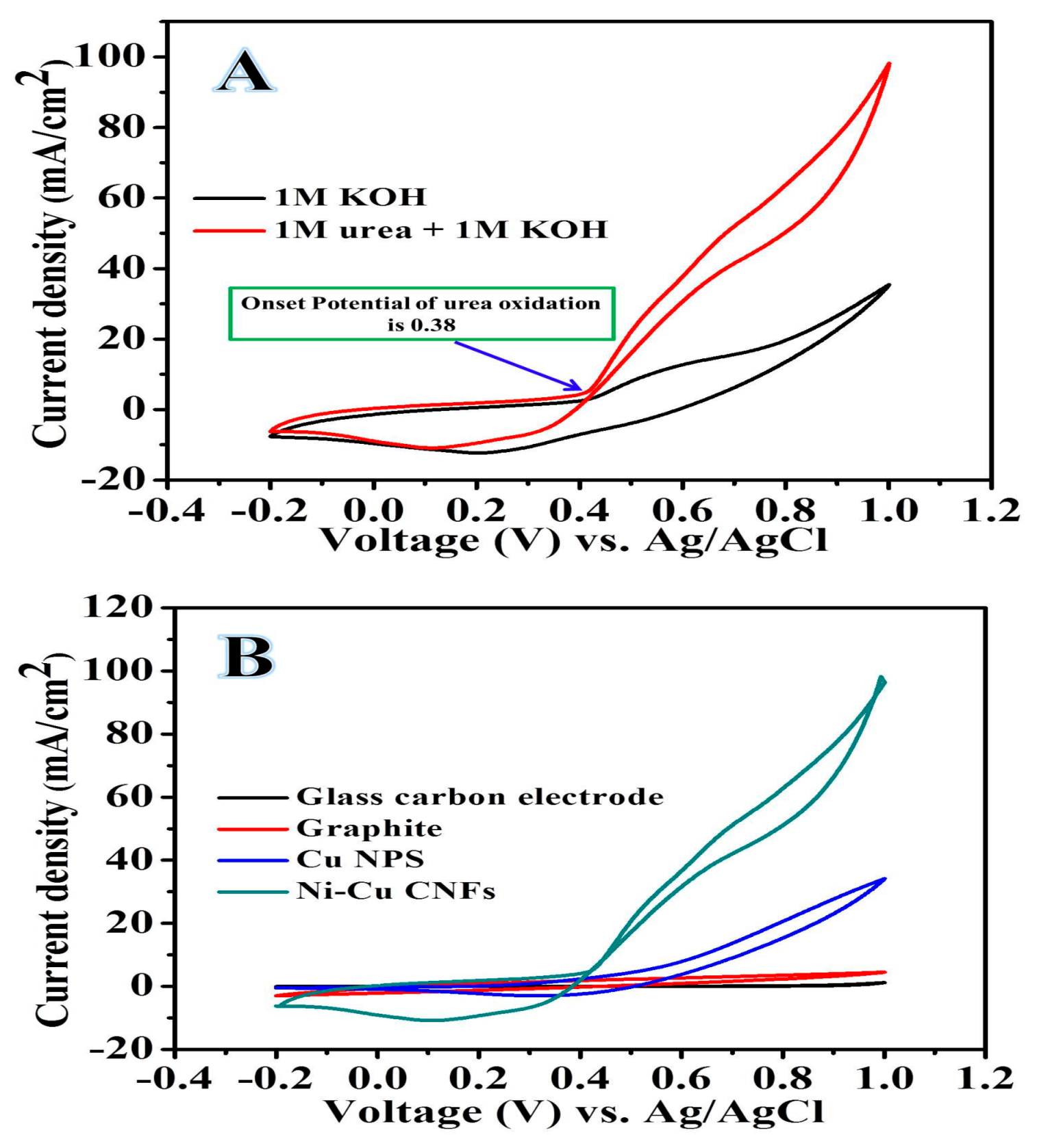
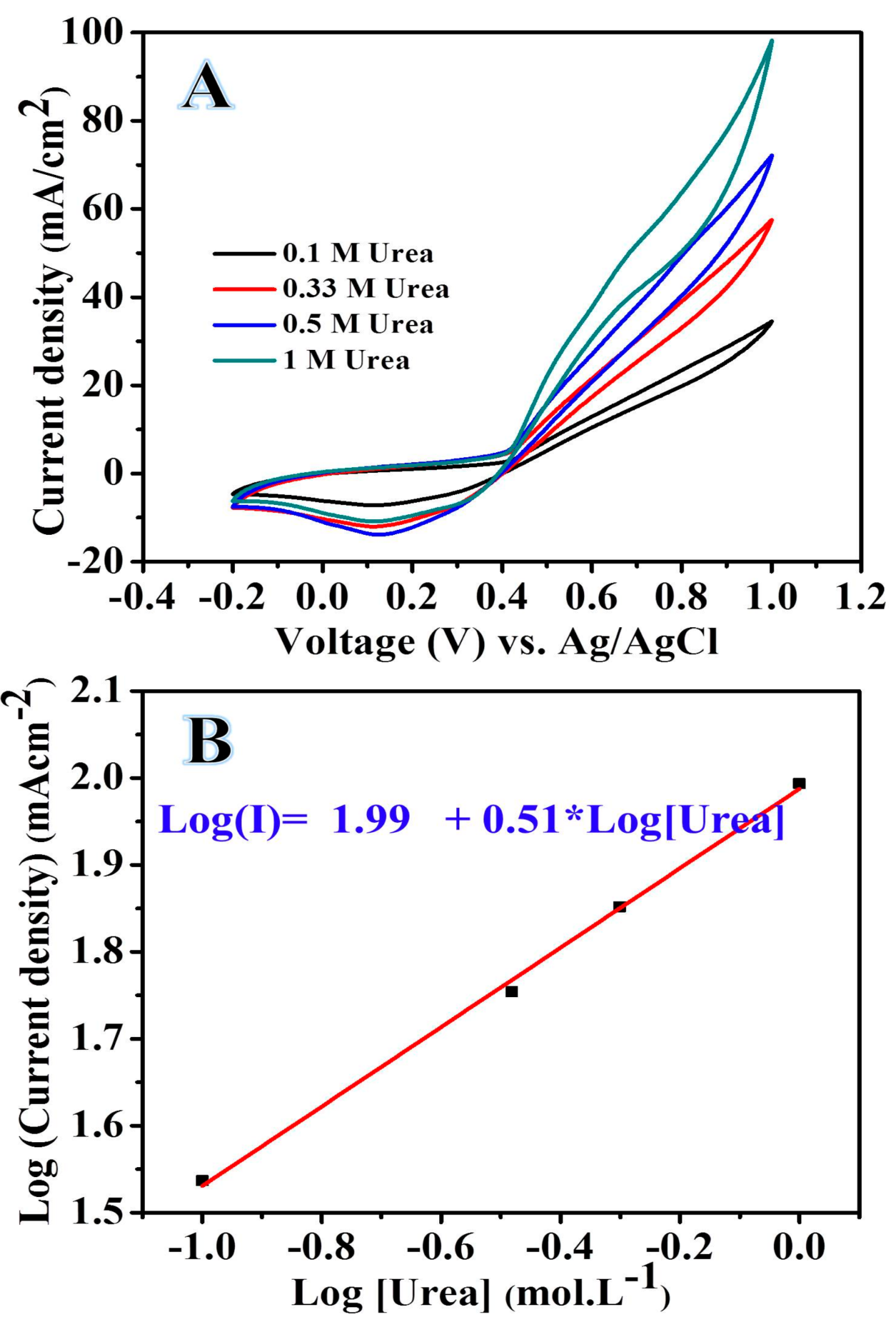
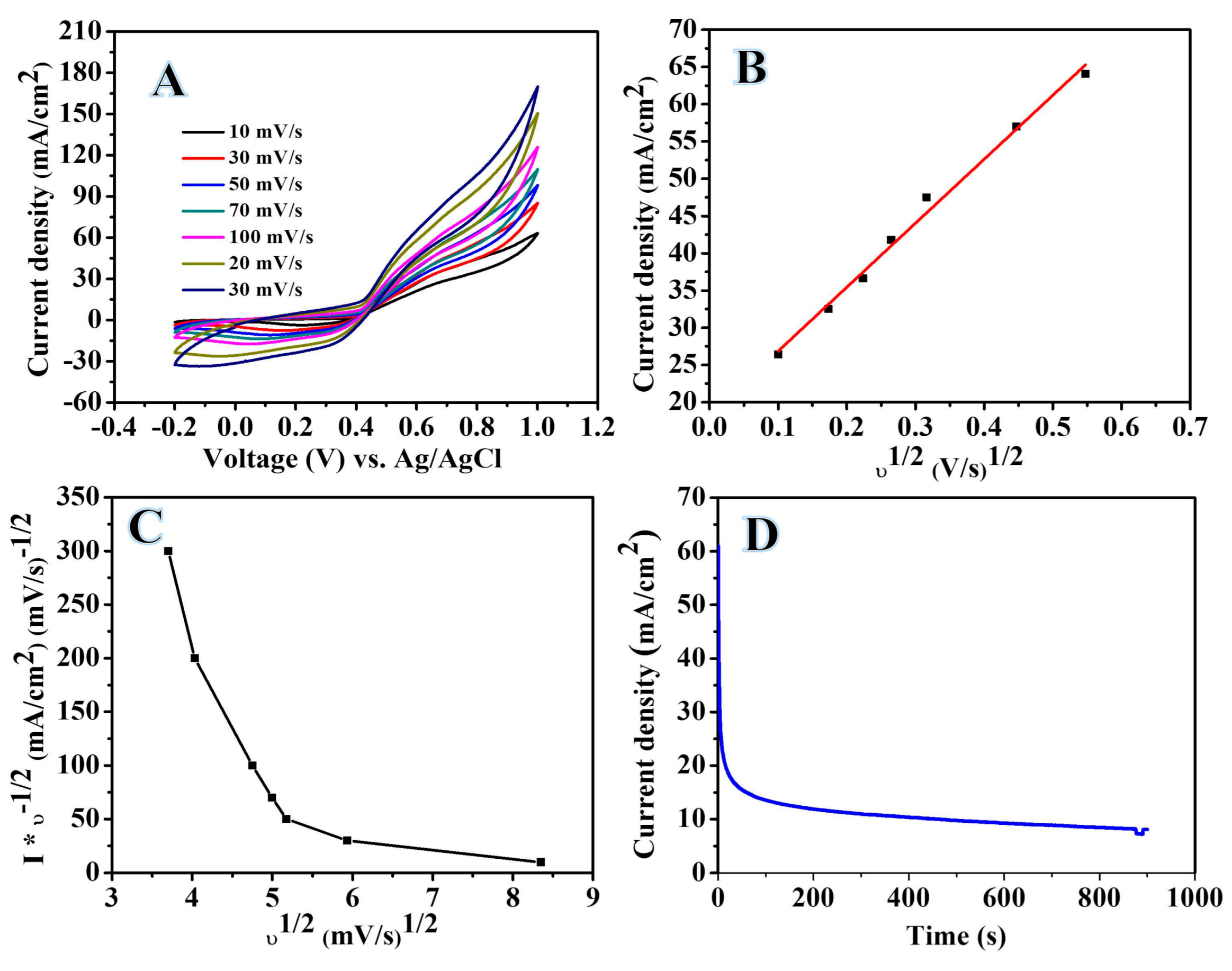
2.2. Electrochemical Analysis
3. Materials and Methods
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdel Hameed, R.M.; Medany, S.S. Enhanced electrocatalytic activity of NiO nanoparticles supported on carbon planes towards urea electro-oxidation in NaOH solution. Int. J. Hydrogen Energy 2017, 42, 24117–24130. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Al Haj, Y.; Alajami, M.; Alawadhi, H.; Barakat, N.A. Ni-Cd carbon nanofibers as an effective catalyst for urea fuel cell. J. Environ. Chem. Eng. 2018, 6, 332–337. [Google Scholar] [CrossRef]
- Wang, L.; Du, T.; Cheng, J.; Xie, X.; Yang, B.; Li, M. Enhanced activity of urea electrooxidation on nickel catalysts supported on tungsten carbides/carbon nanotubes. J. Power Sources 2015, 280, 550–554. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Electrochemical decomposition of urea with Ni-based catalysts. Appl. Catal. B 2012, 127, 221–226. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Diaz, L.A.; Botte, G.G. Nickel nanowires as effective catalysts for urea electro-oxidation. Electrochim. Acta 2014, 134, 266–271. [Google Scholar] [CrossRef]
- Barakat, N.A.; El-Newehy, M.H.; Yasin, A.S.; Ghouri, Z.K.; Al-Deyab, S.S. Ni&Mn nanoparticles-decorated carbon nanofibers as effective electrocatalyst for urea oxidation. Appl. Catal. A 2016, 510, 180–188. [Google Scholar]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea electrolysis: direct hydrogen production from urine. Chem. Commun. 2009, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Zhang, D.; Guo, F.; Cheng, K.; Wang, G.; Cao, D. Highly porous nickel@ carbon sponge as a novel type of three-dimensional anode with low cost for high catalytic performance of urea electro-oxidation in alkaline medium. J. Power Sources 2015, 283, 408–415. [Google Scholar] [CrossRef]
