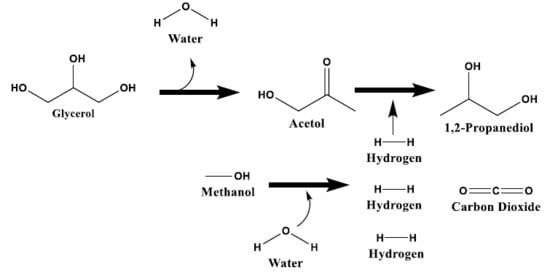
The Promoting Effect of Ni on Glycerol Hydrogenolysis to 1,2-Propanediol with In Situ Hydrogen from Methanol Steam Reforming Using a Cu/ZnO/Al2O3 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
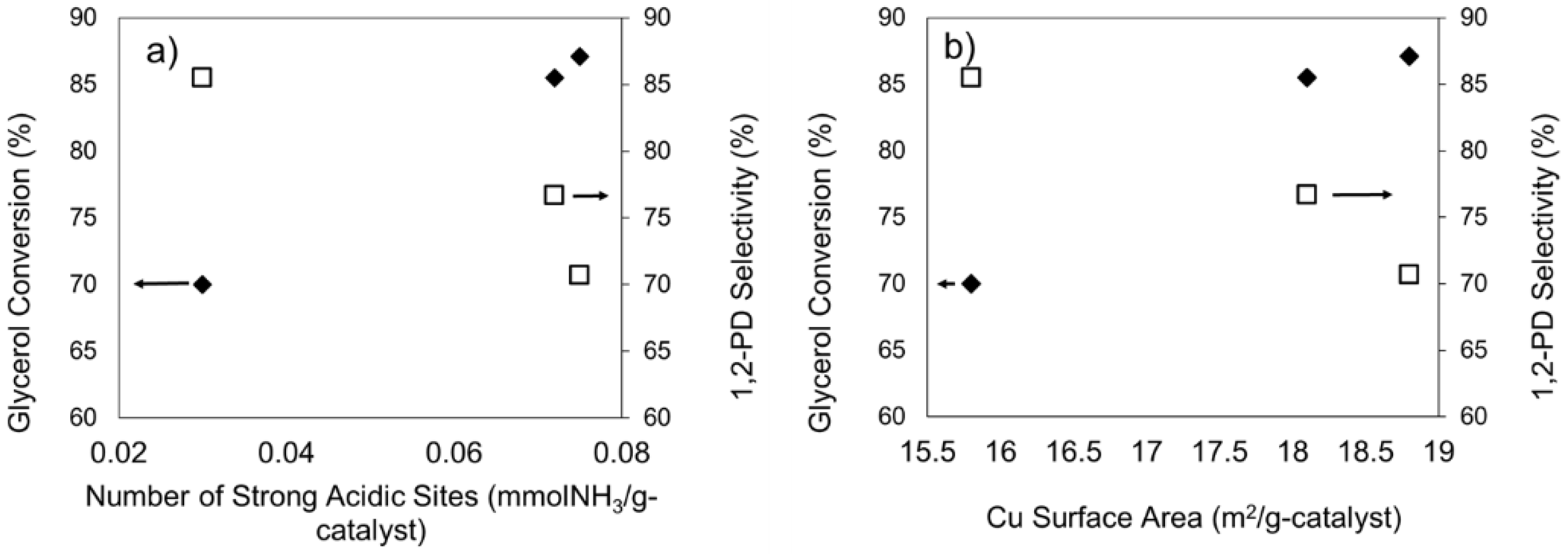
2.1.1. Acidity of the Catalysts
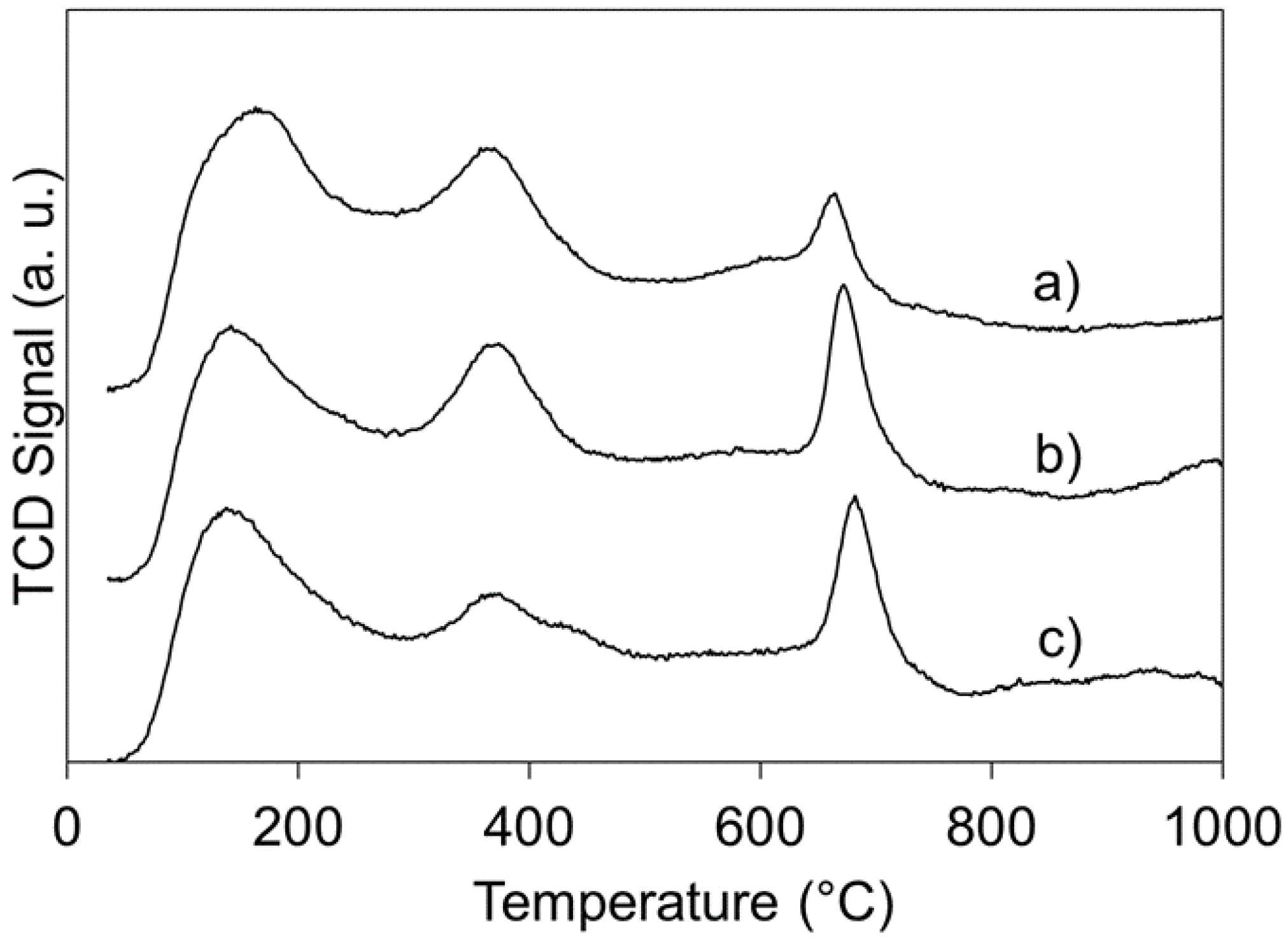
2.1.2. Temperature Programmed Reduction
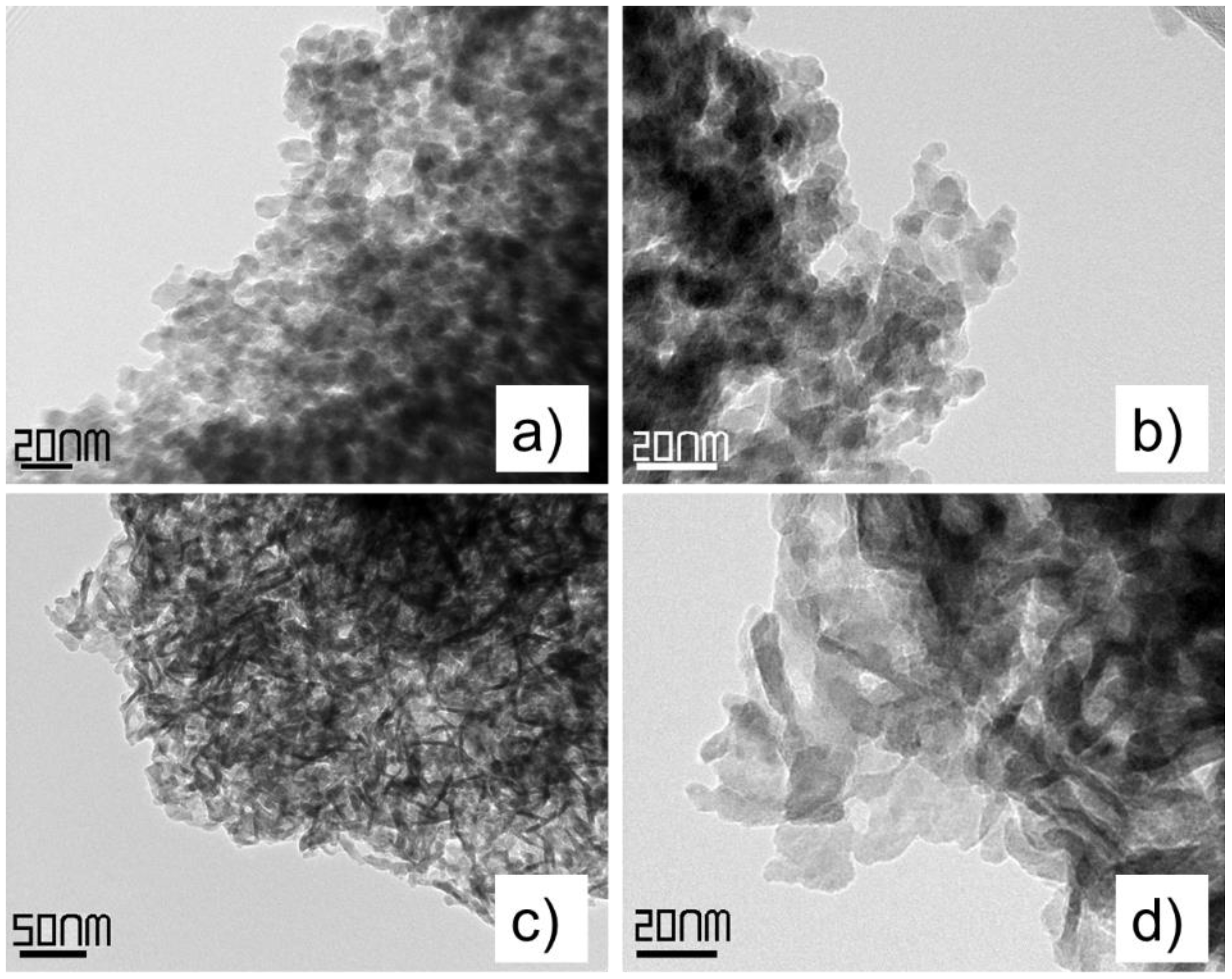
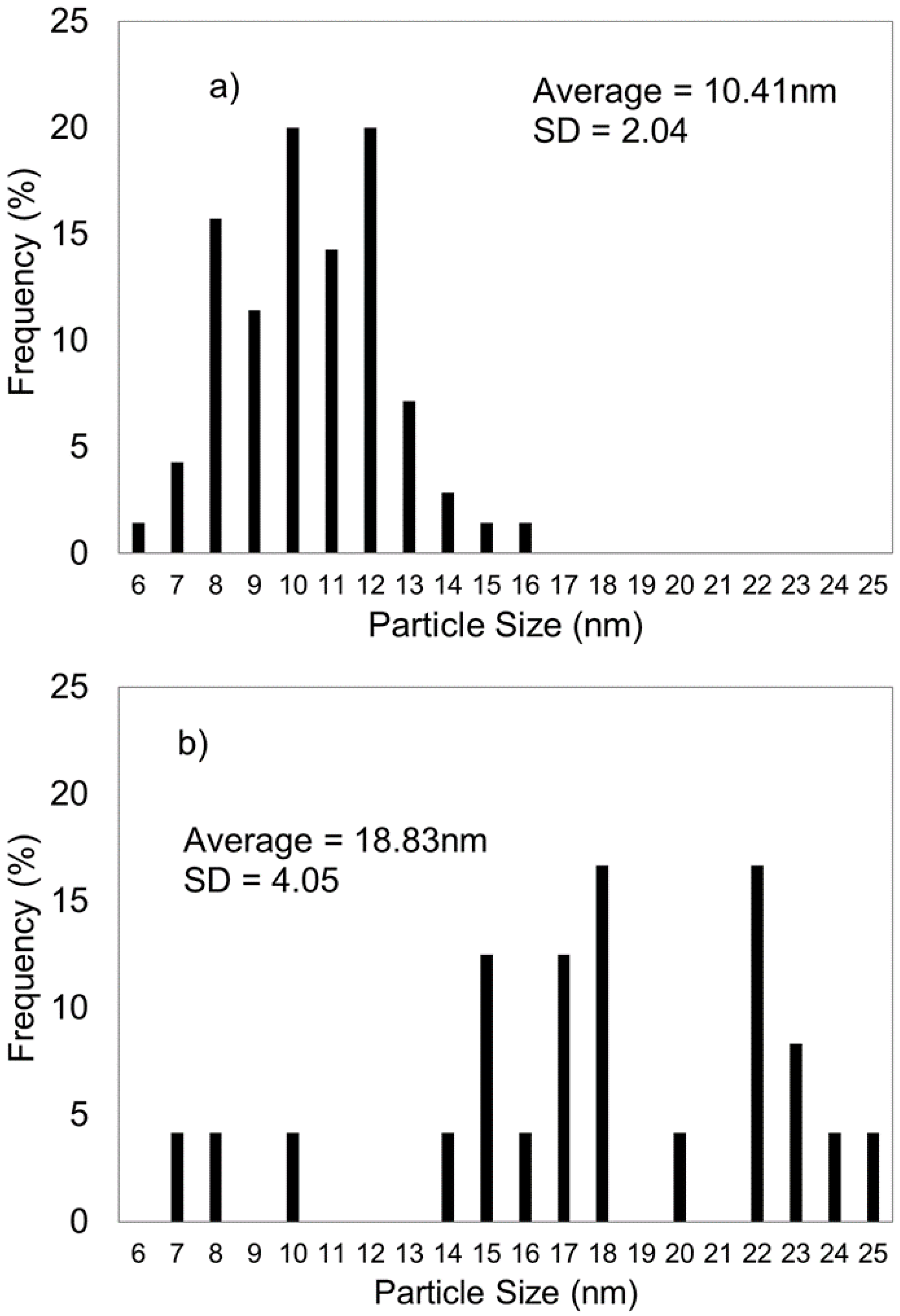
2.1.3. Transmission Electron Microscopy
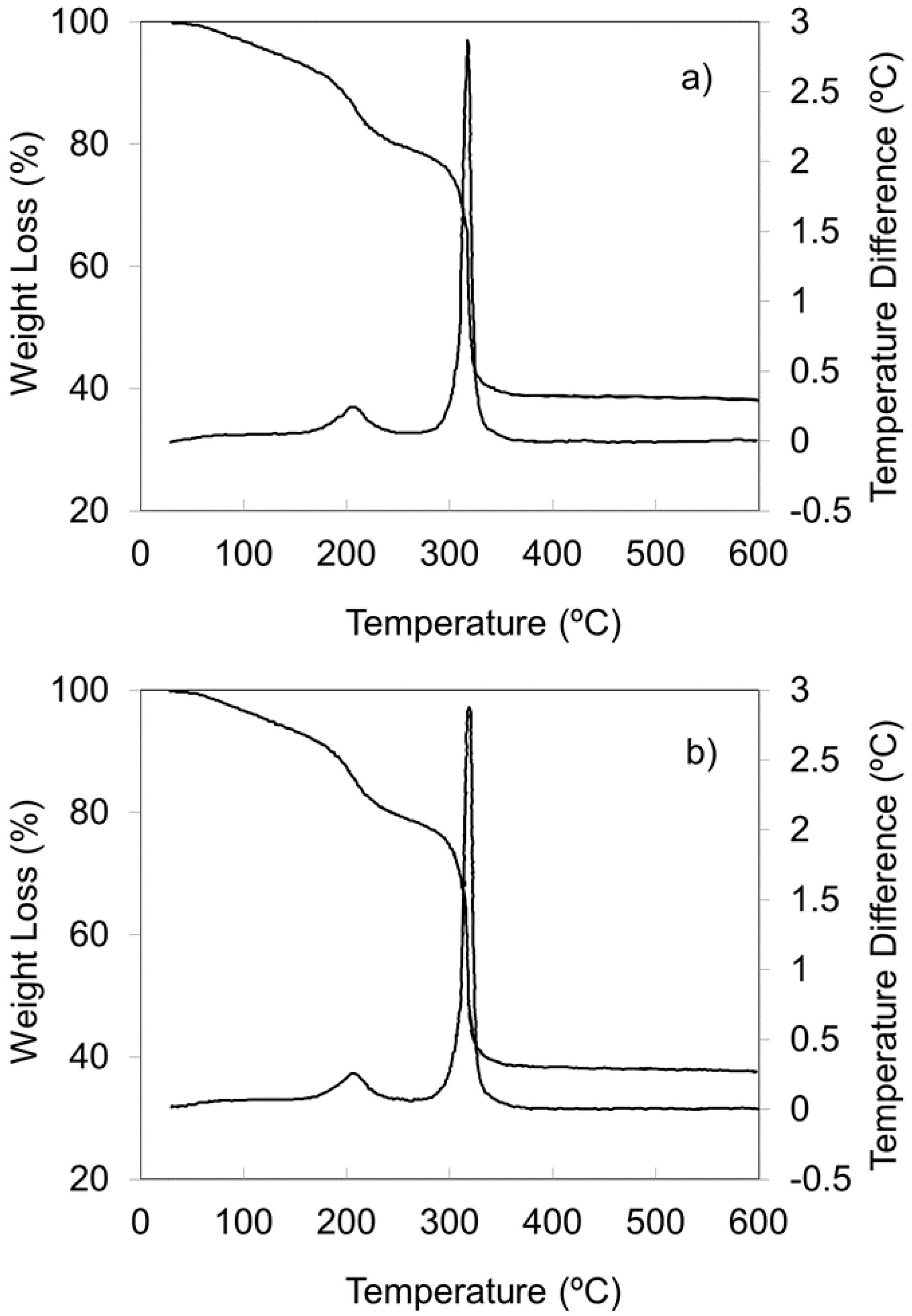
2.1.4. Thermal Gravimetric Analysis
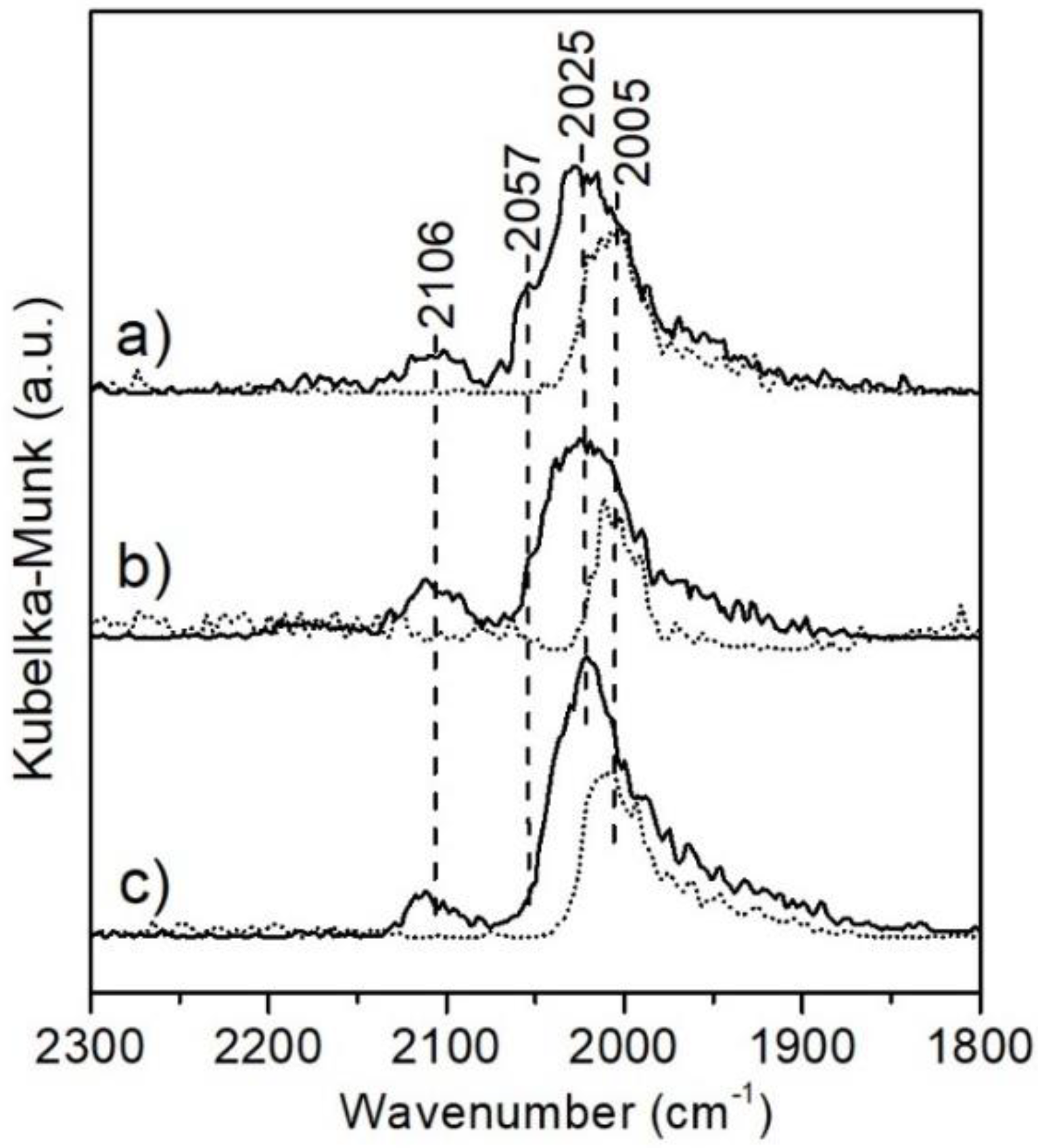
2.1.5. Diffuse Reflectance Infrared Fourier Transform
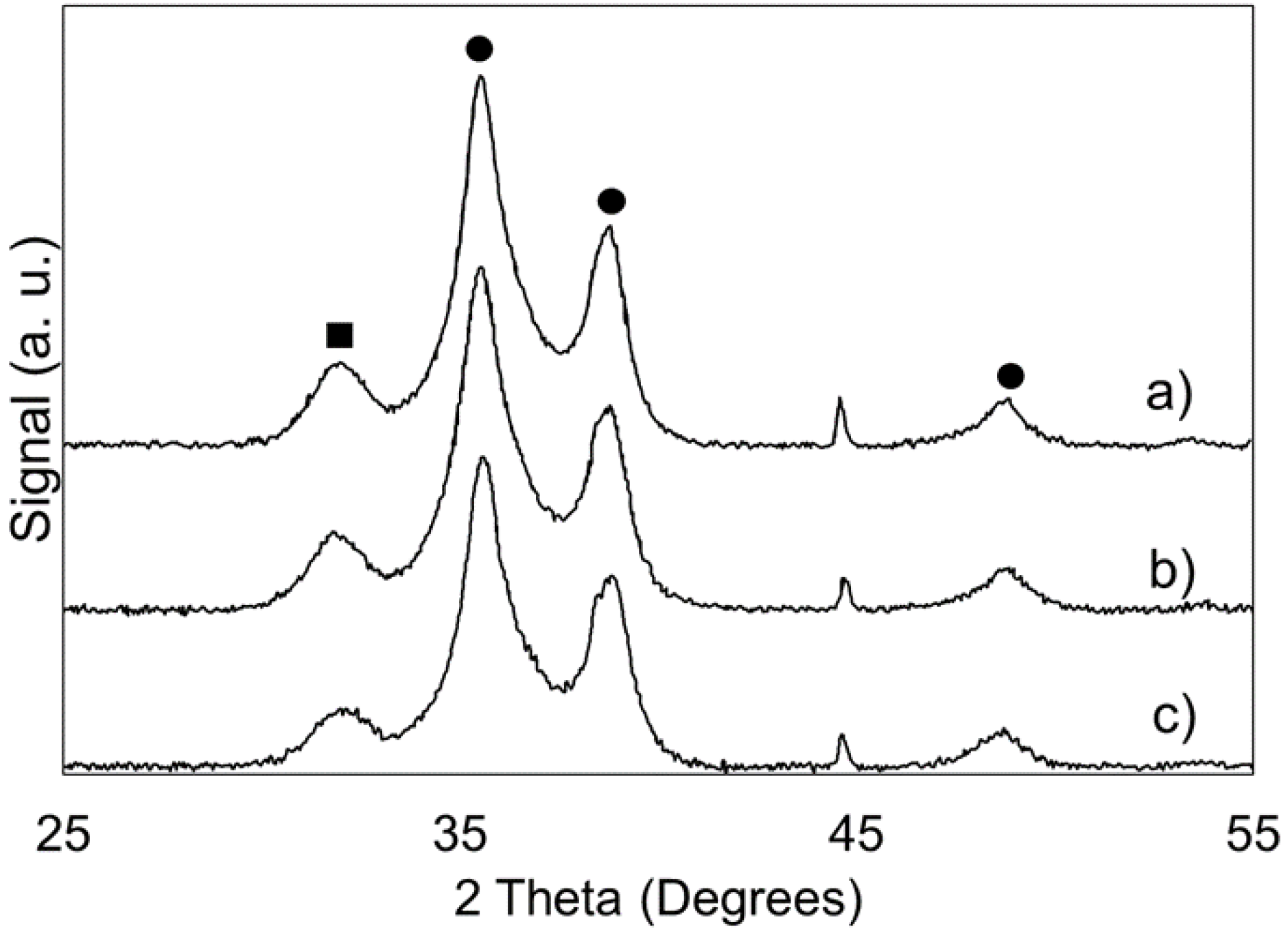
2.1.6. X-ray Diffraction and Other Physicochemical Properties
2.2. Glycerol Hydrogenolysis with In Situ Hydrogen Produced from Methanol Steam Reforming
2.2.1. Effect of Preparation Method for Cu/ZnO/Al2O3 Catalysts
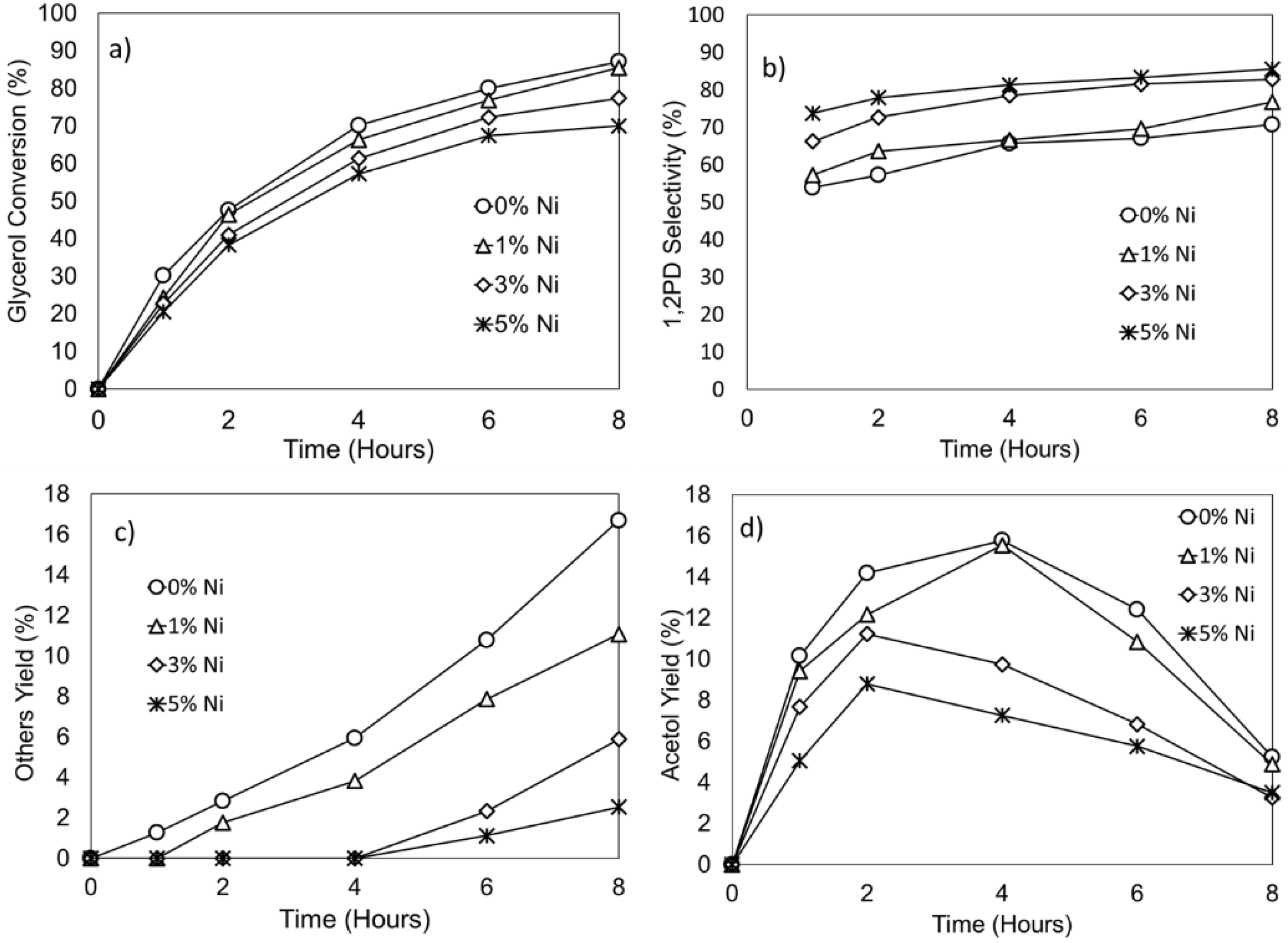
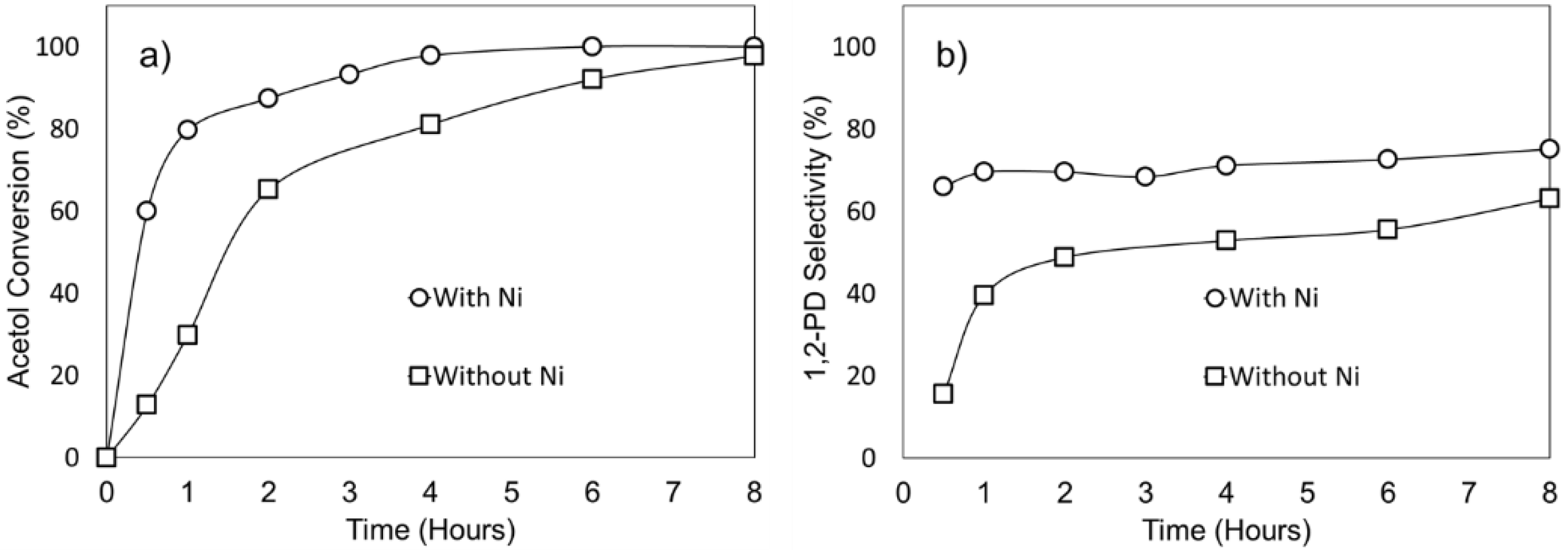
2.2.2. Effect of Ni as a Promoter for the Cu/ZnO/Al2O3-OA Catalysts
3. Materials and Methods
3.1. Materials and Methods for Catalyst Preparation
3.2. Materials and Methods for Catalyst Characterization
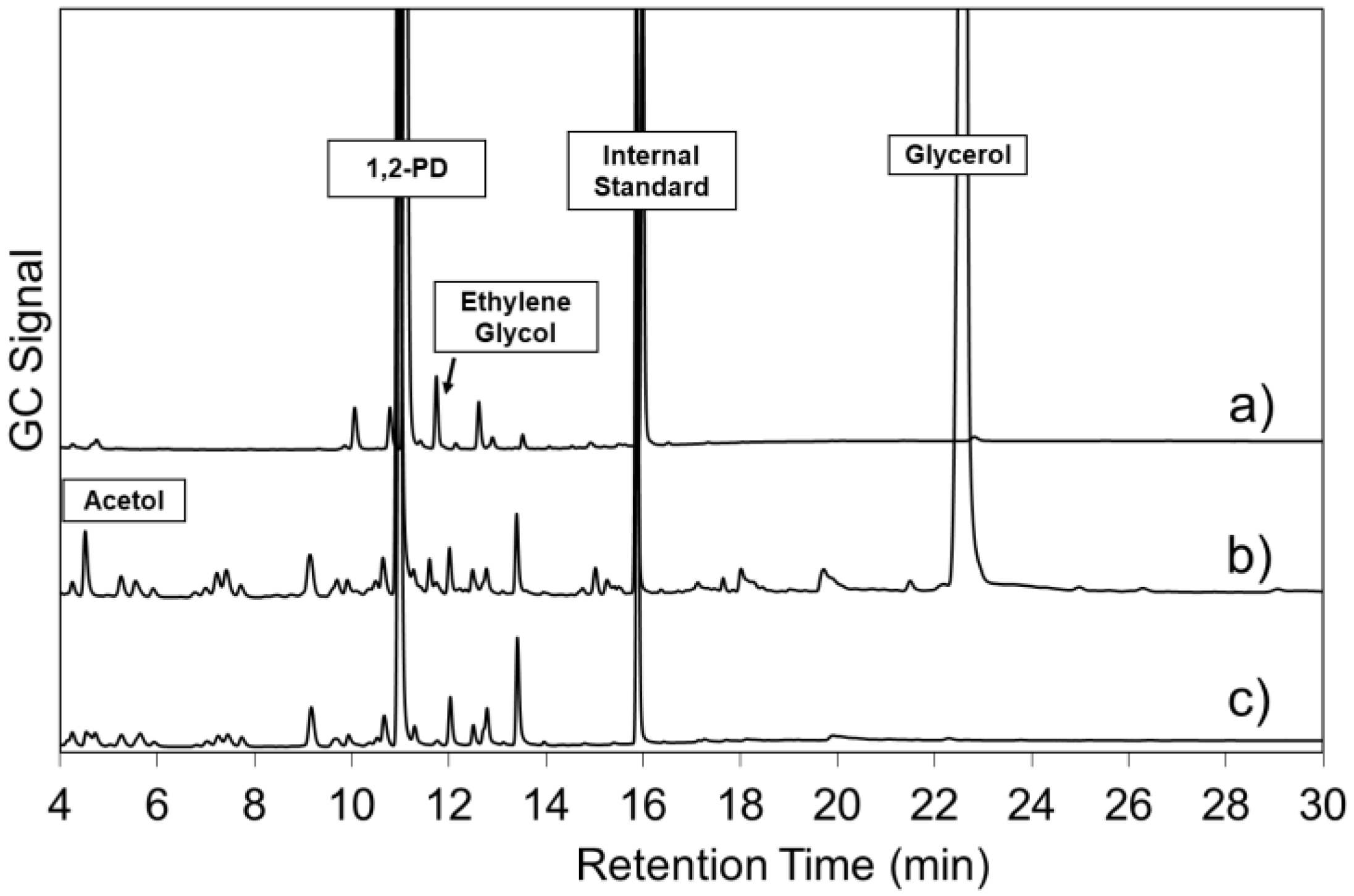
3.3. Materials and Methods for Catalysts Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. The Future of Glycerol, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2010; pp. 1–170. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.; Gallezot, P.; Marion, P.; Pinel, C.; Rosier, C. Glycerol hydrogenolysis on heterogeneous catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H. Selective hydrogenolysis of glycerol to propylene glycol on Cu-ZnO catalysts. Catal. Lett. 2007, 117, 62–67. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol on bimetallic Pd-Cu/solid-base catalysts prepared via layered double hydroxides precursors. Appl. Catal. A 2011, 403, 173–182. [Google Scholar] [CrossRef]
