2.1. XRD Analysis
Figure 1a shows the XRD patterns of the Ni-Mo sulfide/Al
2O
3 catalysts with different Ni/Mo molar ratios. The characteristic diffraction peaks of each catalyst observed at 2θ about 14.4°, 32.7°, and 58.4° were attributed to the (0 0 2), (1 0 0), and (1 1 0) planes of MoS
2 (JCPDS#65-1951), respectively. Similarly, the peaks located at 2θ of 27.2°, 31.5°, 35.3°, 38.8°, 45.1°, 53.5°, 56.1°, 58.6°, and 61.0° were assigned to the (1 1 1), (2 0 0), (2 1 0), (2 1 1), (2 2 0), (3 1 1), (2 2 2), (0 2 3), and (3 2 1) crystal surfaces of the NiS
2 phase (JCPDS#65-3325), respectively. Meanwhile, the diffraction peaks of Al
2O
3 were also detectable. Especially, the XRD patterns also exhibited a variation trend correlated to the chemical compositions. With an increase in the Ni/Mo molar ratio, the diffraction peaks shifted to slightly higher 2θ values, as seen in
Figure 1b. The Ni
2+ ions replaced the position of Mo ions or entered the gap position of MoS
2 to form the Ni-Mo-S
x phase [
15]. Since the radius of Ni
2+ ion is bigger than the radius of Mo
4+ ion, the lattice parameters of Ni-Mo sulfides increased with increasing Ni content. Therefore, it can be deduced that the weak diffraction peaks of MoS
2 with an increase in the content of Ni may be related to the formation of the Ni-Mo-S
x phase. In addition, the other Ni and Mo species were in the states of NiS
2 and MoS
2, respectively. These sulfides were uniformly mixed, owing to the metal ions being uniformly dispersed on the support. In other words, Ni-Mo-S
x, NiS
2, and MoS
2 were well mixed and highly dispersed on the support.
In addition, the weak and broad peaks illustrate that the sulfide particles were highly dispersed with particles in small nanoscale size. Based on the Scherrer equation, the average particle sizes of the Ni-Mo sulfide/Al
2O
3 catalysts were calculated. As shown in
Table 1, the average particle sizes were estimated to be 7.8, 9.1, 10.2, 11.5, and 13.9 nm for the Ni-Mo sulfide/Al
2O
3 catalysts of which Ni/Mo molar ratios were 2/6, 3/5, 4/4, 5/3, and 6/2, respectively, which is agreement with the sizes of the nanoparticles in the TEM analysis. Moreover, as the Ni/Mo molar ratio increased, the average particle sizes gradually increased. The change in the average particle size can be explained by an increase in the Ni content. In addition, the surface areas of the various Ni-Mo sulfide/Al
2O
3 catalysts are compared in
Table 1. It seems that the Ni/Mo molar ratio did not significantly affect the surface areas.
2.2. UV-Visible Analysis
Figure 2a shows the UV-visible spectra of the Ni-Mo sulfide/Al
2O
3 catalysts with different Ni/Mo molar ratios, compared with NiS
2 and MoS
2. From the UV-visible spectra, the band edge of MoS
2 was located around 600−800 nm, which belonged to the absorption of visible-light. Compared with MoS
2, with increasing the Ni/Mo molar ratio, the absorption boundaries of the Ni-Mo sulfide/Al
2O
3 catalysts were gradually red shifted. Continuous shift of the absorption boundaries suggests that the band gaps of the Ni-Mo sulfide/Al
2O
3 catalysts can be controllably adjusted through changing the Ni/Mo molar ratio. The relationship between the incident photon energy and the absorption coefficient of a semiconductor can be determined by the Kubelka–Munk equation [
16,
17]:
where α is the absorption coefficient and its value can be achieved by the equation: α = (1 − R)
2/2R; R is the diffuse reflectance and its relationship with absorbance can be defined by R = 10
−A; A is absorbance. ν is frequency, h is Planck’s constant, and C is a constant. For a direct transition semiconductor, n = 1; for an indirect transition semiconductor, n = 4. The nature of transition is possible to be determined through plotting the graph of (αhν)
2 versus hν; therefore, the band gap energies can be deduced by extrapolating the straight-linear portions of the plot to intersect the photon energy axis. As shown in
Figure 2b and
Table 1, the band gaps obtained in such a way were 2.17, 2.00, 1.81, 1.56, and 1.30 eV for the Ni-Mo sulfide/Al
2O
3 catalysts, of which Ni/Mo molar ratios were 2/6, 3/5, 4/4, 5/3, and 6/2, respectively. For all the catalysts, the influence of the chemical compositions of the Ni-Mo sulfide/Al
2O
3 catalysts on the band gap can be observed. When the Ni/Mo molar ratio increased, the band gap decreased gradually. This indicates that changing the Ni/Mo molar ratio can significantly adjust the band gaps of the Ni-Mo sulfide/Al
2O
3 catalysts. Meanwhile, the changes in band gaps also illustrate that the relative redox abilities of the Ni-Mo sulfide/Al
2O
3 catalysts were effectively changed.