- Wang, D.; Yan, W.; Vijapur, S.H.; Botte, G.G. Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons. J. Power Sources 2012, 217, 498–502. [Google Scholar] [CrossRef]
- Bian, L.; Du, Q.; Luo, M.; Qu, L.; Li, M. Monodisperse nickel nanoparticles supported on multi-walls carbon nanotubes as an effective catalyst for the electro-oxidation of urea. Int. J. Hydrogen Energy 2017, 42, 25244–25250. [Google Scholar] [CrossRef]
- Alajami, M.; Yassin, M.A.; Ghouri, Z.K.; Al-Meer, S.; Barakat, N.A. Influence of bimetallic nanoparticles composition and synthesis temperature on the electrocatalytic activity of NiMn-incorporated carbon nanofibers toward urea oxidation. Int. J. Hydrogen Energy 2018, 43, 5561–5575. [Google Scholar] [CrossRef]
- Barakat, N.A.; Alajami, M.; Al Haj, Y.; Obaid, M.; Al-Meer, S. Enhanced onset potential NiMn-decorated activated carbon as effective and applicable anode in urea fuel cells. Catal. Commun. 2017, 97, 32–36. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Motlak, M.; Ghouri, Z.K.; Yasin, A.S.; El-Newehy, M.H.; Al-Deyab, S.S. Nickel nanoparticles-decorated graphene as highly effective and stable electrocatalyst for urea electrooxidation. J. Mol. Catal. A Chem. 2016, 421, 83–91. [Google Scholar] [CrossRef]
- Guo, F.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. Preparation of nickel nanowire arrays electrode for urea electro-oxidation in alkaline medium. J. Power Sources 2015, 278, 562–568. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Amen, M.T.; Al-Mubaddel, F.S.; Karim, M.R.; Alrashed, M. NiSn nanoparticle-incorporated carbon nanofibers as efficient electrocatalysts for urea oxidation and working anodes in direct urea fuel cells. J. Adv. Res. 2019, 16, 43–53. [Google Scholar] [CrossRef]
- Hameed, R.A.; Tammam, R.H. Nickel oxide nanoparticles grown on mesoporous carbon as an efficient electrocatalyst for urea electro-oxidation. Int. J. Hydrogen Energy 2018, 43, 20591–20606. [Google Scholar] [CrossRef]
