Effect of Co-Feeding Inorganic and Organic Molecules in the Fe and Co Catalyzed Fischer–Tropsch Synthesis: A Review
Abstract
:1. Introduction
2. Co-Feeding in Fischer–Tropsch Synthesis
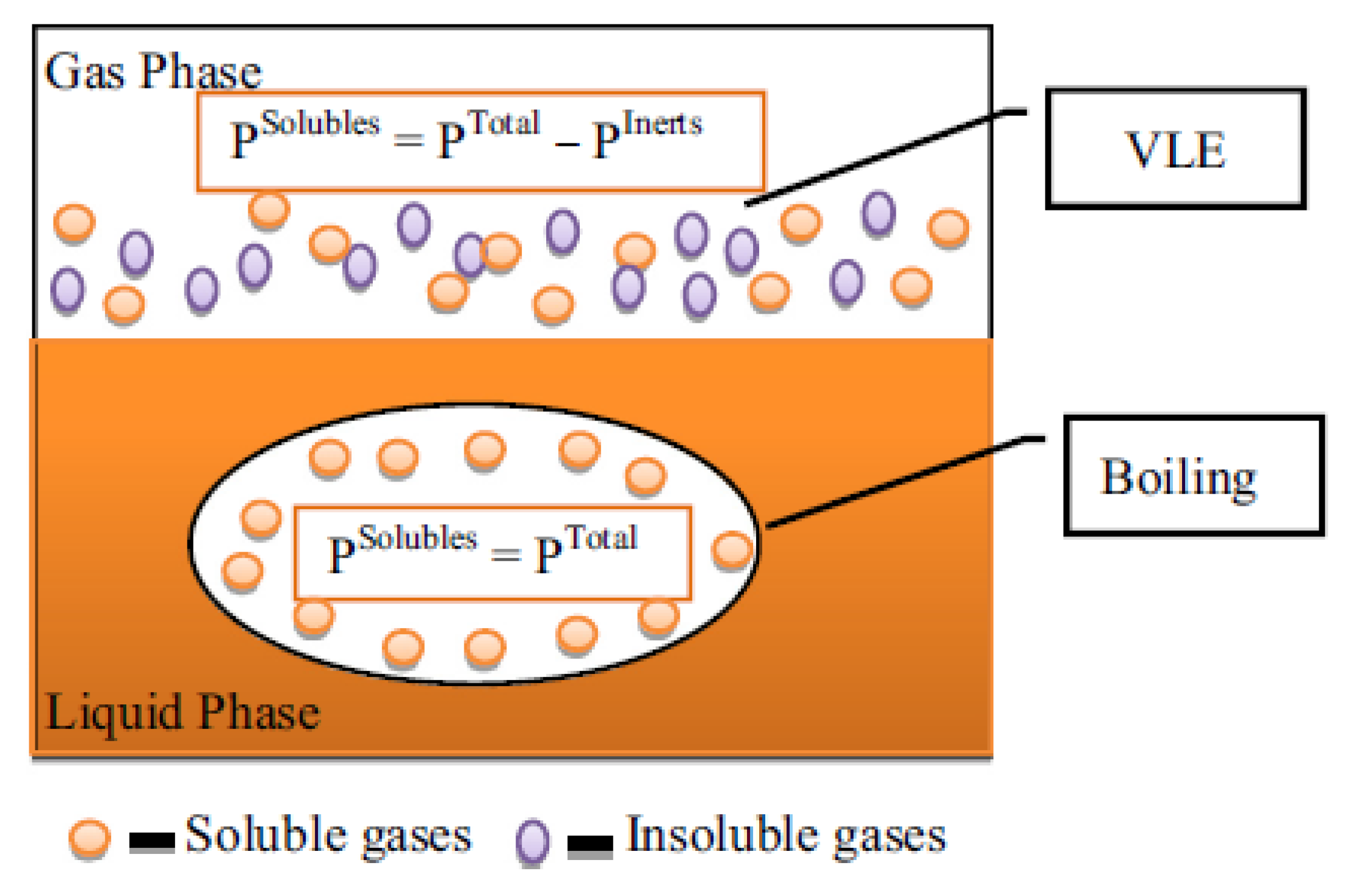
3. Water Co-Feeding in FTS
- (i)
- An oxidation process for the cobalt supported catalyst with the extent of oxidation being a function of features of the cobalt namely the crystallite size but also the ratio values of the reactor partial pressures of hydrogen and water (PH2O/PH2).
- (ii)
- The average support pore diameter influences the water-co-feeding.