2.3. TEM Analysis
The morphologies and microstructures of the Ni-Mo sulfide/Al
2O
3 catalyst with Ni/Mo of 5/3 are presented in
Figure 3. The TEM images suggest that the highly distributed nanoparticles were dispersed on the supports, which can prevent their grain growth. Furthermore, it also reveals that the average particle size was about 10 nm, which was in agreement with the particle size estimated from Scherrer equation (shown in
Table 1). As shown in
Figure 3d, the observed interlayer spacing of 6.1 Å, which was identical to the lattice fringe of Ni-Mo-S
x, was bigger than that of the (0 0 2) plane of MoS
2 (6.0 Å) [
18]. It can be attributed to the bigger radius of Ni
2+ ions. Meanwhile, the interplanar spacings were about 2.8 and 3.2 Å, which correspond to the (2 0 0) and (1 1 1) planes of NiS
2, respectively [
19,
20]. These results of TEM analysis are in good agreement with the XRD analysis.
In addition, the porosity of the catalyst also plays an important role in the generation of an electric field in non-thermal plasma. As shown in
Table 1, the obtained Ni-Mo sulfide/Al
2O
3 catalysts had high surface areas (>200 m
2/g). As pointed by Fridman [
21], the porous material in the gap refracts the electric field, enhancing the local field by a factor of over 10 depending on the porosity of the materials. The electric field can excite the Ni-Mo sulfide semiconductor to generate electron–hole pairs, which plays an extremely important role in converting CO
2 and H
2S. Simultaneously, the strong electric field is beneficial for delaying the recombination of electron–hole pairs, thereby extending their lifetime.
2.4. XPS Analysis
X-ray photoelectron spectroscopy (XPS) analysis was carried out to study the chemical state and surface ratio of MoS
2, NiS
2, and Ni-Mo sulfide/Al
2O
3 catalysts with different Ni/Mo molar ratios.
Figure 4 shows the Mo 3d and Ni 2p spectra of the various catalysts. For Mo element, as shown in
Figure 4a, the observed binding energy (BE) of Mo 3d
5/2 was about 229.0 eV, indicating that the Mo species were Mo
4+ [
22]. In
Figure 4b, for the case of Ni, the main peaks at the BE of about 855.0 eV can be attributed to the Ni 2p
3/2 peaks of Ni
2+ [
22]. However, as the Ni/Mo molar ratio increased, the peak position of Mo 3d
5/2 gradually shifted toward the peak position of the lower BE, accompanied by the BE shift of Ni 2p
3/2. This phenomenon indicates the increased electron density in Mo 3d
5/2, resulting from the electron donating property of Ni 2p
3/2. Therefore, a strong electron interaction between Ni and Mo occurs on the catalyst surface, wherein electrons likely transfer from the Ni species to the Mo species in the Ni-Mo sulfide/Al
2O
3 catalysts.
Table 2 shows the surface and total Ni/Mo ratio of Ni-Mo sulfide/Al
2O
3 catalysts with different Ni/Mo molar ratios. As presented, the total Ni/Mo molar ratio was consistent with the theoretical ratio. However, these total Ni/Mo ratios (0.31İ2.97) were higher than the surface Ni/Mo ratio (0.18-2.41). This is because Ni
2+ ions were intercalated into the gap position of the MoS
2 lattice, and a large number of Mo vacancies could be generated. Therefore, the surface of the Ni-Mo sulfide/Al
2O
3 catalysts became slightly Ni-depleted.
