High Photocatalytic Activity under Visible Light for a New Morphology of Bi2WO6 Microcrystals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystallinity
2.2. Spectroscopic Properties
2.3. Morphology of Bi2WO6 and Bi2WO6-glyc Microcrystals
2.4. Catalytic Performance
3. Materials and Methods
3.1. Synthesis of Bi2WO6
3.2. Obtention of Bi2WO6-glyc Microcrystals
3.3. Characterization
3.4. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. Recent developments in photocatalytic dye degradation upon irradiation with energy-efficient light emitting diodes. Chin. J. Catal. 2014, 35, 1781–1792. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, F.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Chen, Z.; Wong, P.K.; Liu, J. Bi2WO6 micro/nano-structures: Synthesis, modifications and visible-light-driven photocatalytic applications. Appl. Catal. B Environ. 2011, 106, 1–13. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Tong, L.; Zeng, X.H.; Chen, X.B. Green synthesis of flower-like Bi2WO6 microspheres as a visible-light-driven photocatalyst. New J. Chem. 2014, 38, 1973–1979. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Z.; Guo, X.; Liu, C.; Zhai, H.; Wang, Q.; Chang, L. Controllable synthesis and photocatalytic activity of spherical, flower-like and nanofibrous bismuth tungstates. Mater. Sci. Eng. B 2014, 188, 35–42. [Google Scholar] [CrossRef]
- Kadam, S.R.; Park, C.-J.; Kale, B.B.; Tamboli, M.S.; Sethi, Y.A.; Ambekar, J.D.; Nikam, L.K.; Panmand, R.P. Self-assembled hierarchical nanostructures of Bi2WO6 for hydrogen production and dye degradation under solar light. CrystEngComm 2014, 17, 107–115. [Google Scholar]
- Zhang, L.; Zhu, Y. A review of controllable synthesis and enhancement of performances of bismuth tungstate visible-light-driven photocatalysts. Catal. Sci. Technol. 2012, 2, 694–706. [Google Scholar] [CrossRef]
