Microstructural Changes in La0.5Ca0.5Mn0.5Fe0.5O3 Solid Solutions under the Influence of Catalytic Reaction of Methane Combustion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
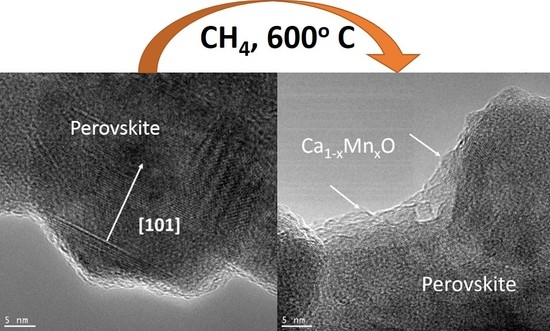
2.2. La0.5Ca0.5Mn0.5Fe0.5O3 Catalytic Activity and Microstructure Modifications in the Methane Combustion Reaction
2.3. Formation Process of the Ca-Mn-O Phase on the Surface of the Perovskite in He Atmosphere
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Najjar, H.; Batis, H. Development of Mn-based perovskite materials: Chemical structure and applications. Catal. Rev. 2016, 58, 371–438. [Google Scholar] [CrossRef]
- Keav, S.; Matam, S.; Ferri, D.; Weidenkaff, A.; Keav, S.; Matam, S.K.; Ferri, D.; Weidenkaff, A. Structured Perovskite-Based Catalysts and Their Application as Three-Way Catalytic Converters—A Review. Catalysts 2014, 4, 226–255. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Liang, L.-Z.; Liang, S.; Zhu, Y.-Y.; Zhu, X.-H. Recent progress on the structural characterizations of domain structures in ferroic and multiferroic perovskite oxides: A review. J. Eur. Ceram. Soc. 2015, 35, 411–441. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Zhang, H.W.; Sun, J.R.; Shen, B.G.; Franco, V. A phenomenological fitting curve for the magnetocaloric effect of materials with a second-order phase transition. J. Appl. Phys. 2008, 103, 116101. [Google Scholar] [CrossRef]
- Izyumov, Y.A.; Skryabin, Y.N. Double exchange model and the unique properties of the manganites. Uspekhi Fiz. Nauk 2001, 171, 121. [Google Scholar]
- Haghiri-Gosnet, A.-M.; Renard, J.-P. CMR manganites: Physics, thin films and devices. J. Phys. D Appl. Phys. 2003, 36, R127–R150. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I.; Kaliaguine, S.; Prellier, W. Perovskites and Related Mixed Oxides: Concepts and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9783527686605. [Google Scholar]
- Takacs, M.; Hoes, M.; Caduff, M.; Cooper, T.; Scheffe, J.R.; Steinfeld, A. Oxygen nonstoichiometry, defect equilibria, and thermodynamic characterization of LaMnO3 perovskites with Ca/Sr A-site and Al B-site doping. Acta Mater. 2016, 103, 700–710. [Google Scholar]
- Elsiddig, Z.A.; Xu, H.; Wang, D.; Zhang, W.; Guo, X.; Zhang, Y.; Sun, Z.; Chen, J. Modulating Mn 4+ Ions and Oxygen Vacancies in Nonstoichiometric LaMnO 3 Perovskite by a Facile Sol-Gel Method as High-Performance Supercapacitor Electrodes. Electrochim. Acta 2017, 253, 422–429. [Google Scholar]
- Malkhandi, S.; Yang, B.; Manohar, A.K.; Manivannan, A.; Prakash, G.K.S.; Narayanan, S.R. Electrocatalytic Properties of Nanocrystalline Calcium-Doped Lanthanum Cobalt Oxide for Bifunctional Oxygen Electrodes. J. Phys. Chem. Lett. 2012, 3, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, B.; Okal, J.; Tylus, W.; Winiarski, J.; Szczygieł, B. The effect of the calcination temperature of LaFeO3 precursors on the properties and catalytic activity of perovskite in methane oxidation. Ceram. Int. 2019, 45, 2779–2788. [Google Scholar] [CrossRef]
- Mueller, D.N.; Machala, M.L.; Bluhm, H.; Chueh, W.C. Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions. Nat. Commun. 2015, 6, 6097. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yuan, F.; Niu, X.; Zhu, Y. Preparation of Pd supported on La(Sr)-Mn-O Perovskite by microwave Irradiation Method and Its Catalytic Performances for the Methane Combustion. Sci. Rep. 2016, 6, 19511. [Google Scholar] [CrossRef] [PubMed]
- De Santana Santos, M.; Neto, R.C.R.; Noronha, F.B.; Bargiela, P.; da Rocha, M.D.G.C.; Resini, C.; Brandão, S.T. Perovskite as catalyst precursors in the partial oxidation of methane: The effect of cobalt, nickel and pretreatment. Catal. Today 2018, 299, 229–241. [Google Scholar]