- Xia, S.; Nie, R.; Lu, X.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol over Cu0.4/Zn5.6−xMgxAl2O8.6 catalysts: The role of basicity and hydrogen spillover. J. Catal. 2012, 296, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Pasupulety, N.; Gunda, K.; Rempel, G.L.; Ng, F.T.T. Glycerol hydrogenolysis to 1,2-propanediol by Cu/ZnO/Al2O3 catalysts. Top. Catal. 2014, 57, 1454–1462. [Google Scholar] [CrossRef]
- Ma, L.; He, D.; Li, Z. Promoting effect of rhenium on catalytic performance of Ru catalysts in hydrogenolysis of glycerol to propanediol. Catal. Commun. 2008, 9, 2489–2495. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, Y.; Liang, S.; Liu, H.; Han, B. Hydrogenolysis of glycerol catalyzed by Ru-Cu bimetallic catalysts supported on clay with the aid of ionic liquids. Green Chem. 2009, 11, 1000–1006. [Google Scholar] [CrossRef]
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef]
- D’Hondt, E.; Van de Vyver, S.; Sels, B.F.; Jacobs, P.A. Catalytic glycerol conversion into 1,2-propanediol in absence of added hydrogen. Chem. Commun. 2008, 6011–6012. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Rodrigues, A.E. Glycerol reforming for hydrogen production: A review. Chem. Eng. Technol. 2009, 32, 1463–1469. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Ma, H.; Xu, Z.; Tian, Z. Production of hydrogen by aqueous-phase reforming of glycerol. Int. J. Hydrogen Energy 2008, 33, 6657–6666. [Google Scholar] [CrossRef]
- Barbelli, M.L.; Santori, G.F.; Nichio, N.N. Aqueous phase hydrogenolysis of glycerol to bio-propylene glycol over Pt–Sn catalysts. Bioresour. Technol. 2012, 111, 500–503. [Google Scholar] [CrossRef]
- Roy, D.; Subramaniam, B.; Chaudhari, R.V. Aqueous phase hydrogenolysis of glycerol to 1,2-propanediol without external hydrogen addition. Catal. Today 2010, 156, 31–37. [Google Scholar] [CrossRef]
- Pendem, C.; Gupta, P.; Chaudhary, N.; Singh, S.; Kumar, J.; Sasaki, T.; Datta, A.; Bal, R. Aqueous phase reforming of glycerol to 1,2-propanediol over Pt-nanoparticles supported on hydrotalcite in the absence of hydrogen. Green Chem. 2012, 14, 3107–3113. [Google Scholar] [CrossRef]
- Musolino, M.G.; Scarpino, L.A.; Mauriello, F.; Pietropaolo, R. Selective transfer hydrogenolysis of glycerol promoted by palladium catalysts in absence of hydrogen. Green Chem. 2009, 11, 1511–1513. [Google Scholar] [CrossRef]