- Zhao, W.; Song, X.; Chen, G.; Tian, G.; Sun, S. Hydrothermal synthesis of PbWO4 uniform hierarchical microspheres. Mater. Lett. 2009, 63, 285–288. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, L.; Zhu, J.; Yuan, Y.; Qian, X. Controlled synthesis of hierarchical Bi2WO6 microspheres with improved visible-light-driven photocatalytic activity. CrystEngComm 2010, 12, 2100–2106. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Xu, H. New Bi2WO6 Nanocages with High Visible-Light-Driven Photocatalytic Activities Prepared in Refluxing EG. Cryst. Growth Des. 2008, 9, 991–996. [Google Scholar] [CrossRef]
- Photocatalysts, V.; Zhang, C.; Zhu, Y. Synthesis of Square Bi2WO6 Nanoplates as High-Activity Visible-Light-Driven Photocatalysts Chuan. Chemistry of Materials. 2005, 17, 3537–3545. [Google Scholar]
- Chen, Z.; Wang, W.; Xu, H.; Zhang, L.; Zhu, W.; Zhou, L. Fabrication of flower-like Bi2WO6 superstructures as high performance visible-light driven photocatalysts. J. Mater. Chem. 2007, 17, 2526–2532. [Google Scholar]
- Dai, X.J.; Luo, Y.S.; Zhang, W.D.; Fu, S.Y. Facile hydrothermal synthesis and photocatalytic activity of bismuth tungstate hierarchical hollow spheres with an ultrahigh surface area. Dalt. Trans. 2010, 39, 3426–3432. [Google Scholar] [CrossRef] [PubMed]
- MacZka, M.; Hanuza, J.; Paraguassu, W.; Filho, A.G.S.; Freire, P.T.C.; Filho, J.M. Phonons in ferroelectric Bi2WO6: Raman and infrared spectra and lattice dynamics. Appl. Phys. Lett. 2008, 92, 112911. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Y.; Zhao, Y.; Hu, X.; Liu, E. Bi2WO6 nanoflowers: An efficient visible light photocatalytic activity for ceftriaxone sodium degradation. Appl. Surf. Sci. 2017, 436, 854–864. [Google Scholar]
- Xia, J.; Di, J.; Yin, S.; Xu, H.; Zhang, J.; Xu, Y.; Xu, L.; Li, H.; Ji, M. Facile fabrication of the visible-light-driven Bi2WO6/BiOBr composite with enhanced photocatalytic activity. RSC Adv. 2014, 4, 82–90. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, R.; Wang, J.; Cui, C.; Wang, J.; Li, Z. A novel hollow-hierarchical structured Bi2WO6 with enhanced photocatalytic activity for CO2 photoreduction. J. Colloid Interface Sci. 2018, 523, 151–158. [Google Scholar] [CrossRef]