2.5. Catalytic Evaluation for the One-Step Conversion of CO2 and H2S to Syngas
The catalytic performances of the various Ni-Mo sulfide/Al
2O
3 catalysts were evaluated through converting CO
2 and H
2S into syngas in non-thermal plasma. For comparison, the performances of NiS
2/Al
2O
3 and MoS
2/Al
2O
3 were also investigated. As seen in
Figure 5a,b, all the Ni-Mo sulfide/Al
2O
3 catalysts possessed better activities in CO
2 and H
2S conversion than NiS
2/Al
2O
3 and MoS
2/Al
2O
3 catalysts, and the CO
2 and H
2S conversions could reach high levels. The experimental results show that the Ni/Mo molar ratio had a great influence on the conversion of CO
2 and H
2S. As the Ni/Mo molar ratio increased, the catalytic activity presented a primary enhancement followed by a decline. The CO
2 and H
2S conversions were strongly dependent on the SEI (Specific energy input). At SEI of 60.0 kJ/L, CO
2 conversions were 25.1%, 45.0%, 46.2%, 46.9%, 47.7%, 56.3%, and 49.0%, and H
2S conversions were 87.8%, 93.7%, 94.8%, 95.7%, 96.4%, 98.9%, and 97.3% when NiS
2/Al
2O
3, MoS
2/Al
2O
3, 2Ni-6Mo/Al
2O
3, 3Ni-5Mo/Al
2O
3, 4Ni-4Mo/Al
2O
3, 5Ni-3Mo/Al
2O
3, and 6Ni-2Mo/Al
2O
3 were filled in the gap, respectively. Especially, the 5Ni-3Mo/Al
2O
3 catalyst exhibited the best catalytic performance and achieved relatively high CO
2 and H
2S conversions with the lowest SEI.
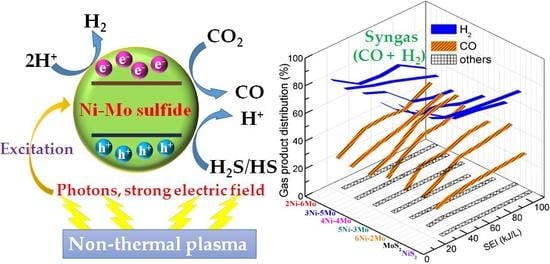
In addition, as seen in
Figure 6a,b, the major products were CO and H
2 in the CO
2 and H
2S conversion. CO and H
2 concentrations were in line with SEI, which indicates that the behavior for CO
2 and H
2S conversion had relatively stronger dependence on the energy input. An increase of SEI could generate more active H species and obviously promote CO
2 activation and CO production, together with the decrease in H
2 yields. Meanwhile, very small amounts of light hydrocarbons (others: CH
4, C
2H
4, and C
2H
6) were also generated. The selectivity to light hydrocarbons was very low (<2%) during the reaction. Furthermore, there were not any C
3+ hydrocarbons. Therefore, this novel method may produce clean syngas. Additionally, it was also found that SEI strongly affected the H
2/CO ratio. In
Figure 7, when SEI was changed from 20 to 110 kJ/L, the H
2/CO ratio considerably decreased from about 4.5 to 1.0, which illustrates that the H
2/CO ratio strongly depends on the energy input. An increase of SEI could induce the decrease in the H
2/CO ratio. Hence, the H
2/CO ratio can be controllably adjusted on a large scale through varying SEI by this method.
A series of characterizations of the Ni-Mo sulfide/Al
2O
3 catalysts displays that the Ni/Mo molar ratio had a significant effect on the physical and chemical properties of the catalyst. We have reported that the synergistic effects of semiconductor catalyst and non-thermal plasma in the H
2S decomposition [
12]. In the present work, the Ni-Mo sulfide/Al
2O
3 catalyst in non-thermal plasma can be excited by both the strong electric field and UV-visible light irradiation, and thus generate highly active hole–electron pairs. The hole–electron pairs will react with the adsorbed surface species, thereby accelerating the conversion of CO
2 and H
2S. Hence, since the generated hole–electron pairs are sufficiently reactive to convert CO
2 and H
2S to H
2 and CO, the rate of CO
2 and H
2S conversion depends on the number of electron–hole pairs generated on the surface of the Ni-Mo sulfide/Al
2O
3 catalyst. A higher number of hole–electron pairs may be linked to the relatively higher behavior on CO
2 and H
2S conversion. From the results of UV-vis spectra (shown in
Figure 2 and
Table 1), the change in the Ni/Mo molar ratio affects the optical properties of the Ni-Mo sulfide/Al
2O
3 catalyst. With increasing the Ni/Mo molar ratio, a monotonous variation in the absorption in visible light region and band gap of Ni-Mo sulfide could be clearly found. For a semiconductor catalyst with a narrower band gap, less energy for electrons is required to jump from valence band (VB) to conduction band (CB). Therefore, a decrease in band gap can lead to the increase in the amount of hole–electron pairs. Moreover, the other optical properties of semiconductor catalyst, such as conduction band position and valence band position, are also related to its chemical compositions. According to the XRD and TEM results, the Ni-Mo sulfide possessed the layer structure, the Ni
2+ ions can replace the position of Mo ions or enter the gap position of MoS
2 to form Ni-Mo-S
x phase. Hence, the suitable impurity energy level could be provided through a proper doping amount of Ni
2+ ions into MoS
2. The presence of impurity levels leads to the easy injection of the excited electrons from VB to CB of MoS
2.
