Wet Peroxide Oxidation of Paracetamol Using Acid Activated and Fe/Co-Pillared Clay Catalysts Prepared from Natural Clays
Abstract
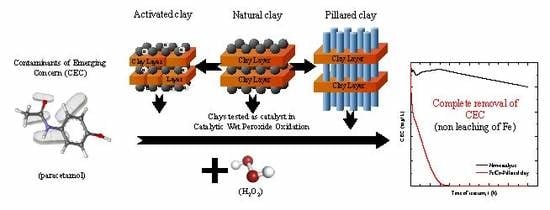
:1. Introduction
2. Results and Discussion
2.1. Characterization of Materials
2.1.1. Textural Properties
2.1.2. Surface Composition
2.1.3. Acid-Base Characterization
2.2. CWPO of Paracetamol
3. Materials and Methods
3.1. Reactants and Materials
3.2. Preparation of Clay-Based Materials
3.3. Techniques of Characterization
3.4. Oxidation Runs
3.5. Analytical Techniques
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total. Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Ratola, N.; Cincinelli, A.; Alves, A.; Katsoyiannis, A. Occurrence of organic microcontaminants in the wastewater treatment process. A mini review. J. Hazard. Mater. 2012, 239, 1–18. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [Green Version]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Rodea-Palomares, I.; Gonzalez-Pleiter, M.; Gonzalo, S.; Rosal, R.; Leganes, F.; Sabater, S.; Casellas, M.; Muñoz-Carpena, R.; Fernández-Piñas, F. Hidden drivers of low-dose pharmaceutical pollutant mixtures revealed by the novel GSA-QHTS screening method. Sci. Adv. 2016, 2, e1601272. [Google Scholar] [CrossRef] [PubMed]
- Christophoridis, C.; Nika, M.-C.; Aalizadeh, R.; Thomaidis, N.S. Ozonation of ranitidine: Effect of experimental parameters and identification of transformation products. Sci. Total Environ. 2016, 557, 170–182. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Off. J. Eur. Union 2013, OJL 226, 1–17. [Google Scholar]
- European Commission. Decision (EU) 2015-495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, OJL 78, 40–42. [Google Scholar]
- Villota, N.; Lomas, J.M.; Camarero, L.M. Kinetic modelling of water-color changes in a photo-Fenton system applied to oxidate paracetamol. J. Photochem. Photobiol. A Chem. 2018, 356, 573–579. [Google Scholar] [CrossRef]
- Slamani, S.; Abdelmalek, F.; Ghezzar, M.R.; Addou, A. Initiation of Fenton process by plasma gliding arc discharge for the degradation of paracetamol in water. J. Photochem. Photobiol. A Chem. 2018, 359, 1–10. [Google Scholar] [CrossRef]
- Akhi, Y.; Irani, M.; Olya, M.E. Simultaneous degradation of phenol and paracetamol using carbon/MWCNT/Fe3O4 composite nanofibers during photo-like-Fenton process. J. Taiwan Inst. Chem. Eng. 2016, 63, 327–335. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.-M.; Al-Shirbini, A.-S.; Mohamed, O.; Nasr, O. Photocatalytic degradation of paracetamol over magnetic flower-like TiO2/Fe2O3 core-shell nanostructures. J. Photochem. Photobiol. A Chem. 2017, 347, 186–198. [Google Scholar] [CrossRef]
- Augusto, T.D.M.; Chagas, P.; Sangiorge, D.L.; Leod, T.C.D.O.M.; Oliveira, L.C.; De Castro, C.S. Iron ore tailings as catalysts for oxidation of the drug paracetamol and dyes by heterogeneous Fenton. J. Environ. Chem. Eng. 2018, 6, 6545–6553. [Google Scholar] [CrossRef]
- Silva, C.P.; Jaria, G.; Otero, M.; Esteves, V.I.; Calisto, V. Waste-based alternative adsorbents for the remediation of pharmaceutical contaminated waters: Has a step forward already been taken? Bioresour. Technol. 2018, 250, 888–901. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, A.; Haghighat, F.; Chen, Z.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Badawy, M.I.; Wahaab, R.A.; El-Kalliny, A. Fenton-biological treatment processes for the removal of some pharmaceuticals from industrial wastewater. J. Hazard. Mater. 2009, 167, 567–574. [Google Scholar] [CrossRef]