- (iii)
- The effects could be kinetic in particular the CO dissociation by direct interaction with co-adsorbed CO can be lowered with water co-feeding while the secondary hydrogenation of olefin products can be inhibited as a result of competitive adsorption of water [46].
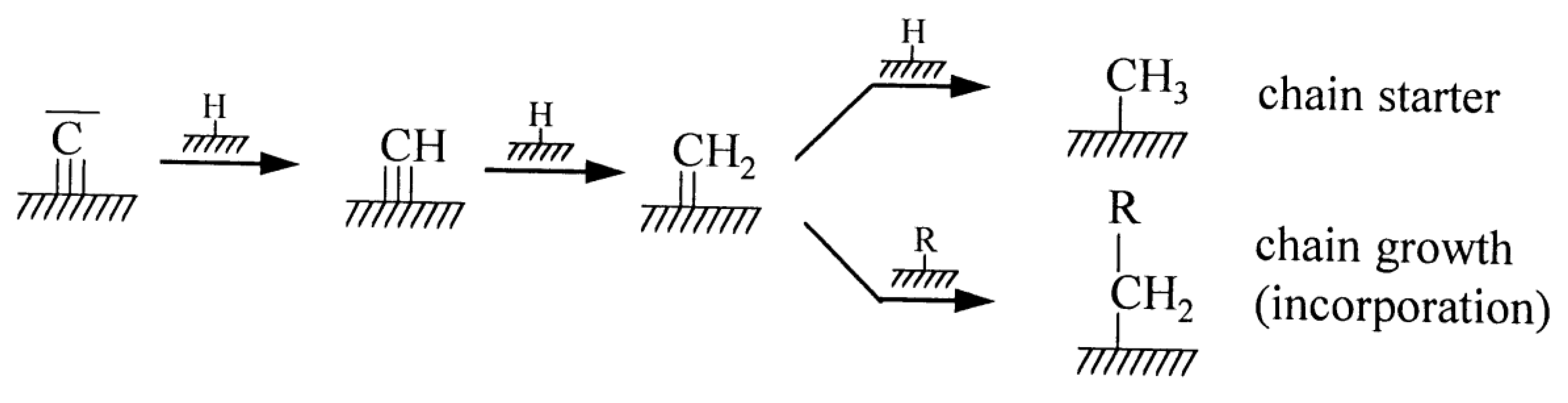
4. Organic Co-Feeds
5. Effect of Nitrogen as a Co-Feed
6. Concluding Remarks and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Storsæter, S.; Borg, Ø.; Blekkan, E.A.; Holmen, A. Study of the effect of water on Fischer–Tropsch synthesis over supported cobalt catalysts. J. Catal. 2005, 231, 405–419. [Google Scholar] [CrossRef]
- Claeys, M.; van Steen, E. On the effect of water during Fischer–Tropsch synthesis with a ruthenium catalyst. Catal. Today 2002, 71, 419–427. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Visconti, C.G.; Lietti, L.; Groppi, G.; Tronconi, E.; Roccaro, E.; Zennaro, R. On the performance of a Co-based catalyst supported on modified γ-Al 2 O 3 during Fischer–Tropsch synthesis in the presence of co-fed water. Catal. Sci. Technol. 2016, 6, 6431–6440. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Tu, M.; Ojeda, M.P.; Pinna, D.; Iglesia, E. An investigation of the effects of water on rate and selectivity for the Fischer–Tropsch synthesis on cobalt-based catalysts. J. Catal. 2002, 211, 422–433. [Google Scholar] [CrossRef]
- Sadeqzadeh, M.; Chambrey, S.; Piché, S.; Fongarland, P.; Luck, F.; Curulla-Ferré, D.; Khodakov, A.Y. Deactivation of a Co/Al2O3 Fischer–Tropsch catalyst by water-induced sintering in slurry reactor: Modeling and experimental investigations. Catal. Today 2013, 215, 52–59. [Google Scholar] [CrossRef]
- Li, J.; Davis, B.H. Effect of water on the catalytic properties of supported cobalt Fischer-Tropsch catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 147, pp. 307–312. [Google Scholar]
- Ma, W.; Jacobs, G.; Sparks, D.E.; Spicer, R.L.; Davis, B.H.; Klettlinger, J.L.; Yen, C.H. Fischer–Tropsch synthesis: Kinetics and water effect study over 25% Co/Al2O3 catalysts. Catal. Today 2014, 228, 158–166. [Google Scholar] [CrossRef]
- Rytter, E.; Borg, Ø.; Enger, B.C.; Holmen, A. α-alumina as catalyst support in Co Fischer-Tropsch synthesis and the effect of added water; encompassing transient effects. J. Catal. 2019, 373, 13–24. [Google Scholar] [CrossRef]
- Lögdberg, S.; Boutonnet, M.; Walmsley, J.C.; Järås, S.; Holmen, A.; Blekkan, E.A. Effect of water on the space-time yield of different supported cobalt catalysts during Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2011, 393, 109–121. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Das, T.K.; Masuku, C.M.; Kang, J.; Pendyala, V.R.R.; Yen, C.H. Fischer–Tropsch Synthesis: Kinetics and Water Effect on Methane Formation over 25% Co/γ-Al2O3 Catalyst. Ind. Eng. Chem. Res. 2014, 53, 2157–2166. [Google Scholar] [CrossRef]