- Musolino, M.G.; Scarpino, L.A.; Mauriello, F.; Pietropaolo, R. Glycerol hydrogenolysis promoted by supported palladium catalysts. ChemSusChem 2011, 4, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Gandarias, I.; Arias, P.L.; Requies, J.; El Doukkali, M.; Güemez, M.B. Liquid-phase glycerol hydrogenolysis to 1,2-propanediol under nitrogen pressure using 2-propanol as hydrogen source. J. Catal. 2011, 282, 237–247. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Fernández, S.G.; Requies, J.; El Doukkali, M.; Güemez, M.B. Hydrogenolysis through catalytic transfer hydrogenation: Glycerol conversion to 1,2-propanediol. Catal. Today 2012, 195, 22–31. [Google Scholar] [CrossRef]
- Gandarias, I.; Requies, J.; Arias, P.L.; Armbruster, U.; Martin, A. Liquid-phase glycerol hydrogenolysis by formic acid over Ni–Cu/Al2O3 catalysts. J. Catal. 2012, 290, 79–89. [Google Scholar] [CrossRef]
- Gandarias, I.; Fernández, S.G.; El Doukkali, M.; Requies, J.; Arias, P.L. Physicochemical study of glycerol hydrogenolysis over a Ni-Cu/Al2O3 catalyst using formic acid as the hydrogen source. Top. Catal. 2013, 56, 995–1007. [Google Scholar] [CrossRef]
- Liu, Y. Catalytic Glycerol Hydrogenolysis to Produce 1,2-Propanediol with Molecular Hydrogen and in situ Hydrogen Produced from Steam Reforming. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2014. [Google Scholar]
- Liu, Y.; Ng, F.T.T.; Rempel, G.L. Glycerol hydrogenolysis to 1,2-propanediol with in situ hydrogen produced from methanol steam reforming. In Advances in Chemistry of Energy & Fuels, 250th ed.; American Chemical Society National Meeting & Exposition, Boston, MA, USA, 19 August 2015; American Chemical Society: Washington, DC, USA, 2015; ENFL356. [Google Scholar]
- Gaurav, A.; Leite, M.L.; Ng, F.T.T.; Rempel, G.L. Transesterification of triglyceride to fatty acid alkyl esters (biodiesel): Comparison of utility requirements and capital costs between reaction separation and catalytic distillation configurations. Energy Fuels 2013, 27, 6847–6857. [Google Scholar] [CrossRef]