- Velichkova, F.; Julcour-Lebigue, C.; Koumanova, B.; Delmas, H. Heterogeneous Fenton oxidation of paracetamol using iron oxide (nano)particles. J. Environ. Chem. Eng. 2013, 1, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Kalmakhanova, M.S.; Diaz de Tuesta, J.L.; Massalimova, B.K.; Gomes, H.T. Pillared clays from natural resources as catalysts for catalytic wet peroxide oxidation: Characterization and kinetic insights. Environ. Eng. Res. 2019. [Google Scholar] [CrossRef]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Catalytic wet peroxide oxidation of phenol over Ce-Zr-modified clays: Effect of the pillaring method. Korean J. Chem. Eng. 2015, 32, 68–73. [Google Scholar] [CrossRef]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Effect of Al and Ce on Zr-pillared bentonite and their performance in catalytic oxidation of phenol. Russ. J. Phys. Chem. A 2016, 90, 1766–1773. [Google Scholar] [CrossRef]
- Tomul, F.; Basoglu, F.T.; Canbay, H. Determination of adsorptive and catalytic properties of copper, silver and iron contain titanium-pillared bentonite for the removal bisphenol A from aqueous solution. Appl. Surf. Sci. 2016, 360, 579–593. [Google Scholar] [CrossRef]
- Galeano, L.-A.; Vicente, M.A.; Gil, A. Catalytic degradation of organic pollutants in aqueous streams by mixed Al/M-pillared clays (M = Fe, Cu, Mn). Catal. Rev. 2014, 56, 239–287. [Google Scholar] [CrossRef]
- Rhodes, C.N.; Franks, M.; Parkes, G.M.B.; Brown, D.R. The effect of acid treatment on the activity of clay supports for ZnCl2 alkylation catalysts. J. Chem. Soc. Chem. Commun. 1991, 804–807. [Google Scholar] [CrossRef]
- Yu, W.; Wang, P.; Zhou, C.; Zhao, H.; Tong, D.; Zhang, H.; Yang, H.; Ji, S.; Wang, H. Acid-activated and WO -loaded montmorillonite catalysts and their catalytic behaviors in glycerol dehydration. Chin. J. Catal. 2017, 38, 1087–1100. [Google Scholar] [CrossRef]
- Cool, P.; Vansant, E.F. Pillared Clays: Preparation, characterization and applications. In Synthesis; Springer: Berlin/Heidelberg, Germany, 2001; Volume 1, pp. 265–288. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Trigueiro, P.; Pereira, F.A.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.-M.; Dos Santos, I.M.; Fonseca, M.G.; Walter, P.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes Pigments 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Sokol, H.; Rafińska, K.; Brzozowska, W.; Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Preparation of AgNPs/saponite nanocomposites without reduction agents and study of its antibacterial activity. Colloids Surf. B Biointerfaces 2019, 180, 457–465. [Google Scholar] [CrossRef]
- Zhu, B.-L.; Qi, C.-L.; Zhang, Y.-H.; Bisson, T.; Xu, Z.; Fan, Y.-J.; Sun, Z.-X. Synthesis, characterization and acid-base properties of kaolinite and metal (Fe, Mn, Co) doped kaolinite. Appl. Clay Sci. 2019, 179, 105138. [Google Scholar] [CrossRef]
- Wu, C.; Wei, X.; Liu, P.; Tan, J.; Liao, C.; Wang, H.; Yin, L.; Zhou, W.; Cui, H.-J. Influence of structural Al species on Cd(II) capture by iron muscovite nanoparticles. Chemosphere 2019, 226, 907–914. [Google Scholar] [CrossRef]
- Yuan, P.; Annabi-Bergaya, F.; Tao, Q.; Fan, M.; Liu, Z.; Zhu, J.; He, H.; Chen, T. A combined study by XRD, FTIR, TG and HRTEM on the structure of delaminated Fe-intercalated/pillared clay. J. Colloid Interface Sci. 2008, 324, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, C.; Wei, H.; Yuan, W.; Li, K. Adsorption of levofloxacin onto an iron-pillared montmorillonite (clay mineral): Kinetics, equilibrium and mechanism. Appl. Clay Sci. 2015, 118, 301–307. [Google Scholar] [CrossRef]
- Widjaya, R.R.; Juwono, A.L.; Rinaldi, N. Bentonite modification with pillarization method using metal stannum. AIP Conf. Proc. 2017, 1904, 020010. [Google Scholar]
- Bahranowski, K.; Włodarczyk, W.; Wisła-Walsh, E.; Gaweł, A.; Matusik, J.; Klimek, A.; Gil, B.; Michalik-Zym, A.; Dula, R.; Socha, R.; et al. [Ti,Zr]-pillared montmorillonite—A new quality with respect to Ti- and Zr-pillared clays. Microporous Mesoporous Mater. 2015, 202, 155–164. [Google Scholar] [CrossRef]