- Kania, A.; Niewiadomski, A.; Kugel, G.E. Dielectric and Raman scattering studies of Bi2WO6 single crystals. Phase Trans. 2013, 86, 290–300. [Google Scholar] [CrossRef]
- Fagundes, N.G.; Nobre, F.X.; Basilio, L.A.L.; Melo, A.D.; Bandeira, B.; Sales, J.C.C., Jr.; Andrade, J.C.S.; Anglada-Rivera, J.; Aguilera, L.; Pérez de la Cruz, J.; et al. Novel and simple way to synthesize Na2Ti6O13 nanoparticles by sonochemical method. Solid State Sci. 2019, 88, 63–66. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Knight, K.S. The crystal structure of ferroelectric Bi2WO6 at 961 K. Ferroelectrics 1993, 150, 319–330. [Google Scholar] [CrossRef]
- Nobre, F.X.; Junior, W.A.G.P.; Ruiz, Y.L.; Bentes, V.L.I.; Silva-Moraes, M.O.; Silva, T.M.C.; Rocco, M.L.M.; González, D.R.L.; de Matos, J.M.E.; da Costa Couceiro, P.R.; et al. Facile synthesis of nTiO2 phase mixture: Characterization and catalytic performance. Material Research Buletin. 2019, 109, 60–71. [Google Scholar] [CrossRef]
- Mishra, R.K.; Weibel, M.; Müller, T.; Heinz, H.; Flatta, R.J. Energy-effective Grinding of Inorganic Solids Using Organic Additives. CHIMIA Int. J. Chem. 2017, 71, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Maczka, M.; Paraguassu, W.; Filho, A.G.S.; Freire, P.T.C.; Filho, J.M.; Hanuza, J. Phonon-instability-driven phase transitions in ferroelectric Bi2WO6: Eu3+: High-pressure Raman and photoluminescence studies. Phys. Rev. B–Condens. Matter Mater. Phys. 2008, 77, 094137. [Google Scholar] [CrossRef]
- Džimbeg-Malčić, V.; Barbarić-Mikočević, Ž.; Itrić, K. Kubelka-Munk theory in describing optical properties of paper (I). Tehnicki Vjesnik. 2011, 18, 117–124. [Google Scholar]