- Wang, G.; Ye, K.; Shao, J.; Zhang, Y.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. Porous Ni2P nanoflower supported on nickel foam as an efficient three-dimensional electrode for urea electro-oxidation in alkaline medium. Int. J. Hydrogen Energy 2018, 43, 9316–9325. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Nickel and cobalt bimetallic hydroxide catalysts for urea electro-oxidation. Electrochim. Acta 2012, 61, 25–30. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Direct evidence of the mechanism for the electro-oxidation of urea on Ni (OH) 2 catalyst in alkaline medium. Electrochim. Acta 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Abutaleb, A.; Lolla, D.; Aljuhani, A.; Shin, H.U.; Rajala, J.W.; Chase, G.G. Effects of Surfactants on the Morphology and Properties of Electrospun Polyetherimide Fibers. Fibers 2017, 5, 33. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Brooks, R.M.; El-Halwany, M.; Yousef, A.; Nafady, A.; Hameed, R.A. CoCr7C3-like nanorods embedded on carbon nanofibers as effective electrocatalyst for methanol electro-oxidation. Int. J. Hydrogen Energy 2018, 43, 9943–9953. [Google Scholar] [CrossRef]
- Lolla, D.; Lolla, M.; Abutaleb, A.; Shin, H.U.; Reneker, D.H.; Chase, G.G. Fabrication, polarization of electrospun polyvinylidene fluoride electret fibers and effect on capturing nanoscale solid aerosols. Materials 2016, 9, 671. [Google Scholar] [CrossRef]
- Shin, H.U.; Abutaleb, A.; Lolla, D.; Chase, G.G. Effect of Calcination Temperature on NO–CO Decomposition by Pd Catalyst Nanoparticles Supported on Alumina Nanofibers. Fibers 2017, 5, 22. [Google Scholar] [CrossRef]
