Insights into the Morphological Effect of Co3O4 Crystallite on Catalytic Oxidation of Vinyl Chloride
Abstract
:1. Introduction
2. Results
2.1. Characterization Results
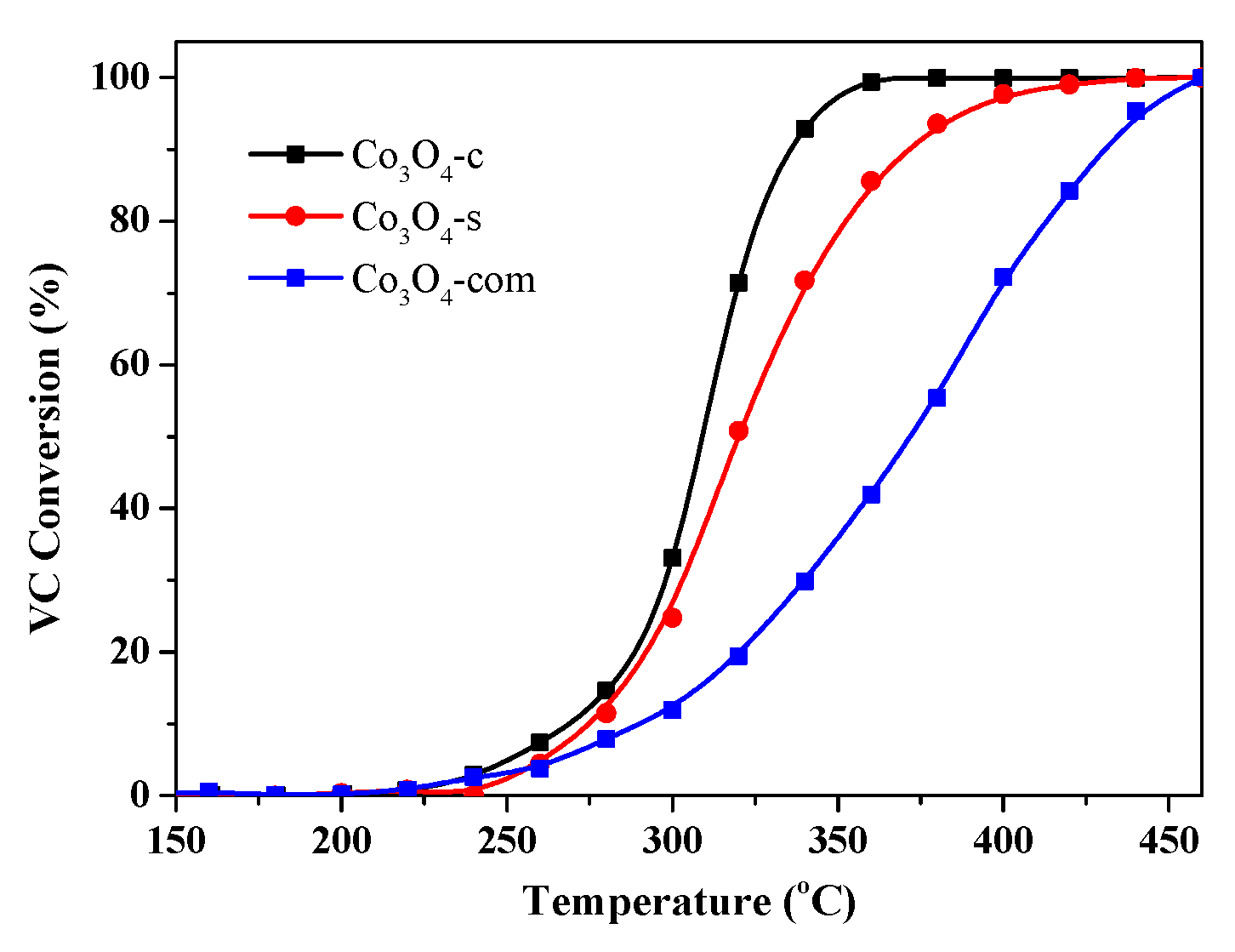
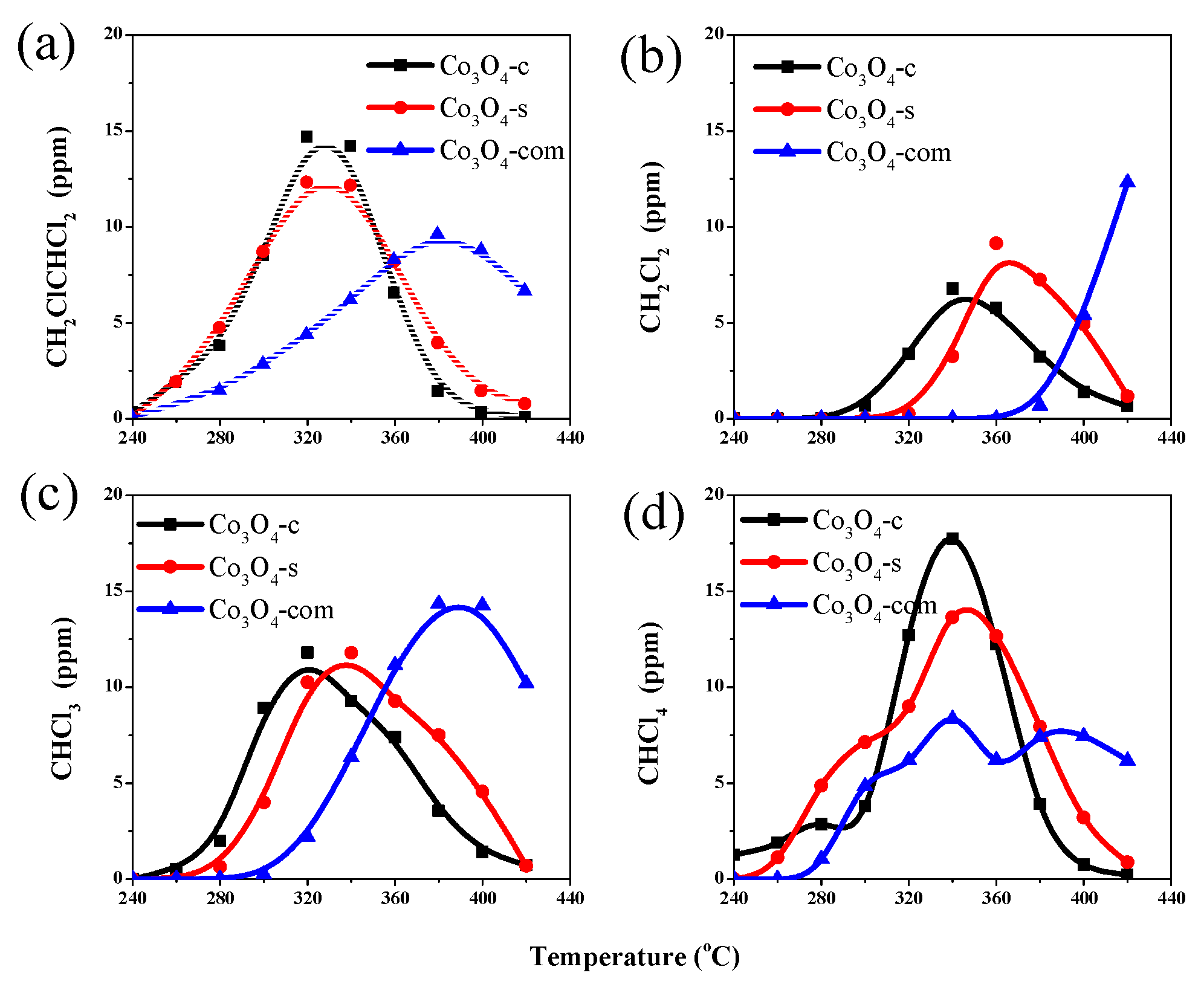
2.2. Catalytic Performances for VC Oxidation
3. Materials and Methods
3.1. Co3O4 Preparation
3.2. Characterizations
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, Q.; Wang, C.; Li, X.; Li, Z.; Wang, L.; Zhang, Q.; Wu, G.; Li, Z. Engineering an effective MnO2 catalyst from LaMnO3 for catalytic methane combustion. Fuel 2019, 239, 1240–1245. [Google Scholar] [CrossRef]
- Luo, D.; Chen, B.; Li, X.; Liu, Z.; Liu, X.; Liu, X.; Shi, C.; Zhao, X.S. Three-dimensional nitrogen-doped porous carbon anchored CeO2 quantum dots as an efficient catalyst for formaldehyde oxidation. J. Mater. Chem. A 2018, 6, 7897–7902. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Li, G.; Wang, L.; Song, L.; Li, X. Zeolitic acidity as a promoter for the catalytic oxidation of toluene over MnOx/HZSM-5 catalysts. Catal. Today 2019, 327, 374–381. [Google Scholar] [CrossRef]