- Zhou, Y.; Zhang, Y.; Lin, M.; Long, J.; Zhang, Z.; Lin, H.; Wu, J.C.S.; Wang, X. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 2015, 6, 8340. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Ohtani, B. Enhanced photocatalytic activity of bismuth-tungsten mixed oxides for oxidative decomposition of acetaldehyde under visible light irradiation. Catal. Commun. 2012, 20, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Phuruangrat, A.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Hydrothermal synthesis of I-doped Bi2WO6 for using as a visible-light-driven photocatalyst. Mater. Lett. 2018, 224, 67–70. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Chen, X.; Zhang, J.; Jiang, L.; Wu, Z.; Zeng, G.; Wang, H. Highly efficient photocatalysis toward tetracycline of nitrogen doped carbon quantum dots sensitized bismuth tungstate based on interfacial charge transfer. J. Colloid Interface Sci. 2017, 511, 296–306. [Google Scholar]
- Sezancoski, J.C.; Bomio, M.D.R.; Cavalcante, L.S.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Longo, E.; Li, M.S.; Andrés, J.A. Morphology and blue photoluminescence emission of PbMoO4 processed in conventional hydrothermal. J. Phys. Chem. C 2009, 113, 5812–5822. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Hu, J.; Li, Z. Synthesis and characterization of Bi2WO6 nanoplates using egg white as a biotemplate through sol-gel method. Mater Lett. 2015, 139, 401–404. [Google Scholar] [CrossRef]
- Zhang, X.; Gai, W. Effect of surfactant on the photocatalytic activity of Bi2WO6 nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 9777–9781. [Google Scholar] [CrossRef]
- Ge, M.; Liu, L. Sunlight-induced photocatalytic performance of Bi2WO6 hierarchical microspheres synthesized via a relatively green hydrothermal route. Mater. Sci. Semiconduct. Process. 2014, 25, 258–263. [Google Scholar] [CrossRef]
- Regulska, E.; Breczko, J.; Basa, A. Pristine and Graphene-Quantum-Dots-Decorated Spinel Nickel Aluminate for Water Remediation from Dyes and Toxic Pollutants. Water 2019, 11, 953. [Google Scholar] [CrossRef]