- Roseno, K.T.C.; Brackmann, R.; da Silva, M.A.; Schmal, M. Investigation of LaCoO3, LaFeO3 and LaCo0.5Fe0.5O3 perovskites as catalyst precursors for syngas production by partial oxidation of methane. Int. J. Hydrogen Energy 2016, 41, 18178–18192. [Google Scholar] [CrossRef]
- De, K.T.; Schmal, M.; Brackmann, R.; Alves, R.M.B.; Giudici, R. Partial oxidation of methane on neodymium and lanthanium chromate based perovskites for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 8166–8177. [Google Scholar]
- Alifanti, M.; Auer, R.; Kirchnerova, J.; Thyrion, F.; Grange, P.; Delmon, B. Activity in methane combustion and sensitivity to sulfur poisoning of La1−xCexMn1−yCoyO3 perovskite oxides. Appl. Catal. B Environ. 2003, 41, 71–81. [Google Scholar] [CrossRef]
- Marchetti, L.; Forni, L. Catalytic combustion of methane over perovskites. Appl. Catal. B Environ. 1998, 15, 179–187. [Google Scholar] [CrossRef]
- Szabo, V.; Bassir, M.; Van Neste, A.; Kaliaguine, S. Perovskite-type oxides synthesized by reactive grinding: Part IV. Catalytic properties of LaCo1−xFexO3 in methane oxidation. Appl. Catal. B Environ. 2003, 43, 81–92. [Google Scholar]
- Banerjee, A.; McGuire, J.; Lawnick, O.; Bozack, M. Low-Temperature Activity and PdO-PdOx Transition in Methane Combustion by a PdO-PdOx/γ-Al2O3 Catalyst. Catalysts 2018, 8, 266. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Support Interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Bassil, J.; AlBarazi, A.; Boutros, M. Catalytic combustion of methane over mesoporous silica supported palladium. Catal. Today 2011, 176, 36–40. [Google Scholar] [CrossRef]
- Choudhary, T.; Banerjee, S.; Choudhary, V. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A Gen. 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y. Nanostructured perovskite oxides as promising substitutes of noble metals catalysts for catalytic combustion of methane. Chin. Chem. Lett. 2018, 29, 252–260. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Melo Jorge, M.; Correia dos Santos, A.; Nunes, M. Effects of synthesis method on stoichiometry, structure and electrical conductivity of CaMnO3−δ. Int. J. Inorg. Mater. 2001, 3, 915–921. [Google Scholar] [CrossRef]
- Tan, L.; Gu, X.; Yang, L.; Jin, W.; Zhang, L.; Xu, N. Influence of powder synthesis methods on microstructure and oxygen permeation performance of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite-type membranes. J. Membr. Sci. 2003, 212, 157–165. [Google Scholar] [CrossRef]
- Leontiou, A.; Ladavos, A.; Bakas, T.; Vaimakis, T.; Pomonis, P. Reverse uptake of oxygen from La1−xSrx (Fe3+/Fe4+)O3±δ perovskite-type mixed oxides (x = 0.00, 0.15, 0.30, 0.40, 0.60, 0.70, 0.80, 0.90). Appl. Catal. A Gen. 2003, 241, 143–154. [Google Scholar] [CrossRef]
- Ponce, S.; Peña, M.; Fierro, J.L. Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Appl. Catal. B Environ. 2000, 24, 193–205. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Wei, G.; Zheng, A.; Li, H.; Zhao, Z. Perovskite-type LaFe1−x MnxO3 (x=0, 0.3, 0.5, 0.7, 1.0) oxygen carriers for chemical-looping steam methane reforming: Oxidation activity and resistance to carbon formation. Korean J. Chem. Eng. 2017, 34, 1651–1660. [Google Scholar] [CrossRef]
- Zheng, S.; Hua, Q.; Gu, W.; Liu, B. Catalytic oxidation of CO on LaMn1−xFexO3 perovskites solid solution. J. Mol. Catal. A Chem. 2014, 391, 7–11. [Google Scholar] [CrossRef]
- Isupova, L.A.; Gerasimov, E.Y.; Zaikovskii, V.I.; Tsybulya, S.V.; Kulikovskaya, N.A.; Saputina, N.F. Synthesis of homogeneous La1–xCaxMnO3 solid solutions by the Pechini method and their activity in methane oxidation. Kinet. Catal. 2009, 50, 886–891. [Google Scholar] [CrossRef]