- De Sousa Maia, A.C.; e Silva, I.S.; Stragevitch, L. Liquid-liquid equilibrium of methyl esters of fatty acid/methanol/glycerol and fatty acid ethyl esters/ethanol/glycerol: A case study for biodiesel application. Int. J. Chem. Eng. Appl. 2013, 4, 285–289. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Yfanti, V.-L.; Lemonidou, A.A. One-pot tandem processing of glycerol stream to 1,2-propanediol with methanol reforming as hydrogen donor reaction. Appl. Catal. B 2015, 163, 258–266. [Google Scholar] [CrossRef]
- Yfanti, V.-L.; Vasiliadou, E.S.; Lemonidou, A.A. Glycerol hydro-deoxygenation aided by in situ H2 generation via methanol aqueous phase reforming over a Cu-ZnO-Al2O3 catalyst. Catal. Sci. Technol. 2016, 6, 5415–5426. [Google Scholar] [CrossRef]
- Yfanti, V.-L.; Lemonidou, A.A. Mechanistic study of liquid phase glycerol hydrodeoxygenation with in-situ generated hydrogen. J. Catal. 2018, 368, 98–111. [Google Scholar] [CrossRef]
- Yfanti, V.-L.; Ipsakis, D.; Lemonidou, A.A. Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst. React. Chem. Eng. 2018, 3, 559–571. [Google Scholar] [CrossRef]
- Yfanti, V.-L.; Ipsakis, D.; Lemonidou, A.A. Kinetic model of glycerol hydrodeoxygenation under inert conditions over copper catalyst. Mater. Today Proc. 2018, 5, 27482–27490. [Google Scholar] [CrossRef]
- Chiu, C.; Tekeei, A.; Ronco, J.M.; Banks, M.; Suppes, G.J. Reducing byproduct formation during conversion of glycerol to propylene glycol. Ind. Eng. Chem. Res. 2008, 47, 6878–6884. [Google Scholar] [CrossRef]
- Van Ryneveld, E.; Mahomed, A.S.; van Heerden, P.S.; Friedrich, H.B. Direct hydrogenolysis of highly concentrated glycerol solutions over supported Ru, Pd and Pt catalyst systems. Catal. Lett. 2011, 141, 958–967. [Google Scholar] [CrossRef]
- Bienholz, A.; Schwab, F.; Claus, P. Hydrogenolysis of glycerol over a highly active CuO/ZnO catalyst prepared by an oxalate gel method: Influence of solvent and reaction temperature on catalyst deactivation. Green Chem. 2010, 12, 290–295. [Google Scholar] [CrossRef]