- Bruckman, V.J.; Wriessnig, K. Improved soil carbonate determination by FT-IR and X-ray analysis. Environ. Chem. Lett. 2013, 11, 65–70. [Google Scholar] [CrossRef]
- Li, T.; Zhao, L.; Zheng, Z.; Zhang, M.; Sun, Y.; Tian, Q.; Zhang, S. Design and preparation acid-activated montmorillonite sustained-release drug delivery system for dexibuprofen in vitro and in vivo evaluations. Appl. Clay Sci. 2018, 163, 178–185. [Google Scholar] [CrossRef]
- Jain, S.; Datta, M. Montmorillonite-alginate microspheres as a delivery vehicle for oral extended release of Venlafaxine hydrochloride. J. Drug Deliv. Sci. Technol. 2016, 33, 149–156. [Google Scholar] [CrossRef]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Eren, E.; Afsin, B. An investigation of Cu(II) adsorption by raw and acid-activated bentonite: A combined potentiometric, thermodynamic, XRD, IR, DTA study. J. Hazard. Mater. 2008, 151, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, Y.; He, M.; Chen, L.; Yu, X. Characterization of GMZ bentonite and its application in the adsorption of Pb(II) from aqueous solutions. Appl. Clay Sci. 2009, 43, 164–171. [Google Scholar] [CrossRef]
- De Tuesta, J.D.; Quintanilla, A.; Casas, J.; Rodriguez, J. P-, B- and N-doped carbon black for the catalytic wet peroxide oxidation of phenol: Activity, stability and kinetic studies. Catal. Commun. 2017, 102, 131–135. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Degradation of four pharmaceuticals by solar photo-Fenton process: Kinetics and costs estimation. J. Environ. Chem. Eng. 2015, 3, 46–51. [Google Scholar] [CrossRef]
- Trovó, A.G.; Nogueira, R.F.P.; Agüera, A.; Fernández-Alba, A.R.; Malato, S. Paracetamol degradation intermediates and toxicity during photo-Fenton treatment using different iron species. Water Res. 2012, 46, 5374–5380. [Google Scholar] [CrossRef] [PubMed]
- De Tuesta, J.L.D.; Silva, A.M.; Faria, J.L.; Gomes, H.T. Removal of Sudan IV from a simulated biphasic oily wastewater by using lipophilic carbon adsorbents. Chem. Eng. J. 2018, 347, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Diaz de Tuesta, J.L.; Machado, B.F.; Serp, P.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Janus amphiphilic carbon nanotubes as Pickering interfacial catalysts for the treatment of oily wastewater by selective oxidation with hydrogen peroxide. Catal. Today 2019. [Google Scholar] [CrossRef]






| Sample | SBET (m2 g−1) | VTotal (mm3 g−1) |
|---|---|---|
| KO-N | 26 | 69.2 |
| KO-A | 28 | 94.6 |
| KO-C | 24 | 74.0 |
| KO-PILC | 19 | 72.3 |
| AK-PILC | 13 | 27.8 |
| AS-PILC | 11 | 22.1 |
| KA-PILC | 13 | 46.6 |
| Sample | pHPZC | Acidity (μmol g−1) | Basicity (μmol g−1) |
|---|---|---|---|
| KO-N | 7.8 | 350 | 245 |
| KO-A | 7.2 | 987 | 614 |
| KO-C | 8.0 | 338 | 270 |
| KO-PILC | 7.4 | 950 | 652 |
| AK-PILC | 7.2 | 812 | 538 |
| AS-PILC | 7.6 | 475 | 372 |
| KA-PILC | 7.4 | 687 | 627 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos Silva, A.; Seitovna Kalmakhanova, M.; Kabykenovna Massalimova, B.; G. Sgorlon, J.; Jose Luis, D.d.T.; T. Gomes, H. Wet Peroxide Oxidation of Paracetamol Using Acid Activated and Fe/Co-Pillared Clay Catalysts Prepared from Natural Clays. Catalysts 2019, 9, 705. https://doi.org/10.3390/catal9090705
Santos Silva A, Seitovna Kalmakhanova M, Kabykenovna Massalimova B, G. Sgorlon J, Jose Luis DdT, T. Gomes H. Wet Peroxide Oxidation of Paracetamol Using Acid Activated and Fe/Co-Pillared Clay Catalysts Prepared from Natural Clays. Catalysts. 2019; 9(9):705. https://doi.org/10.3390/catal9090705
Chicago/Turabian StyleSantos Silva, Adriano, Marzhan Seitovna Kalmakhanova, Bakytgul Kabykenovna Massalimova, Juliana G. Sgorlon, Diaz de Tuesta Jose Luis, and Helder T. Gomes. 2019. "Wet Peroxide Oxidation of Paracetamol Using Acid Activated and Fe/Co-Pillared Clay Catalysts Prepared from Natural Clays" Catalysts 9, no. 9: 705. https://doi.org/10.3390/catal9090705