Pollution-Free Approaches for Highly Efficient Sapphire Substrate Processing by Mechanical Chemical Polishing
Abstract
:1. Introduction
2. Results and discussion
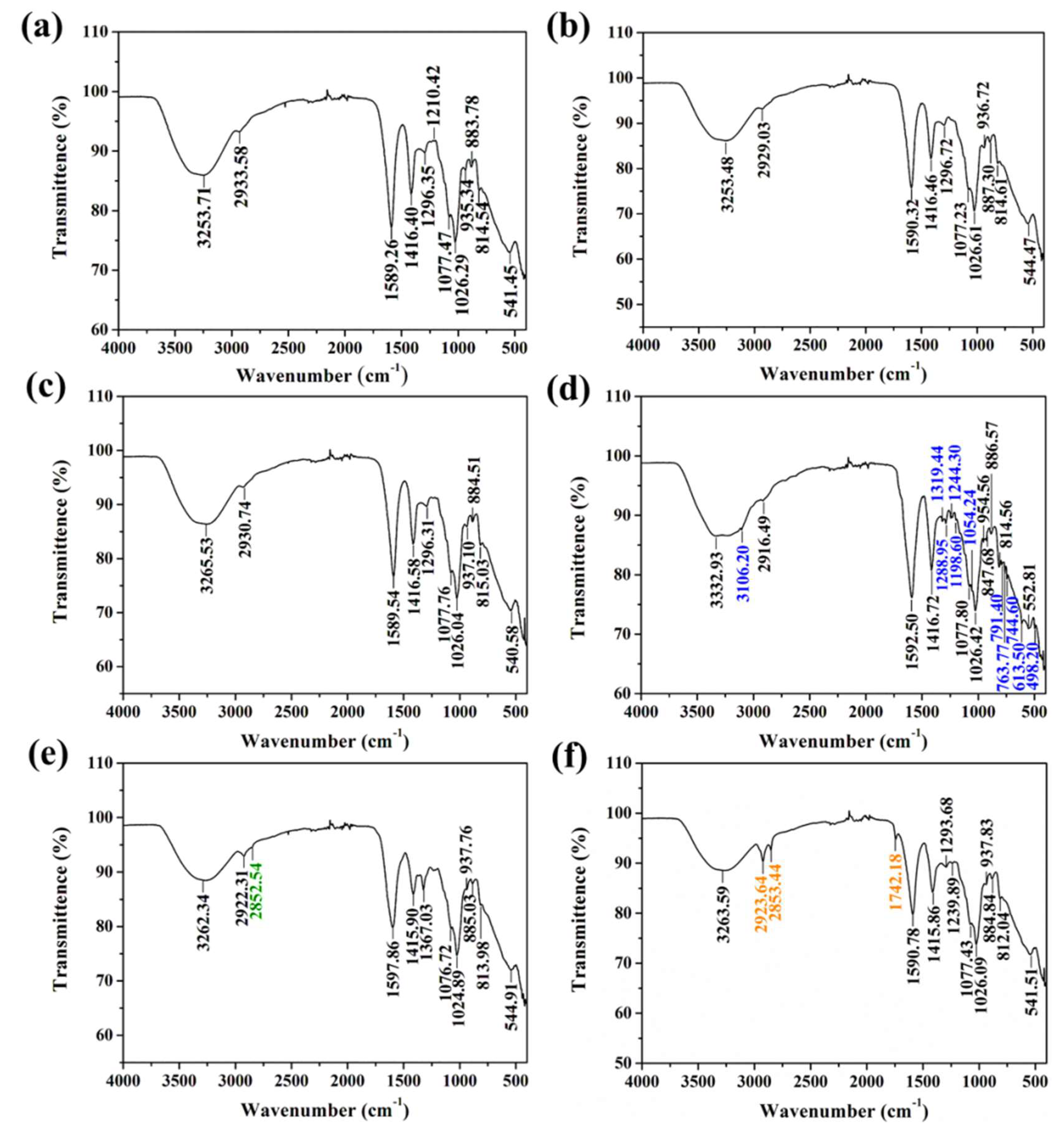
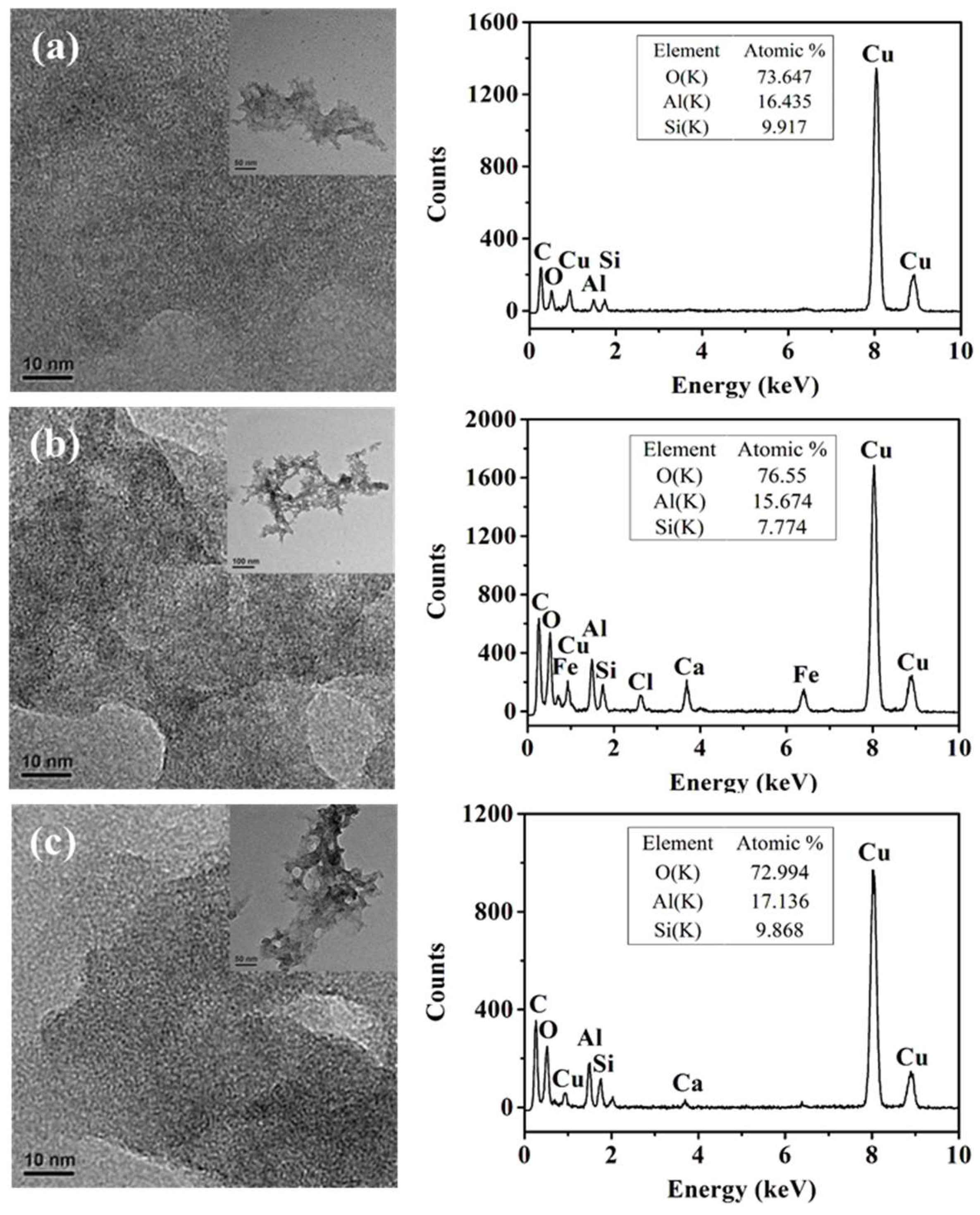
2.1. Characterization of Polishing Pad with Different Catalysts
2.2. The Polishing Performance on Sapphire Substrate under Different Catalyzing Modes
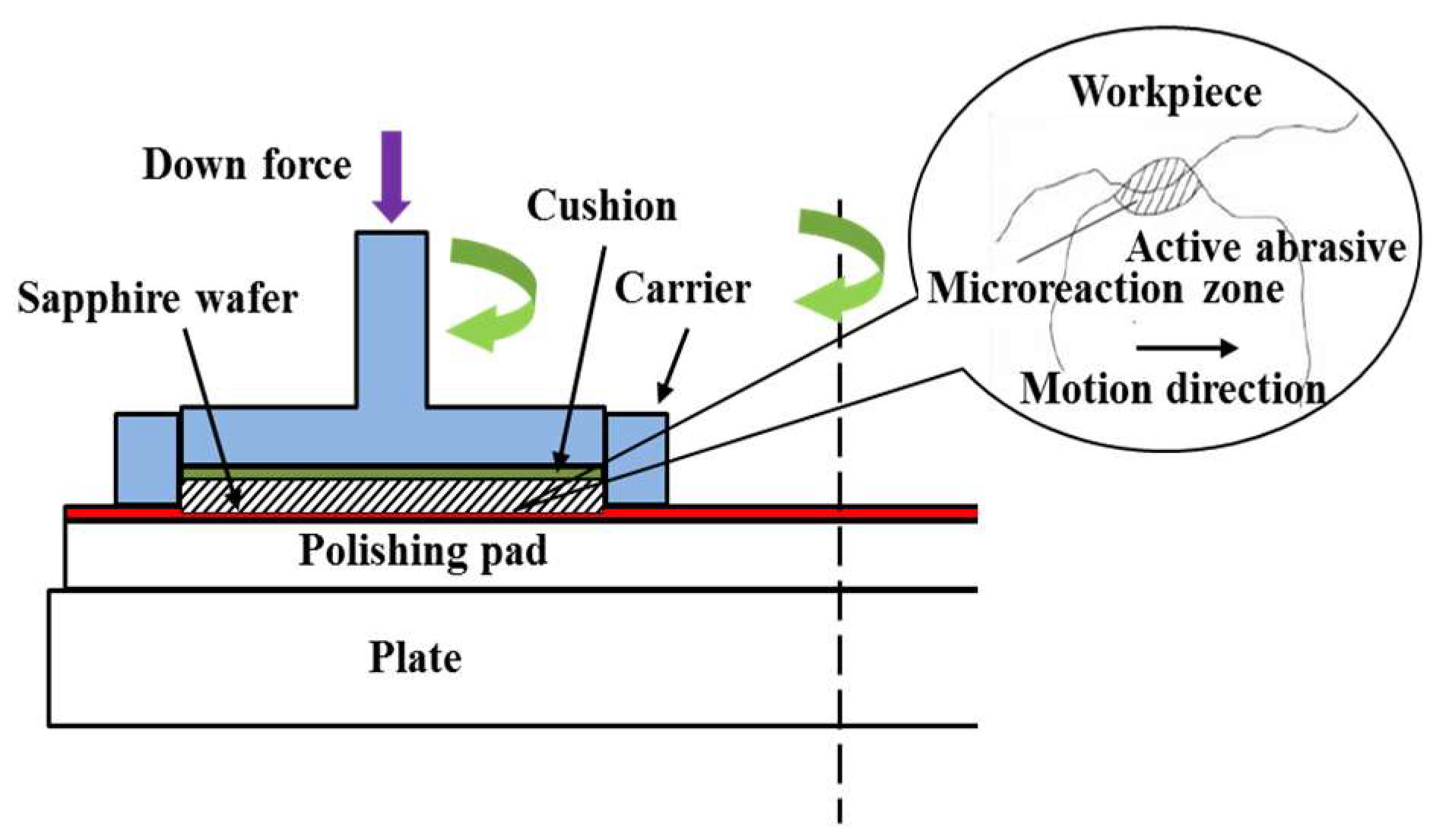
2.3. The Sapphire Substrate Removal Mechanism
3. Experimental Section
3.1. Synthesis of Soft Abrasives with High Reactivity
3.2. Organic Acid- and Base-Assisted Polishing
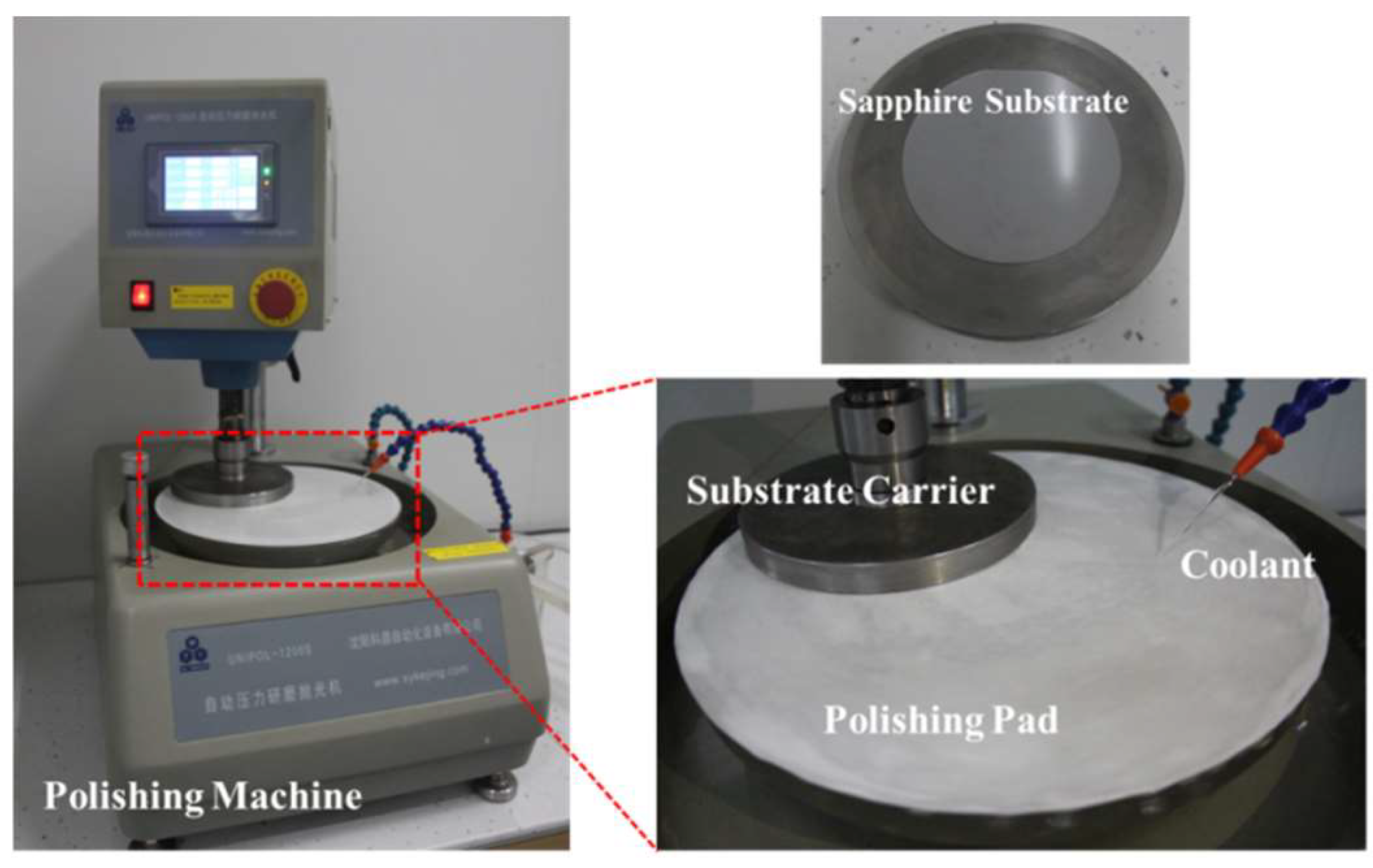
3.3. Polishing Setup and Process Control
3.4. Characterization Methods
4. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Kim, H.; Lee, S.; Jeong, H. Effect of initial deflection of diamond wire on thickness variation of sapphire wafer in multi-wire saw. Int. J. Pr. Eng. 2015, 2, 117–121. [Google Scholar] [CrossRef]