- Shen, J.; Song, C. Influence of preparation method on performance of Cu/Zn-based catalysts for low-temperature steam reforming and oxidative steam reforming of methanol for H2 production for fuel cells. Catal. Today 2002, 77, 89–98. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Yao, C.; Cao, Y.; Dai, W.; He, H.; Fan, K. A highly efficient Cu/ZnO/Al2O3 catalyst via gel-coprecipitation of oxalate precursors for low-temperature steam reforming of methanol. Catal. Lett. 2005, 102, 183–190. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Takezawa, N. Steam reforming of methanol over Ni, Co, Pd and Pt supported on ZnO. React. Kinet. Catal. Lett. 1995, 55, 349–353. [Google Scholar] [CrossRef]
- Qi, C.; Amphlett, J.C.; Peppley, B.A. Methanol steam reforming over NiAl and Ni (M) Al layered double hydroxides (M = Au, Rh, Ir) derived catalysts. Catal. Lett. 2005, 104, 57–62. [Google Scholar] [CrossRef]
- Jiang, T.; Huai, Q.; Geng, T.; Ying, W.; Xiao, T.; Cao, F. Catalytic performance of Pd–Ni bimetallic catalyst for glycerol hydrogenolysis. Biomass Bioenergy 2015, 78, 71–79. [Google Scholar] [CrossRef]
- Banu, M.; Sivasanker, S.; Sankaranarayanan, T.M.; Venuvanalingam, P. Hydrogenolysis of sorbitol over Ni and Pt loaded on NaY. Catal. Commun. 2011, 12, 673–677. [Google Scholar] [CrossRef]
- Pompeo, F.; Santori, G.F.; Nichio, N.N. Hydrogen production by glycerol steam reforming with Pt/SiO2 and Ni/SiO2 catalysts. Catal. Today 2011, 172, 183–188. [Google Scholar] [CrossRef]
- Damyanova, S.; Spojakina, A.; Jiratova, K. Effect of mixed titania-alumina supports on the phase composition of NiMo/TiO2-Al2O3 catalysts. Appl. Catal. A 1995, 125, 257–269. [Google Scholar] [CrossRef]
- Niemantsverdriet, J.W. Spectroscopy in Catalysis: An Introduction, Third, Completely Revised and Enlarged, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 13–21. [Google Scholar]
- Sun, Q.; Zhang, Y.; Chen, H.; Deng, J.; Wu, D.; Chen, S. A novel process for the preparation of Cu/ZnO and Cu/ZnO/Al2O3 ultrafine catalyst: Structure, surface properties, and activity for methanol synthesis from CO2+H2. J. Catal. 1997, 167, 92–105. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Chen, M.; Liu, Y.; Cao, Y.; Fan, K.N. Structural evolution and catalytic properties of nanostructured Cu/ZrO2 catalysts prepared by oxalate gel-coprecipitation technique. J. Phys. Chem. C 2007, 111, 16549–16557. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Venkov, T.; Knözinger, H. FTIR spectroscopic study of CO adsorption on Cu/SiO2: Formation of new types of copper carbonyls. Catal. Lett. 2001, 75, 55–59. [Google Scholar] [CrossRef]
- Li, D.; Sakai, S.; Nakagawa, Y.; Tomishige, K. FTIR study of CO adsorption on Rh/MgO modified with Co, Ni, Fe, or CeO2 for the catalytic partial oxidation of methane. Phys. Chem. Chem. Phys. 2012, 14, 9204–9213. [Google Scholar] [CrossRef]
- Poncelet, G.; Centeno, M.A.; Molina, R. Characterization of reduced α-alumina-supported nickel catalysts by spectroscopic and chemisorption measurements. Appl. Catal. A 2005, 288, 232–242. [Google Scholar] [CrossRef]
- Garland, C.W.; Lord, R.C.; Troiano, P.F. Infrared spectrum of carbon monoxide chemisorbed on evaporated nickel films. J. Phys. Chem. 1965, 69, 1195–1203. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Z.; Lu, G. Improvement of Cu/Zn-based catalysts by nickel additive in methanol decomposition. Appl. Catal. A 2002, 225, 77–86. [Google Scholar] [CrossRef]
- Cheng, W. Reaction and XRD studies on Cu based methanol decomposition catalysts: Role of constituents and development of high-activity multicomponent catalysts. Appl. Catal. A 1995, 130, 13–30. [Google Scholar] [CrossRef]
- Wu, H.; Chung, S. Kinetics of hydrogen production of methanol reformation using Cu/ZnO/Al2O3 catalyst. J. Comb. Chem. 2007, 9, 990–997. [Google Scholar] [CrossRef]
- Meher, L.C.; Gopinath, R.; Naik, S.N.; Dalai, A.K. Catalytic hydrogenolysis of glycerol to propylene glycol over mixed oxides derived from a hydrotalcite-type precursor. Ind. Eng. Chem. Res. 2009, 48, 1840–1846. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Liu, H. Selective hydrogenolysis of glycerol to propylene glycol on Cu–ZnO composite catalysts: Structural requirements and reaction mechanism. Chem. Asian J. 2010, 5, 1100–1111. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Vila, F.; Mariscal, R.; Jiménez-López, A. Hydrogenolysis of glycerol to obtain 1,2-propanediol on Ce-promoted Ni/SBA-15 catalysts. Appl. Catal. B 2012, 117–118, 253–259. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, J.; Ma, H.; Miao, H.; Song, Q.; Xu, J. Aqueous hydrogenolysis of glycerol over Ni–Ce/AC catalyst: Promoting effect of Ce on catalytic performance. Appl. Catal. A 2010, 383, 73–78. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Investigating the performance and deactivation behaviour of silica-supported copper catalysts in glycerol hydrogenolysis. Appl. Catal. A 2011, 396, 177–185. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Eggenhuisen, T.M.; Munnik, P.; de Jongh, P.E.; de Jong, K.P.; Lemonidou, A.A. Synthesis and performance of highly dispersed Cu/SiO2 catalysts for the hydrogenolysis of glycerol. Appl. Catal. B 2014, 145, 108–119. [Google Scholar] [CrossRef]
- Sato, S.; Akiyama, M.; Takahashi, R.; Hara, T.; Inui, K.; Yokota, M. Vapor-phase reaction of polyols over copper catalysts. Appl. Catal. A 2008, 347, 186–191. [Google Scholar] [CrossRef]


| Catalysts | Number of Acidic Sites | Total Acidic Sites |
|---|---|---|
| mmolNH3/g-cat | mmolNH3/g-cat | |
| Cu/ZnO/Al2O3-Na 1 | 0.04 (107.0–392.0 °C) | 0.040 |
| Cu/ZnO/Al2O3-OA 1 | 0.216 (50.0–290.0 °C) | 0.347 |
| 0.056 (290.0–470.0 °C) | ||
| 0.075 (590.0–800.0 °C) | ||
| 1% (molar) Ni/Cu/ZnO/Al2O3-OA 2 | 0.198 (50.0–290.0 °C) | 0.342 |
| 0.072 (290.0–470.0 °C) | ||
| 0.072 (590.0–800.0 °C) | ||
| 5% (molar) Ni/Cu/ZnO/Al2O3-OA 3 | 0.197 (50.0–590.0 °C) | 0.298 |
| 0.071 (290.0–470.0 °C) | ||
| 0.030 (590.0–800.0 °C) |
| Crystal Size of CuO 1 nm | Crystal Size of ZnO 1 nm | Cu Surface Area m2/g-cat | Metal Composition 2 (Theoretical Value) | |
|---|---|---|---|---|
| 0% Ni | 14.7 | 10.2 | 18.8 | 37.1/36.4/26.5 (35.0/35.0/30.0) |
| 1% Ni | 14.2 | 11.6 | 18.1 | 0.9/36.2/35.6/27.3 (1.0/34.5/34.5/30.0) |
| 5% Ni | 15.0 | 10.9 | 15.8 | 4.7/33.1/34.4/27.8 (5.0/32.5/32.5/30.0) |
| Catalysts | Glycerol Conversion | 1,2-PD Selectivity | 1,2-PD Yield | Acetol Yield | EG 5 Yield | Propanol Yield | Others Yield |
|---|---|---|---|---|---|---|---|
| Cu/ZnO/Al2O3-Na 2 | 60.3 | 29.1 | 17.5 | 16.1 | 0.0 | 0.0 | 26.7 |