- Du, Y.; Huang, W.; Hua, Z.; Wang, Y.; Cui, X.; Wu, M.; Shu, Z.; Zhang, L.; Wang, J.; Chen, H.; et al. A facile ultrasonic process for the preparation of Co3O4 nanoflowers for room-temperature removal of low-concentration NOx. Catal. Commun. 2014, 57, 73–77. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, Y.-J.; Karandikar, P.R.; Jun, K.-W.; Ha, K.-S.; Park, H.-G. Fischer–Tropsch catalysts deposited with size-controlled Co3O4 nanocrystals: Effect of Co particle size on catalytic activity and stability. Appl. Catal. A 2012, 411–412, 15–23. [Google Scholar] [CrossRef]
- del Río, L.; López, I.; Marbán, G. Stainless steel wire mesh-supported Co3O4 catalysts in the steam reforming of ethanol. Appl. Catal. B 2014, 150–151, 370–379. [Google Scholar] [CrossRef]
- Bi, L.; Shafi, S.P.; Da’as, E.H.; Traversa, E. Tailoring the Cathode–Electrolyte Interface with Nanoparticles for Boosting the Solid Oxide Fuel Cell Performance of Chemically Stable Proton-Conducting Electrolytes. Small 2018, 14, 1801231. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bi, L.; Zhao, X. Liquid-phase synthesis of SrCo0. 9Nb0. 1O3-δ cathode material for proton-conducting solid oxide fuel cells. Ceram. Int. 2018, 44, 5139–5144. [Google Scholar] [CrossRef]
- Teng, F.; Chen, M.; Li, G.; Teng, Y.; Xu, T.; Hang, Y.; Yao, W.; Santhanagopalan, S.; Meng, D.D.; Zhu, Y. High combustion activity of CH4 and catalluminescence properties of CO oxidation over porous Co3O4 nanorods. Appl. Catal. B 2011, 110, 133–140. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Sánchez-Royo, J.F.; Murillo, R.; Mastral, A.M.; Aranda, A.; Vázquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A 2010, 386, 16–27. [Google Scholar] [CrossRef]
- de Rivas, B.; López-Fonseca, R.; Jiménez-González, C.; Gutiérrez-Ortiz, J.I. Synthesis, characterisation and catalytic performance of nanocrystalline Co3O4 for gas-phase chlorinated VOC abatement. J. Catal. 2011, 281, 88–97. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Wang, Z.; Huang, H.; Peng, H.; Li, X. Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene oxidation. Appl. Catal. A 2018, 550, 67–76. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B 2013, 142–143, 677–683. [Google Scholar] [CrossRef]
- Cai, T.; Huang, H.; Deng, W.; Dai, Q.; Liu, W.; Wang, X. Catalytic combustion of 1,2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts. Appl. Catal. B 2015, 166–167, 393–405. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Gao, L.; Zheng, H.; Zhang, Z.; Qian, Y. One-dimensional arrays of Co3O4 nanoparticles: synthesis, characterization, and optical and electrochemical properties. J. Phys. Chem. B 2004, 108, 16401–16404. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Selective synthesis of Co3O4 nanocrystal with different shape and crystal plane effect on catalytic property for methane combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts. Chem. Eng. J. 2017, 315, 392–402. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G.; Baylet, A.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emission over LaMnO3 and LaB0.2Mn0.8O3(B=Co, Ni, Fe) catalysts. Appl. Catal. B 2013, 129, 509–516. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Low-temperature catalytic oxidation of vinyl chloride over Ru modified Co3O4 catalysts. RSC Adv. 2016, 6, 99577–99585. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef]
- Hu, L.; Sun, K.; Peng, Q.; Xu, B.; Li, Y. Surface active sites on Co3O4 nanobelt and nanocube model catalysts for CO oxidation. Nano Res. 2010, 3, 363–368. [Google Scholar] [CrossRef]
- Xia, S.; Yu, M.; Hu, J.; Feng, J.; Chen, J.; Shi, M.; Weng, X. A model of interface-related enhancement based on the contrast between Co3O4 sphere and cube for electrochemical detection of hydrogen peroxide. Electrochem. Commun. 2014, 40, 67–70. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Zhao, H.; Chen, D.; Liu, F.; Xiang, J.; Hu, Z.; Li, Y. Facile shape control of Co3O4 and the effect of the crystal plane on electrochemical performance. Adv. Mater. 2012, 24, 5762–5766. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, F.; Deng, C.; Zhen, S.; Li, X.; Xue, Y.; Yan, Y.-M.; Sun, K. Crystal plane-dependent electrocatalytic activity of Co3O4 toward oxygen evolution reaction. Catal. Commun. 2015, 67, 78–82. [Google Scholar] [CrossRef]
- Konsolakis, M.; Sgourakis, M.; Carabineiro, S.A.C. Surface and redox properties of cobalt-ceria binary oxides: on the effect of Co content and pretreatment conditions. Appl. Surf. Sci. 2015, 341, 48–54. [Google Scholar] [CrossRef]
- Barreca, D.; Massignan, C.; Daolio, S.; Fabrizio, M.; Piccirillo, C.; Armelao, L.; Tondello, E. Composition and microstructure of cobalt oxide thin films obtained from a novel cobalt(II) precursor by chemical vapor deposition. Chem. Mater. 2001, 13, 588–593. [Google Scholar] [CrossRef]
- Wei, W.; Chen, W.; Ivey, D.G. Rock salt-spinel structural transformation in anodically electrodeposited Mn-Co-O nanocrystals. Chem. Mater. 2008, 20, 1941–1947. [Google Scholar] [CrossRef]
- Tian, Z.; Tchoua Ngamou, P.H.; Vannier, V.; Kohse-Höinghaus, K.; Bahlawane, N. Catalytic oxidation of VOCs over mixed Co-Mn oxides. Appl. Catal. B 2012, 117–118, 125–134. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Díaz-Fernández, D.; Méndez, J.; Bomatí-Miguel, O.; Yubero, F.; Mossanek, R.J.O.; Abbate, M.; Domínguez-Cañizares, G.; Gutiérrez, A.; Tougaard, S.; Soriano, L. The growth of cobalt oxides on HOPG and SiO2 surfaces: A comparative study. Surf. Sci. 2014, 624, 145–153. [Google Scholar] [CrossRef]