- Yousef, A.; Akhtar, M.S.; Barakat, N.A.M.; Motlak, M.; Yang, O.B.; Kim, H.Y. Effective NiCu NPs-doped carbon nanofibers as counter electrodes for dye-sensitized solar cells. Electrochim. Acta 2013, 102, 142–148. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.M.; El-Newehy, M.; Kim, H.Y. Chemically stable electrospun NiCu nanorods@ carbon nanofibers for highly efficient dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2012, 37, 17715–17723. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; Abdelkareem, M.A.; Khamaj, J.A.; El-Halwany, M.; Barakat, N.A.; EL-Newehy, M.H.; Kim, H.Y. Electrospun NiCu Nanoalloy Decorated on Carbon Nanofibers as Chemical Stable Electrocatalyst for Methanol Oxidation. ECS Electrochem. Lett. 2015, 4, F51–F55. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.M.; Abdelkareem, M.A.; Khamaj, J.A.; EL-Newehy, M.H.; Barakat, N.A.M.; Kim, H.Y. Fabrication of Electrical Conductive NiCu– Carbon Nanocomposite for Direct Ethanol Fuel Cells. Int. J. Electrochem. Sci. 2015, 10, 7025–7032. [Google Scholar]
- Yousef, A.; Brooks, R.M.; Abutaleb, A.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. One-step synthesis of Co-TiC-carbon composite nanofibers at low temperature. Ceram. Int. 2017, 43, 15735–15742. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.M.; Abutaleb, A.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. Electrospun CoCr7C3-supported C nanofibers: Effective, durable, and chemically stable catalyst for H2 gas generation from ammonia borane. Mol. Catal. 2017, 434, 32–38. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. Electrospun Co-TiC nanoparticles embedded on carbon nanofibers: Active and chemically stable counter electrode for methanol fuel cells and dye-sensitized solar cells. Int. J. Hydrogen Energy 2017, 42, 10407–10415. [Google Scholar] [CrossRef]
- Barakat, N.A.; Abdelkareem, M.A.; El-Newehy, M.; Kim, H.Y. Influence of the nanofibrous morphology on the catalytic activity of NiO nanostructures: an effective impact toward methanol electrooxidation. Nanoscale Res. Lett. 2013, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Boskovic, B.O.; Stolojan, V.; Zeze, D.A.; Forrest, R.D.; Silva, S.R.P.; Haq, S. Branched carbon nanofiber network synthesis at room temperature using radio frequency supported microwave plasmas. J. Appl. Phys. 2004, 96, 3443. [Google Scholar] [CrossRef] [Green Version]