- Wang, J.; Feng, P.; Zhang, J.; Zhang, C.; Pei, Z. Modeling the dependency of edge chipping size on the material properties and cutting force for rotary ultrasonic drilling of brittle materials. Int. J. Mach. Tool. Manuf. 2016, 101, 18–27. [Google Scholar] [CrossRef]
- Lee, T.; Jeong, H.; Kim, H.; Lee, S.; Kim, D. Effect of platen shape on evolution of total thickness variation in single-sided lapping of sapphire wafer. Int. J. Pr. Eng. 2016, 3, 225–229. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, D.; Zhang, F.; Gan, Y. AFM characterization of patterned sapphire substrate with dense cone arrays: Image artifacts and tip-cone convolution effect. Appl. Surf. Sci. 2018, 433, 358–366. [Google Scholar] [CrossRef]
- Alombert-Goget, G.; Li, H.; Guyot, Y.; Lebbou, K. Luminescence and coloration of undoped and Ti-doped sapphire crystals grown by Czochralski technique. J. Lumin. 2016, 169, 516–519. [Google Scholar] [CrossRef]
- Jang, S.; Jeong, H.; Yuh, M.; Park, I.; Park, J. Effect of glycine on copper CMP. Int. J. Pr. Eng. 2016, 3, 155–159. [Google Scholar] [CrossRef]
- Lei, H.; Tong, K. Preparation of La-doped colloidal SiO2 composite abrasives and their chemical mechanical polishing behavior on sapphire substrates. Precis. Eng. 2016, 44, 124–130. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, G.; Shi, X.; Zhang, S.; Gong, H. Effects of ultra-smooth surface atomic step morphology on chemical mechanical polishing (CMP) performances of sapphire and SiC wafers. Tribol. Int. 2015, 87, 145–150. [Google Scholar] [CrossRef]
- Lee, H. Environmental impact of concentration of slurry components in thick copper CMP. Int. J. Pr. Eng. 2017, 1, 13–18. [Google Scholar] [CrossRef]