- Lykhach, Y.; Faisal, F.; Skála, T.; Neitzel, A.; Tsud, N.; Vorokhta, M.; Dvořák, F.; Beranová, K.; Kosto, Y.; Prince, K.C.; et al. Interplay between the metal-support interaction and stability in Pt/Co3O4 (111) model catalysts. J. Mater. Chem. A 2018, 6, 23078–23086. [Google Scholar] [CrossRef]
- Chen, J.; Shi, W.; Yang, S.; Arandiyan, H.; Li, J. Distinguished roles with various vanadium loadings of CoCr2–xVxO4 (x=0-0.20) for methane combustion. J. Phys. Chem. C 2011, 115, 17400–17408. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, A.; Luo, M.; Lu, J. Highly active spinel type CoCr2O4 catalysts for dichloromethane oxidation. Appl. Catal. B 2015, 165, 477–486. [Google Scholar] [CrossRef]
- Gautier, J.L.; Rios, E.; Gracia, M.; Marco, J.F.; Gancedo, J.R. Characterisation by X-ray photoelectron spectroscopy of thin MnxCo3−xO4(1≥x≥0) spinel films prepared by low-temperature spray pyrolysis. Thin Solid Films 1997, 311, 51–57. [Google Scholar] [CrossRef]
- Aristizábal, B.H.; de Correa, C.M.; Serykh, A.I.; Hetrick, C.E.; Amiridis, M.D. In situ FTIR study of the adsorption and reaction of ortho-dichlorobenzene over Pd-promoted Co-HMOR. Microporous Mesoporous Mater. 2008, 112, 432–440. [Google Scholar] [CrossRef]