- Ud Din, I.; Shaharun, M.S.; Subbarao, D.; Naeem, A. Surface modification of carbon nanofibers by HNO3 treatment. Ceram. Int. 2016, 42, 966–970. [Google Scholar] [CrossRef]
- Rahim, M.A.; Hameed, R.A.; Khalil, M. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Barakat, N.A.M.; Abdelkareem, M.A.; Al-Deyab, S.S.; Kim, H.Y. Influence of Nitrogen doping on the Catalytic Activity of Ni-incorporated Carbon Nanofibers for Alkaline Direct Methanol Fuel Cells. Electrochim. Acta 2014, 142, 228–239. [Google Scholar] [CrossRef]
- Jafarian, M.; Moghaddam, R.; Mahjani, M.; Gobal, F. Electro-Catalytic Oxidation of Methanol on a Ni–Cu Alloy in Alkaline Medium. J. Appl. Electrochem. 2006, 36, 913–918. [Google Scholar] [CrossRef]
- Ding, R.; Li, X.; Shi, W.; Xu, Q.; Wang, L.; Jiang, H.; Yang, Z.; Liu, E. Mesoporous Ni-P nanocatalysts for alkaline urea elect oxidation. Electrochim. Acta 2016, 222, 455–462. [Google Scholar] [CrossRef]
- Abdel Hameed, R.M.; Medany, S.S. Influence of support material on the electrocatalytic activity of nickel oxide nanoparticles for urea electro-oxidation reaction. J. Colloid Interface Sci. 2018, 513, 536–548. [Google Scholar] [CrossRef]
- Periyasamy, S.; Subramanian, P.; Levi, E.; Aurbach, D.; Gedanken, A.; Schechter, A. Exceptionally Active and Stable Spinel Nickel Manganese Oxide Electrocatalysts for Urea Oxidation Reaction. Appl. Mater. Interfaces 2016, 8, 12176–12185. [Google Scholar] [CrossRef]
- Wang, D.; Yan, W.; Vijapur, S.H.; Botte, G.G. Electrochimica Acta Electrochemically reduced graphene oxide – nickel nanocomposites for urea electrolysis. Electrochim. Acta 2013, 89, 732–736. [Google Scholar] [CrossRef]
- Basumatary, P.; Konwar, D.; Yoon, Y.S. A novel NieCu/ZnO@MWCNT anode employed in urea fuel cell to attain superior performances. Electrochim. Acta 2018, 261, 78–85. [Google Scholar] [CrossRef]