- Isupova, L.A.; Kulikovskaya, N.A.; Saputina, N.F.; Gerasimov, E.Y.; Tsybulya, S.V. La1-xCaxFeO3-δ (x = 0-1) perovskites prepared by the Pechini method: Catalytic activity in deep methane and CO oxidation. Kinet. Catal. 2015, 56, 781–787. [Google Scholar] [CrossRef]
- Shaheen, R.; Bashir, J.; Rundlöf, H.; Rennie, A.R. The crystal structure of CaLaMnFeO6 double perovskite. Mater. Lett. 2005, 59, 2296–2299. [Google Scholar] [CrossRef]
- Othmani, S.; Balli, M.; Cheikhrouhou, A. Structural, magnetic and magnetocaloric properties of La0.6Ca0.4Mn1−xFexO3 (x=0, 0.05, 0.1, 0.15 and 0.2) manganites. Solid State Commun. 2014, 192, 51–55. [Google Scholar] [CrossRef]
- Gerasimov, E.Y.; Isupova, L.A.; Tsybulya, S.V. Microstructural features of the La1−xCaxFeO3−δ solid solutions prepared via Pechini route. Mater. Res. Bull. 2015, 70, 291–295. [Google Scholar] [CrossRef]
- Isupova, L.A.; Gerasimov, E.Y.; Zaikovskii, V.I.; Tsybulya, S.V. Effect of the reaction medium on the structure of the La1−xCaxMnO3 (x = 0–1) solid solutions prepared by the pechini method. Kinet. Catal. 2011, 52, 104–110. [Google Scholar] [CrossRef]
- Jay, A.H.; Andrews, K.W. Note on oxide systems pertaining to steel - making furnace slags: FeO-MnO, FeO-MgO, CaO-MnO, MgO-MnO. J. Iron Steel Inst. 1946, 152, 15–18. [Google Scholar]
- Yakovleva, I.S.; Isupova, L.A.; Rogov, V.A.; Sadykov, V.A. Forms of oxygen in La1−xCaxMnO3 + δ (x = 0–1) perovskites and their reactivities in oxidation reactions. Kinet. Catal. 2008, 49, 261–270. [Google Scholar] [CrossRef]
- Isupova, L.A.; Yakovleva, I.S.; Rogov, V.A.; Alikina, G.M.; Sadykov, V.A. Oxygen States in Oxides with a Perovskite Structure and Their Catalytic Activity in Complete Oxidation Reactions: System La1 – xCaxFeO3 – y (x = 0–1). Kinet. Catal. 2004, 45, 446–453. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]









| Sample Name/Mass % | La | Ca | Mn | Fe |
|---|---|---|---|---|
| La0.5Ca0.5Mn0.5Fe0.5O3 | 32.8 | 8.7 | 13.8 | 14.2 |
| Temperature, °C | СН4 Conversion, % |
|---|---|
| 400 | 25.46 |
| 450 | 60.71 |
| 500 | 93.72 |
| 550 | 99.52 |
| 600 | 100 |
| 500 | 90.02 |
| Temperature, °C | a, Å | b, Å | c, Å | V, Å3 |
|---|---|---|---|---|
| 30 | 5.43 | 7.67 | 5.47 | 227.92 |
| 300 | 5.46 | 7.71 | 5.47 | 230.51 |
| 600 | 5.53 | 7.83 | 5.54 | 239.66 |
| 750 | 5.57 | 7.87 | 5.57 | 244.18 |
| cooling | 5.53 | 7.81 | 5.54 | 238.72 |
| Samples | Mn/Fe | Fe/La | Mn/La | Mn/Ca | Fe/Ca | La/Ca |
|---|---|---|---|---|---|---|
| Initial | 1.15 | 0.69 | 0.80 | 0.36 | 0.31 | 0.45 |
| He treatment | 1.16 | 0.61 | 0.70 | 0.21 | 0.18 | 0.30 |
| After reaction | 0.97 | 0.76 | 0.73 | 0.26 | 0.27 | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimov, E.Y.; Rogov, V.A.; Prosvirin, I.P.; Isupova, L.A.; Tsybulya, S.V. Microstructural Changes in La0.5Ca0.5Mn0.5Fe0.5O3 Solid Solutions under the Influence of Catalytic Reaction of Methane Combustion. Catalysts 2019, 9, 563. https://doi.org/10.3390/catal9060563
Gerasimov EY, Rogov VA, Prosvirin IP, Isupova LA, Tsybulya SV. Microstructural Changes in La0.5Ca0.5Mn0.5Fe0.5O3 Solid Solutions under the Influence of Catalytic Reaction of Methane Combustion. Catalysts. 2019; 9(6):563. https://doi.org/10.3390/catal9060563
Chicago/Turabian StyleGerasimov, Evgeny Yu., Vladimir A. Rogov, Igor P. Prosvirin, Lyubov A. Isupova, and Sergey V. Tsybulya. 2019. "Microstructural Changes in La0.5Ca0.5Mn0.5Fe0.5O3 Solid Solutions under the Influence of Catalytic Reaction of Methane Combustion" Catalysts 9, no. 6: 563. https://doi.org/10.3390/catal9060563