- Valeri, S.; Borghi, A.; Gazzadi, G.C.; di Bona, A. Growth and structure of cobalt oxide on (001) bct cobalt film. Surf. Sci. 1999, 423, 346–356. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Relationship between catalytic deactivation and physicochemical properties of LaMnO3 perovskite catalyst during catalytic oxidation of vinyl chloride. Appl. Catal. B 2016, 186, 173–183. [Google Scholar] [CrossRef]
- De Rivas, B.; López-Fonseca, R.; Jiménez-González, C.; Gutiérrez-Ortiz, J.I. Highly active behaviour of nanocrystalline Co3O4 from oxalate nanorods in the oxidation of chlorinated short chain alkanes. Chem. Eng. J. 2012, 184, 184–192. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Zhao, Z.; Au, C.T. Controlled preparation and high catalytic performance of three-dimensionally ordered macroporous LaMnO3 with nanovoid skeletons for the combustion of toluene. J. Catal. 2012, 287, 149–160. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Zhao, Z. Lysine-aided PMMA-templating preparation and high performance of three-dimensionally ordered macroporous LaMnO3 with mesoporous walls for the catalytic combustion of toluene. Appl. Catal. B 2012, 119–120, 20–31. [Google Scholar] [CrossRef]
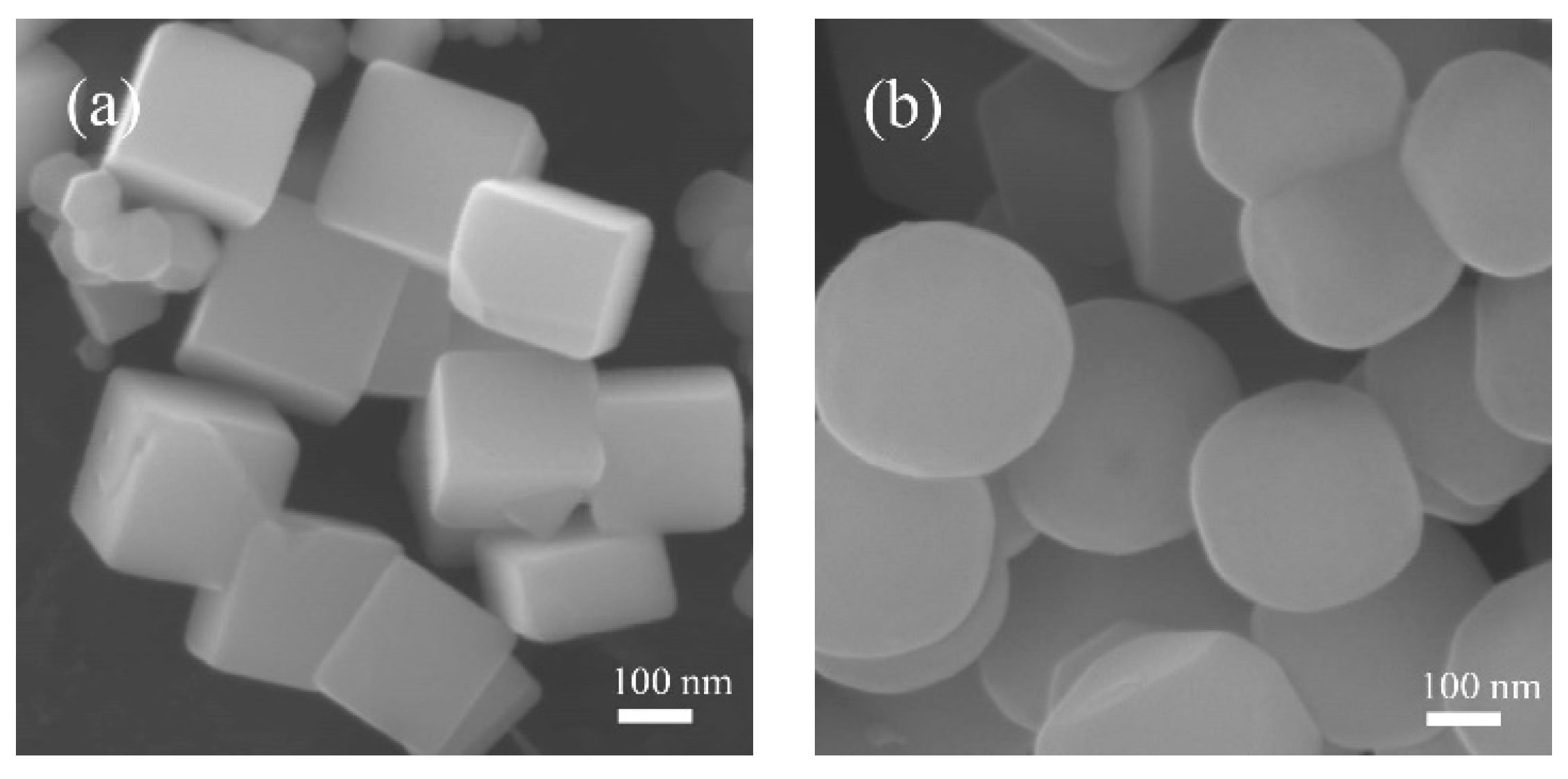

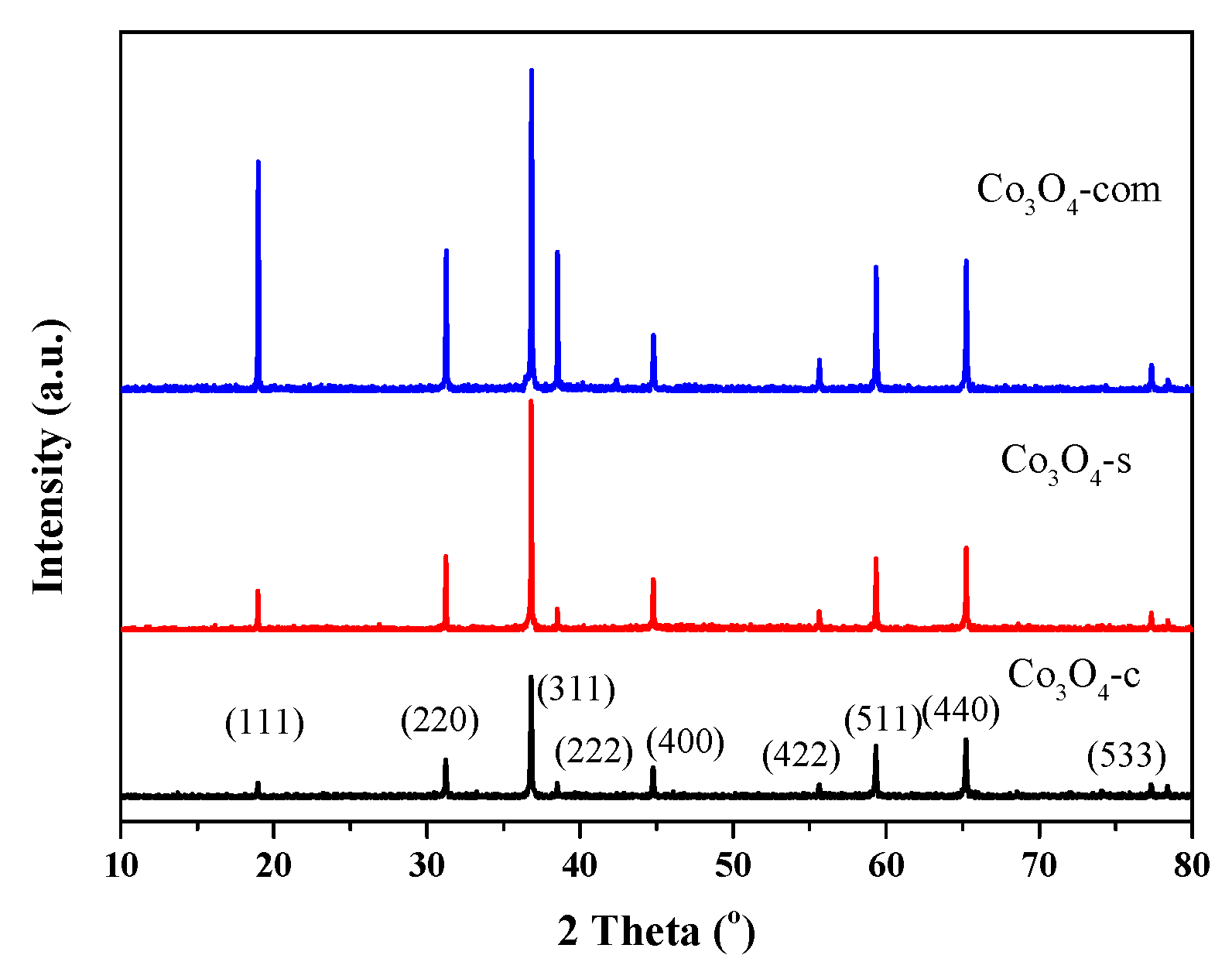
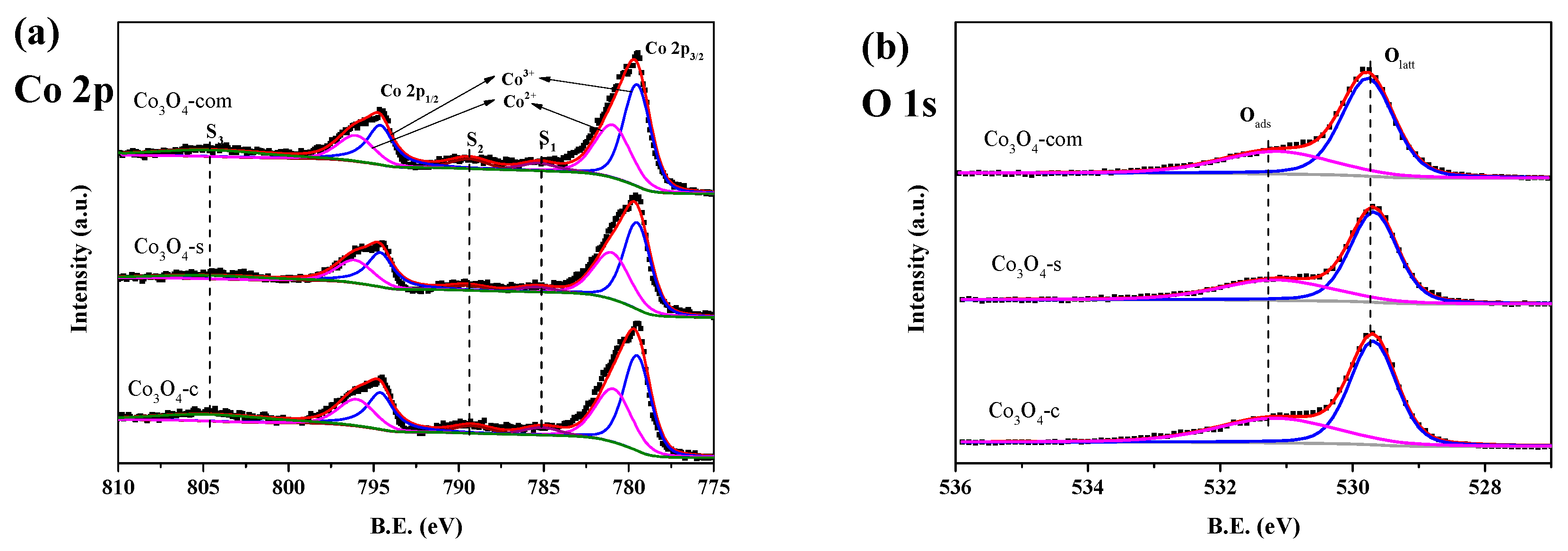
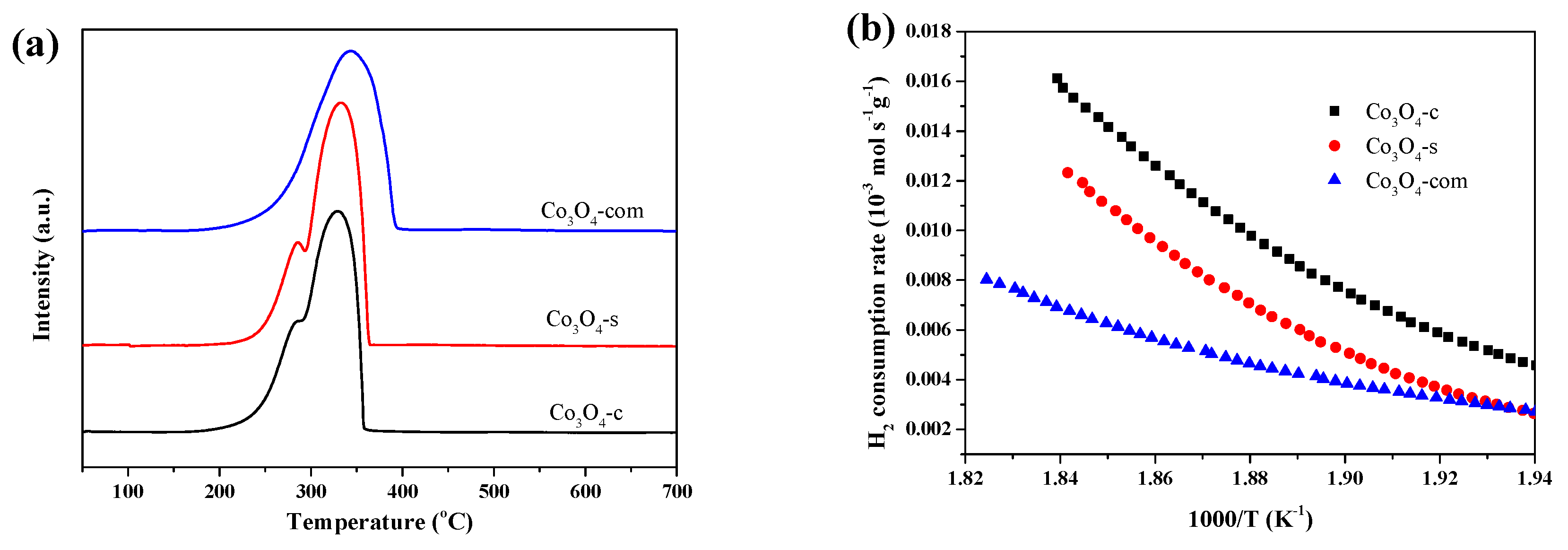


| Catalysts | Co content (wt. %) | Crystallite size (nm) | Co3+/Co2+ (at./at.) | Oads/Olatt (at./at.) |
|---|---|---|---|---|
| Co3O4-c | 71.6 | 62 | 1.35 | 0.60 |
| Co3O4-s | 71.6 | 100 | 1.41 | 0.53 |
| Co3O4-com | 70.2 | 93 | 1.46 | 0.49 |
| Catalysts | T50 (°C) | T90 (°C) |
|---|---|---|
| Co3O4-c | 308 | 340 |
| Co3O4-s | 320 | 372 |
| Co3O4-com | 372 | 431 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Hua, W.; Chai, G.; Zhang, C.; Guo, Y. Insights into the Morphological Effect of Co3O4 Crystallite on Catalytic Oxidation of Vinyl Chloride. Catalysts 2019, 9, 408. https://doi.org/10.3390/catal9050408
Wang C, Hua W, Chai G, Zhang C, Guo Y. Insights into the Morphological Effect of Co3O4 Crystallite on Catalytic Oxidation of Vinyl Chloride. Catalysts. 2019; 9(5):408. https://doi.org/10.3390/catal9050408
Chicago/Turabian StyleWang, Chao, Wenchao Hua, Guangtao Chai, Chuanhui Zhang, and Yanglong Guo. 2019. "Insights into the Morphological Effect of Co3O4 Crystallite on Catalytic Oxidation of Vinyl Chloride" Catalysts 9, no. 5: 408. https://doi.org/10.3390/catal9050408