In addition, all the Ni-Mo sulfide/Al
2O
3 catalysts exhibited relatively high BET surface areas (shown in
Table 1). The high surface area facilitates photon absorption, provides more active sites, and reduces the distance of generated carriers from the catalyst surface [
23]. Moreover, the average particle size was around 10 nm. The small nanoparticles with low crystallinity are favorable for the fast electron transportation from bulk to surface, which prevent the recombination of the generated electrons and holes of the catalyst [
24]. Therefore, the reduction in the particle size of the Ni-Mo sulfide/Al
2O
3 catalyst also contributes to the improvement of the catalytic activity.
Additionally, the Ni2+ ions can be evenly incorporated into the MoS2 lattice to form Ni-Mo-Sx phase, which would bring about the Mo vacancies formation. Ideally, the incorporation of two Ni2+ ions may generate one Mo vacancy. Therefore, the incorporation of Ni2+ ions can produce a large amount of Mo vacancies. Mo vacancies favor the separation of the energy-induced electrons and holes, which induce the high catalytic performance in CO2 and H2S conversion, compared to the MoS2/Al2O3 catalyst. Nevertheless, the 6Ni-2Mo/Al2O3 catalyst with higher Ni content possessed relatively stronger visible light absorption capacity than other Ni-Mo sulfide/Al2O3 catalysts, but the CO2 and H2S conversions were lower. The reason for the low catalytic activity over the 6Ni-2Mo/Al2O3 catalyst may be that the excessive amount of Ni would result in the unevenly distributed Ni2+ ions. Owing to the higher concentration of Ni, the probability of electron–hole recombination was regarded to become comparably high. Consequently, a superfluous increase in the Ni/Mo molar ratio not only encumbered the light absorption, but also offered more recombination sites for hole–electron pairs, so the catalytic activity was repressed. In particular, when the Ni/Mo molar ratio was 5/3, the Ni-Mo sulfide/Al2O3 catalyst exhibited the best catalytic activity for CO2 and H2S conversion with the most proper optical and structural properties.
Figure 8 presents the CO
2 and H
2S conversion, and H
2/CO molar ratio variations over 5Ni-3Mo/Al
2O
3 during the long-term test. The results demonstrate that the catalytic activity did not exhibit loss in the runs. The XPS spectra and SEM images were taken before and after evaluation, as shown in
Figures S1 and S2 (Supplementary Materials), respectively. There was no obvious difference detected in the spent 5Ni-3Mo/Al
2O
3 catalyst after the reaction test. Moreover,
Figure S3 (Supplementary Materials) shows a comparison between the fresh 5Ni-3Mo/Al
2O
3 catalyst and the spent one after reaction tests in the XRD patterns. According to the Scherrer equation, the average particle size of the 5Ni-3Mo/Al
2O
3 catalyst increased from 11.5 to 13.2 nm after the 50 h long-term test, proving high stability of the active phases on the 5Ni-3Mo/Al
2O
3 catalyst in the CO
2 and H
2S conversion process. Furthermore, the surface area decreased only from about 250 to 231 m
2/g. The active phases were highly dispersed in Al
2O
3 support, which could also prevent the agglomeration formation and inhibit the growth of the particles. Hence, it is clear that Ni-Mo sulfide underwent no variation in non-thermal plasma and can maintain stable structures in the plasma-induced CO
2 and H
2S conversion.