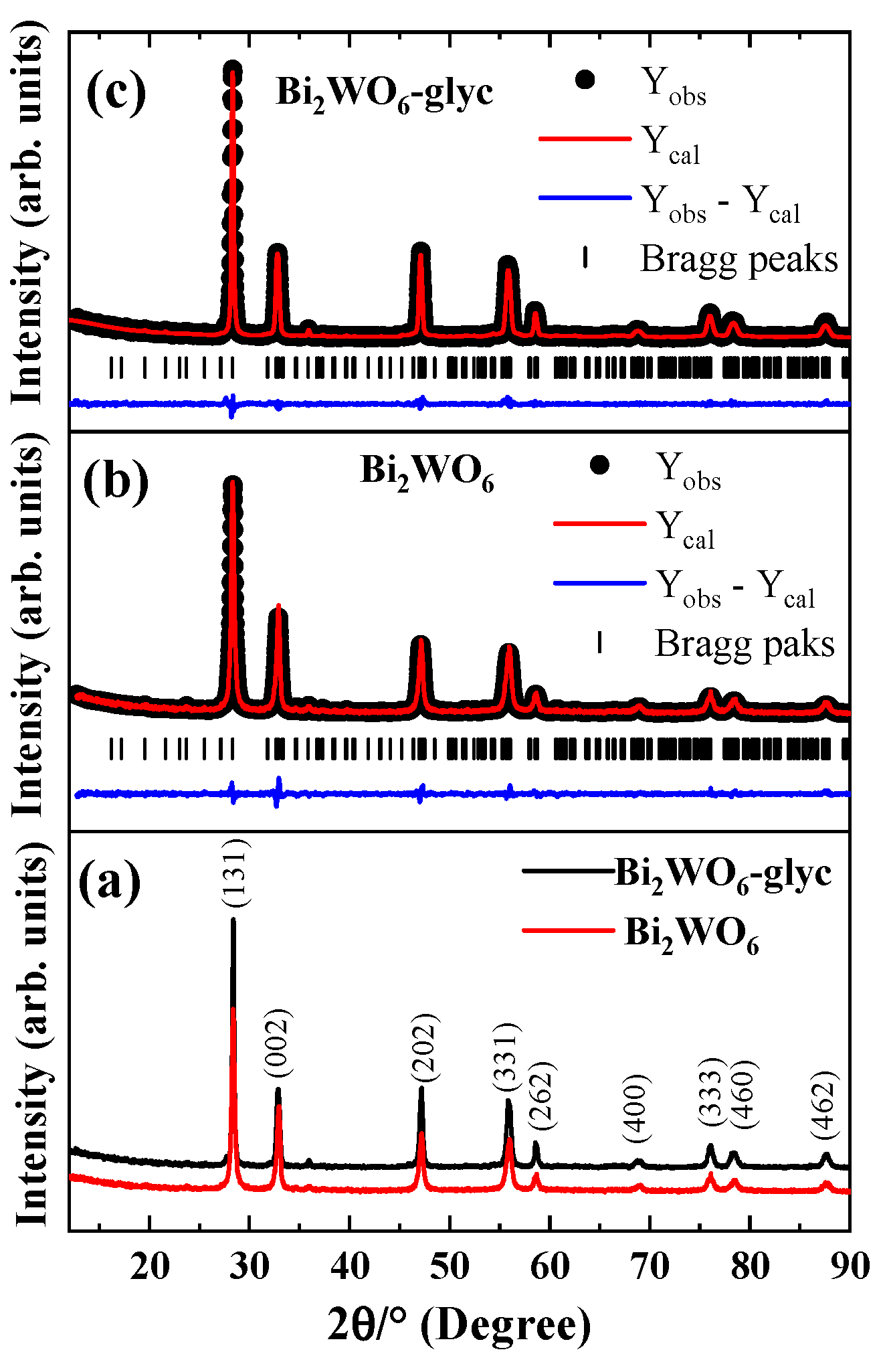
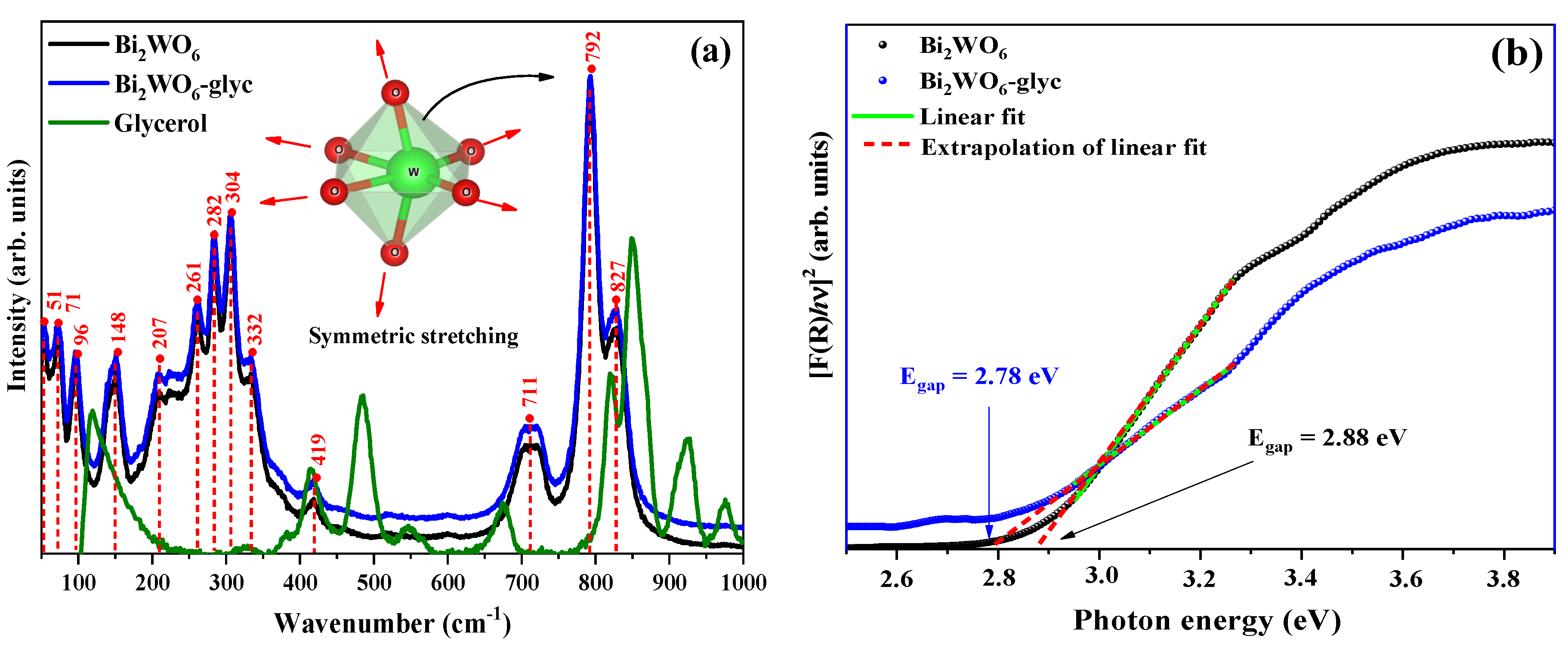
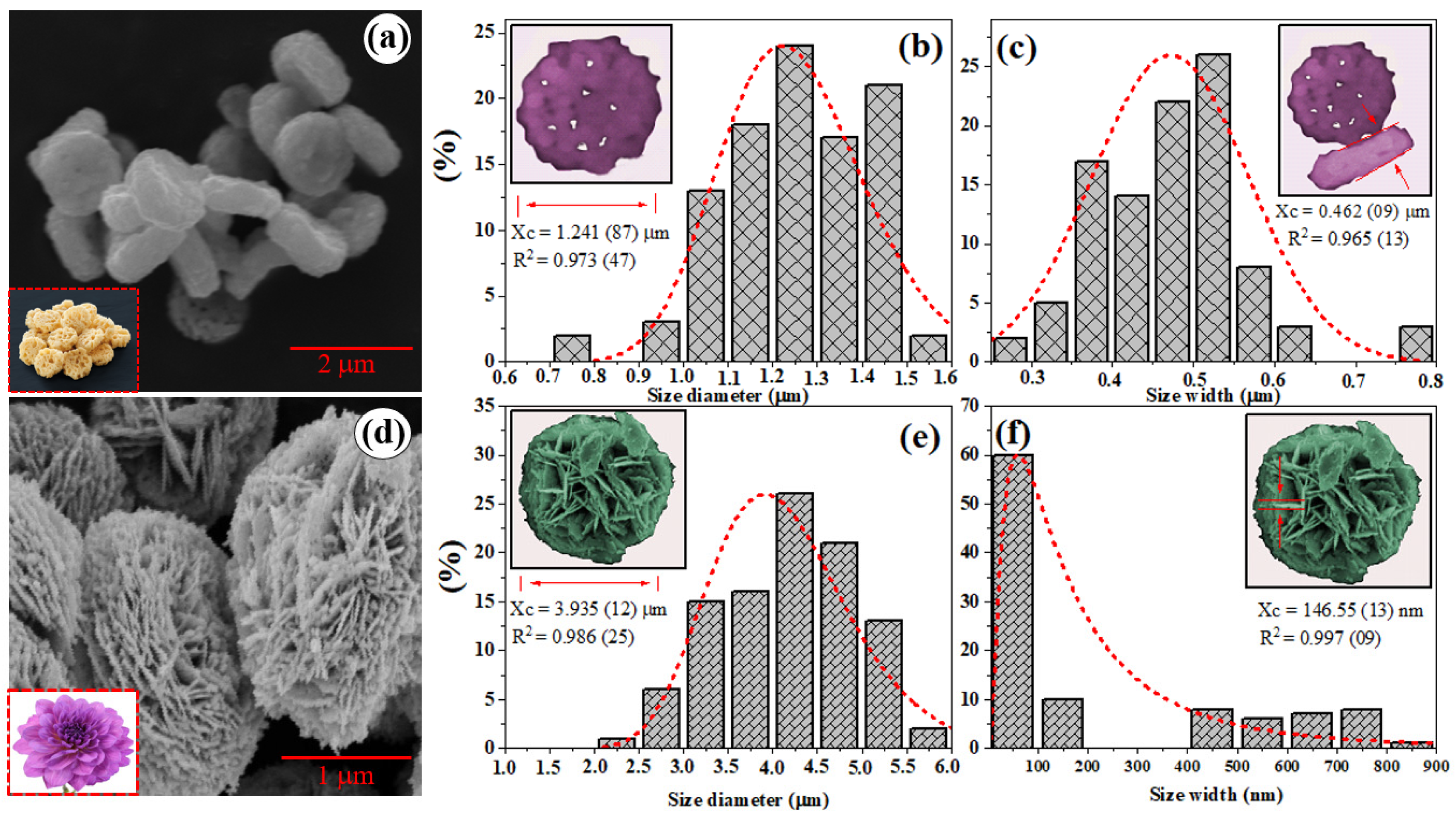

| ID | Lattice Parameters (Å) | V (Å3) | Dhkl (nm) | Ref. | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Bi2WO6 | 5.443(3) | 16.428(1) | 5.451(2) | 487.45(8) | 24.761(78) | This work |
| Bi2WO6-glyc | 5.437(1) | 16.433(4) | 5.457(9) | 487.66(4) | 20.153(80) | This work |
| - | 5.437(3) | 16.430(2) | 5.458(4) | 487.63(3) | - | ♣ |
| - | 5.457 | 16.435 | 5.438 | 487.71(1) | 70 | [11] |
| ID | Egap (eV) | Degradation (%) | kobs x 10−3 (min−1) | t1/2 (min) | Ref. |
|---|---|---|---|---|---|
| Bi2WO6 | 2.88 | 81.3(3) | 22.68(02) | 30.56 | This work |
| Bi2WO6-glyc | 2.78 | 94.2(8) | 55.89(01) | 12.40 | This work |
| Photolysis | - | 8.2(5) | 4.54(09) | 152.67 | This work |
| Bi2WO6 | 2.75 | - | 7.5 | 92.41 | [13] |
| Bi2WO6 | - | - | 12 | 57.76 | [29] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, W.E.O.; Nobre, F.X.; da Rocha Filho, G.N.; da Silva, M.A.R.; da Costa, C.E.F.; do Nascimento, L.A.S.; Zamian, J.R. High Photocatalytic Activity under Visible Light for a New Morphology of Bi2WO6 Microcrystals. Catalysts 2019, 9, 667. https://doi.org/10.3390/catal9080667
Campos WEO, Nobre FX, da Rocha Filho GN, da Silva MAR, da Costa CEF, do Nascimento LAS, Zamian JR. High Photocatalytic Activity under Visible Light for a New Morphology of Bi2WO6 Microcrystals. Catalysts. 2019; 9(8):667. https://doi.org/10.3390/catal9080667
Chicago/Turabian StyleCampos, Willison Eduardo Oliveira, Francisco Xavier Nobre, Geraldo Narciso da Rocha Filho, Marcos Augusto Ribeiro da Silva, Carlos Emmerson Ferreira da Costa, Luís Adriano Santos do Nascimento, and José Roberto Zamian. 2019. "High Photocatalytic Activity under Visible Light for a New Morphology of Bi2WO6 Microcrystals" Catalysts 9, no. 8: 667. https://doi.org/10.3390/catal9080667