- Park, C.; Kim, H.; Lee, S.; Jeong, H. The Influence of abrasive size on high-pressure chemical mechanical polishing of sapphire wafer. Int. J. Pr. Eng. 2015, 2, 157–162. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Xu, X. The Research of reactivity between nano-abrasives and sapphire during polishing process. Integr. Ferroelectr. 2015, 159, 41–48. [Google Scholar] [CrossRef]
- Yasunaga, N.; Obara, A.; Imanaka, O. Effect of solid state reaction on wear of sapphire sliding on steel. Int. J. Jpn. S. Prec. Eng. 1978, 44, 717–723. [Google Scholar] [CrossRef]
- Liu, C.M.; Chen, J.C.; Chen, C.J. The growth of an epitaxial Mg-Al spinel layer on sapphire by solid-state reactions. J. Cryst. Growth 2005, 285, 275–283. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Xu, X. Study on planarization machining of sapphire wafer with soft-hard mixed sbrasive through mechanical chemical polishing. Appl. Surf. Sci. 2016, 389, 713–720. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Zhang, D.; Xu, X. The synthesis of the core/shell structured diamond/akageneite hybrid particles with enhanced polishing performance. Materials 2017, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, Y.; Zhang, Y.; Xu, X. The effects of SiO2 coating on diamond abrasives in sol-gel tool for SiC substrate polishing. Diam. Relat. Mater. 2017, 76, 123–131. [Google Scholar] [CrossRef]
- Lu, J.; Luo, Q.; Xu, X.; Huang, H.; Jiang, F. Removal mechanism of 4H- and 6H-SiC substrates (0001 and 0001) in mechanical planarization machining. Proc. Inst. Mech. Eng. J. Eng. Manuf. 2017, 1–8. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Xu, X.; Jiang, F. Removal mechanism of sapphire substrates (0001, 1120 and 1010) in mechanical planarization machining. Ceram. Int. 2017, 43, 16178–16184. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, G.; Gong, H.; Shi, X.; Zou, C. Characterization of sapphire chemical mechanical polishing performances using silica with different sizes and their removal mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 153–159. [Google Scholar] [CrossRef]
- Xu, L.; Zou, C.; Shi, X.; Pan, G.; Luo, G. Fe-Nx/C assisted chemical-mechanical polishing for improving the removal rate of sapphire. Appl. Surf. Sci. 2015, 343, 115–120. [Google Scholar] [CrossRef]
- Shi, X.; Pan, G.; Zhou, Y.; Xu, L.; Zou, C. A Study of chemical products formed on sapphire (0001) during chemical-mechanical polishing. Surf. Coat. Technol. 2015, 270, 206–220. [Google Scholar] [CrossRef]
- Vovk, E.A.; Budnikov, A.T.; Dobrotvorskaya, M.V.; Krivonogov, S.I.; Dan’ko, A.Y. Mechanism of the Interaction between Al2O3 and SiO2 during the Chemical Mechanical Polishing of Sapphire with Silicon Dioxide. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2012, 6, 115–121. [Google Scholar] [CrossRef]

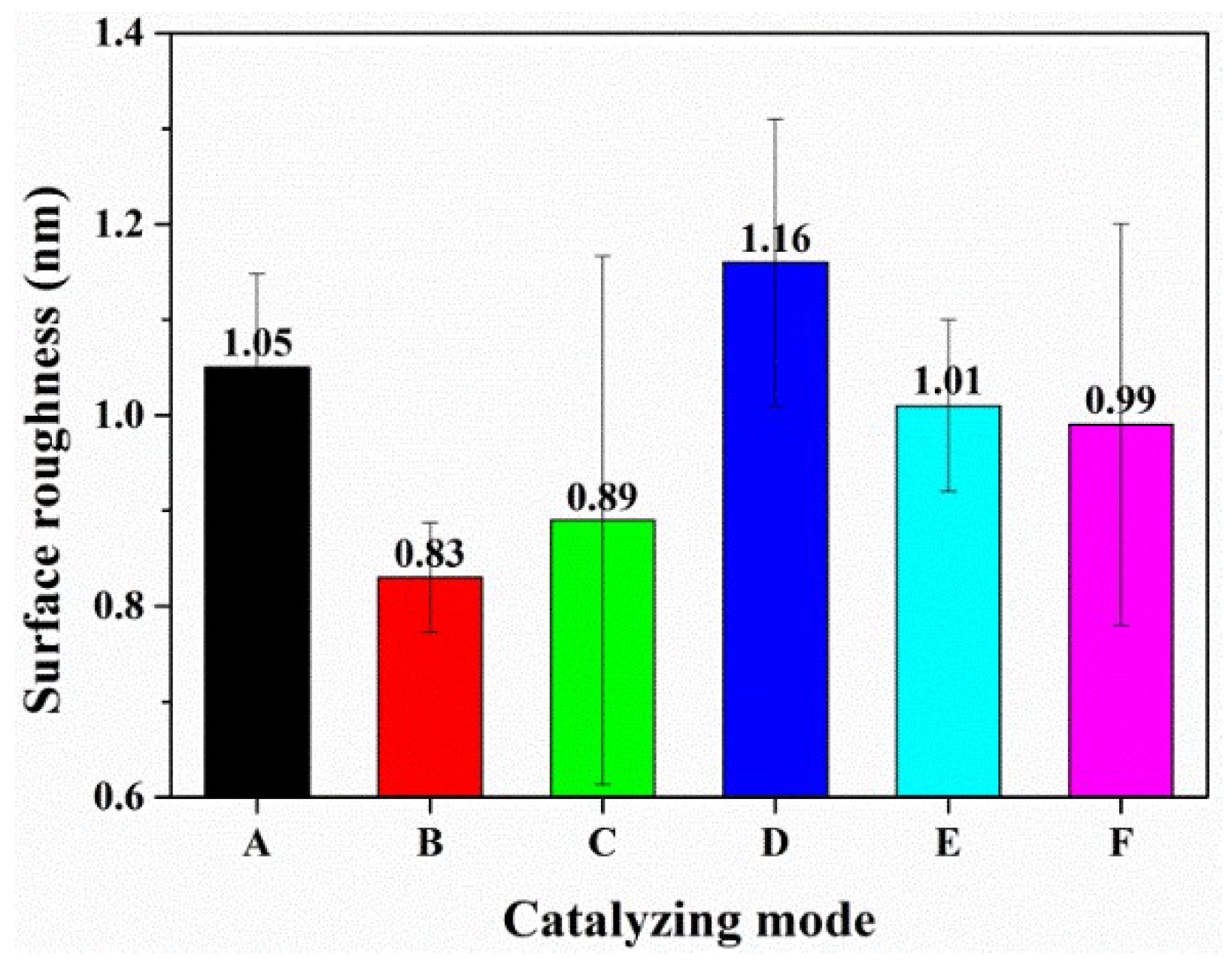