- Yousef, A.; El-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N.A. Facile synthesis of Ni-decorated multi-layers graphene sheets as effective anode for direct urea fuel cells. Arabian J. Chem. 2017, 10, 811–822. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.; Xu, C.; Jiang, S.P.; Tong, Y. Kinetics of ethanol electrooxidation at Pd electrodeposited on Ti. Electrochem. Commun. 2007, 9, 2334–2339. [Google Scholar] [CrossRef]



| Electrocatalyst | Onset Potential | CD (mA/cm2) | Anodic Peak Potential (V) | Reference Electrode | Urea Concentration (M) in KOH (M) | Reference |
|---|---|---|---|---|---|---|
| Ni | 0.39 | 22 | 0.5 | Hg/HgO | 0.33 in 1 | [4] |
| Ni-Zn-Co | 0.35 | 24 | 0.5 | Hg/HgO | 0.33 in 1 | |
| Ni-P | 1.37 | ~40 | 1.5 | RHE | 0.33 in 1 | [37] |
| NiO/Gt | 0.345 | 17 | 0.62 | Ag/AgCl | 0.33 in 0.5M NaOH | [38] |
| NiO/Gt-15 | 0.345 | 17 | 0.64 | Ag/AgCl | [1] | |
| Ni1.5Mn1.5O4 | 0.29 | 6.9 | 0.5 | Ag/AgCl | 0.33 in 1 | [39] |
| Ni-ERGO | 0.4 | 35 | Hg/HgO | 0.33 in 1 | [40] | |
| NiCo(OH)2 | 0.25 | ~20 | ~0.42 | Hg/HgO | 0.33 in 1 | [18] |
| Ni-Cu/ZnO@MWCNT | ~30 | 32 | ~0.45 | Ag/AgCl | 0.07 in 0.4 | [41] |
| NiMn-CNFs | 0.29 | 67 | 0.58 | Ag/AgCl | 2 in 1 | [6] |
| Ni-Cu-CNFs | 0.38 | 25 | 0.6 | Ag/AgCl | 1 in 1 | This study |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abutaleb, A. Electrochemical Oxidation of Urea on NiCu Alloy Nanoparticles Decorated Carbon Nanofibers. Catalysts 2019, 9, 397. https://doi.org/10.3390/catal9050397
Abutaleb A. Electrochemical Oxidation of Urea on NiCu Alloy Nanoparticles Decorated Carbon Nanofibers. Catalysts. 2019; 9(5):397. https://doi.org/10.3390/catal9050397
Chicago/Turabian StyleAbutaleb, Ahmed. 2019. "Electrochemical Oxidation of Urea on NiCu Alloy Nanoparticles Decorated Carbon Nanofibers" Catalysts 9, no. 5: 397. https://doi.org/10.3390/catal9050397