| Cu/ZnO/Al2O3-OA 2 | 87.1 | 70.7 | 61.6 | 5.2 | 2.9 | 0.7 | 16.7 |
| Cu/ZnO/Al2O3-OA 3 | 80.2 | 65.7 | 52.7 | 9.5 | 2.6 | 0.5 | 14.9 |
| Cu/ZnO/Al2O3-OA 4 | 82.7 | 69.9 | 57.8 | 9.2 | 2.0 | 0.9 | 12.7 |
| Ni Molar Content | Glycerol Conversion | 1,2-PD Selectivity | 1,2-PD Yield | Acetol Yield | EG Yield | Propanol Yield | Others Yield |
|---|---|---|---|---|---|---|---|
| 0% Ni 2 | 87.1 | 70.7 | 61.6 | 5.2 | 2.9 | 0.7 | 16.7 |
| 1% Ni 3 | 85.5 | 76.7 | 65.6 | 4.9 | 3.4 | 0.6 | 11.1 |
| 3% Ni 4 | 77.5 | 82.8 | 64.2 | 3.2 | 3.6 | 0.5 | 5.9 |
| 5% Ni 5 | 70.0 | 85.5 | 59.9 | 3.5 | 3.5 | 0.6 | 2.5 |
| 5% Ni 5,6 | 80.8 | 81.2 | 64.8 | 2.5 | 6.8 | 1.1 | 5.6 |
| 5%Ni 5,7 | 87.0 | 82.9 | 72.1 | 1.3 | 7.7 | 1.2 | 4.7 |
| Catalysts | Glycerol Conversion | 1,2-PD Selectivity | 1,2-PD Yield | Methanol Conversion | Mole Balance |
|---|---|---|---|---|---|
| Cu/ZnO/Al2O3-OA 2 | 100.0 | 77.0 | 77.0 | 17.7 | 96.5 |
| Ni/Cu/ZnO/Al2O3-OA 3 | 97.4 | 86.0 | 83.8 | 21.6 | 100.4 |
| Ni/ZnO/Al2O3-OA 4 | 4.8 | 0.0 | 0.0 | 23.3 | 94.6 |
| Catalysts | Acetol Conversion | 1,2-PD Selectivity | 1,2-PD Yield | Others Yield | Rate Constant (s−1) |
|---|---|---|---|---|---|
| Cu/ZnO/Al2O3-OA 2 | 97.7 | 63.1 | 61.6 | 36.1 | 1.231 × 10−4 |
| 5% Ni/Cu/ZnO/Al2O3-OA 3 | 100.0 | 75.1 | 75.1 | 24.9 | 2.721 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Guo, X.; Rempel, G.L.; Ng, F.T.T. The Promoting Effect of Ni on Glycerol Hydrogenolysis to 1,2-Propanediol with In Situ Hydrogen from Methanol Steam Reforming Using a Cu/ZnO/Al2O3 Catalyst. Catalysts 2019, 9, 412. https://doi.org/10.3390/catal9050412
Liu Y, Guo X, Rempel GL, Ng FTT. The Promoting Effect of Ni on Glycerol Hydrogenolysis to 1,2-Propanediol with In Situ Hydrogen from Methanol Steam Reforming Using a Cu/ZnO/Al2O3 Catalyst. Catalysts. 2019; 9(5):412. https://doi.org/10.3390/catal9050412
Chicago/Turabian StyleLiu, Yuanqing, Xiaoming Guo, Garry L. Rempel, and Flora T. T. Ng. 2019. "The Promoting Effect of Ni on Glycerol Hydrogenolysis to 1,2-Propanediol with In Situ Hydrogen from Methanol Steam Reforming Using a Cu/ZnO/Al2O3 Catalyst" Catalysts 9, no. 5: 412. https://doi.org/10.3390/catal9050412