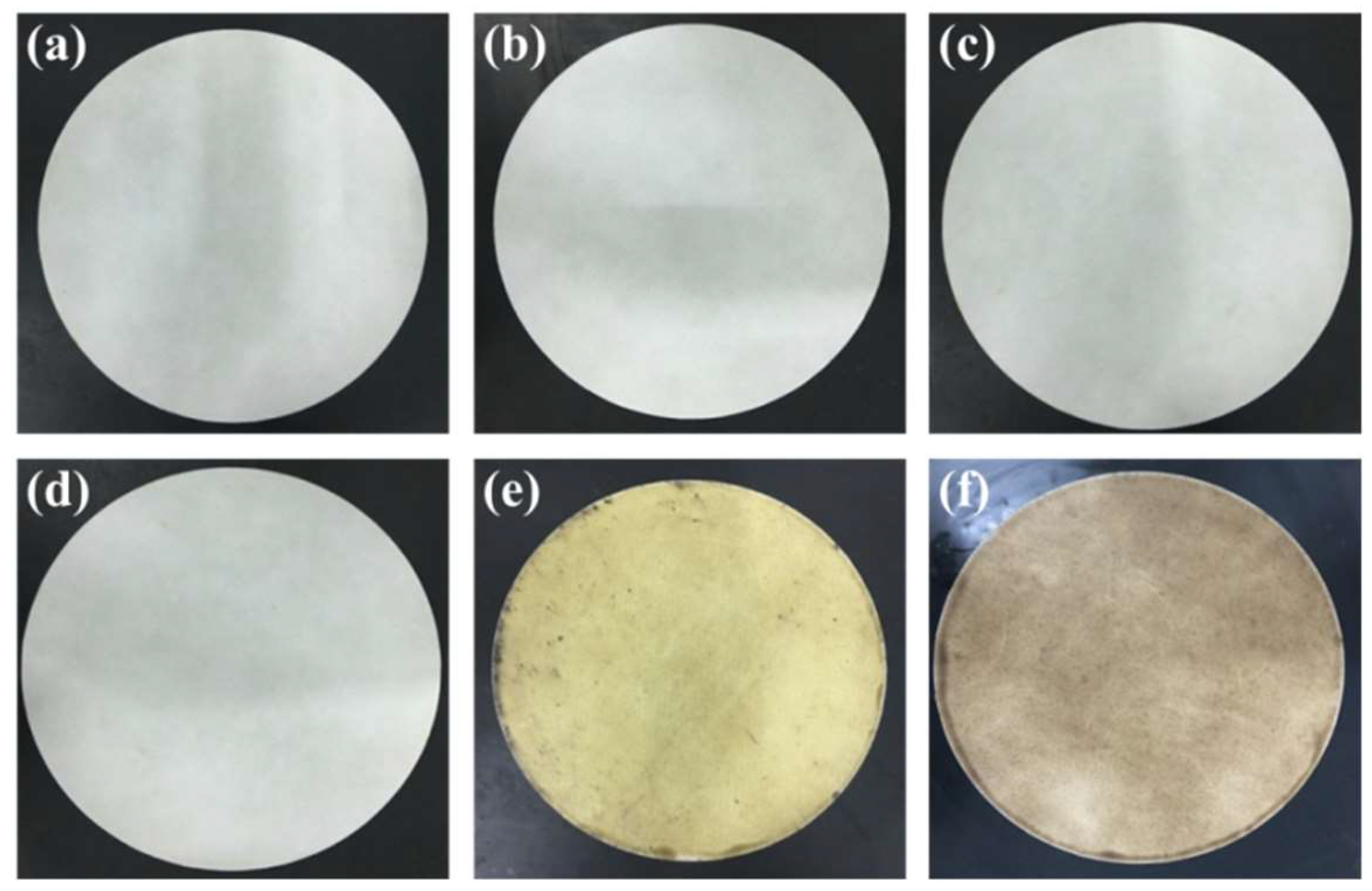
| Experiment | Catalyzing Mode | Experiment | Catalyzing Mode |
|---|---|---|---|
| A | Nothing | B | Synthetic silica |
| C | Citric acid | D | Theophylline |
| E | Green tea | F | Coffee |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Lu, J.; Xu, X. Pollution-Free Approaches for Highly Efficient Sapphire Substrate Processing by Mechanical Chemical Polishing. Catalysts 2019, 9, 594. https://doi.org/10.3390/catal9070594
Xu Y, Lu J, Xu X. Pollution-Free Approaches for Highly Efficient Sapphire Substrate Processing by Mechanical Chemical Polishing. Catalysts. 2019; 9(7):594. https://doi.org/10.3390/catal9070594
Chicago/Turabian StyleXu, Yongchao, Jing Lu, and Xipeng Xu. 2019. "Pollution-Free Approaches for Highly Efficient Sapphire Substrate Processing by Mechanical Chemical Polishing" Catalysts 9, no. 7: 594. https://doi.org/10.3390/catal9070594