- Li, J.; Zhan, X.; Zhang, Y.; Jacobs, G.; Das, T.; Davis, B.H. Fischer–Tropsch synthesis: Effect of water on the deactivation of Pt promoted Co/Al2O3 catalysts. Appl. Catal. A Gen. 2002, 228, 203–212. [Google Scholar] [CrossRef]
- Sage, V.; Burke, N. Use of probe molecules for Fischer–Tropsch mechanistic investigations: A short review. Catal. Today 2011, 178, 137–141. [Google Scholar] [CrossRef]
- Gaube, J.; Klein, H.F. Studies on the reaction mechanism of the Fischer–Tropsch synthesis on iron and cobalt. J. Mol. Catal. A Chem. 2008, 283, 60–68. [Google Scholar] [CrossRef]
- Jordan, D.S.; Bell, A.T. Influence of ethylene on the hydrogenation of carbon monoxide over ruthenium. J. Phys. Chem. 1986, 90, 4797–4805. [Google Scholar] [CrossRef]
- Boelee, J.H.; Cüsters, J.M.G.; Van Der Wiele, K. Influence of reaction conditions on the effect of Co-feeding ethene in the Fischer-Tropsch synthesis on a fused-iron catalyst in the liquid phase. Appl. Catal. 1989, 53, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Adesina, A.A.; Hudgins, R.R.; Silveston, P.L. Effect of ethene addition during the Fischer-Tropsch reaction. Appl. Catal. 1990, 62, 295–308. [Google Scholar] [CrossRef]
- Schulz, H.; Claeys, M. Reactions of α-olefins of different chain length added during Fischer–Tropsch synthesis on a cobalt catalyst in a slurry reactor. Appl. Catal. A Gen. 1999, 186, 71–90. [Google Scholar] [CrossRef]
- Kummer, J.T.; Emmett, P.H. Fischer—Tropsch synthesis mechanism studies. The addition of radioactive alcohols to the synthesis gas. J. Am. Chem. Soc. 1953, 75, 5177–5183. [Google Scholar] [CrossRef]
- Jalama, K.; Coville, N.J.; Hildebrandt, D.; Glasser, D.; Jewell, L.L. Fischer–Tröpsch synthesis over Co/TiO2: Effect of ethanol addition. Fuel 2007, 86, 73–80. [Google Scholar] [CrossRef]
- Jacobs, G.; Patterson, P.M.; Das, T.K.; Luo, M.; Davis, B.H. Fischer–Tropsch synthesis: Effect of water on Co/Al2O3 catalysts and XAFS characterization of reoxidation phenomena. Appl. Catal. A Gen. 2004, 270, 65–76. [Google Scholar] [CrossRef]
- Rytter, E.; Borg, Ø.; Tsakoumis, N.E.; Holmen, A. Water as key to activity and selectivity in Co Fischer-Tropsch synthesis: γ-alumina based structure-performance relationships. J. Catal. 2018, 365, 334–343. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Perspectives on the effect of water in cobalt Fischer–Tropsch synthesis. ACS Catal. 2017, 7, 5321–5328. [Google Scholar] [CrossRef]
- Borg, Ø.; Storsæter, S.; Eri, S.; Wigum, H.; Rytter, E.; Holmen, A. The effect of water on the activity and selectivity for γ-alumina supported cobalt Fischer–Tropsch catalysts with different pore sizes. Catal. Lett. 2006, 107, 95–102. [Google Scholar] [CrossRef]
- Hibbitts, D.D.; Loveless, B.T.; Neurock, M.; Iglesia, E. Mechanistic role of water on the rate and selectivity of Fischer–Tropsch synthesis on ruthenium catalysts. Angew. Chem. Int. Ed. 2013, 52, 12273–12278. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, W.; Chen, D.; Holmen, A.; Davis, B.H. Fischer–Tropsch synthesis: A review of the effect of CO conversion on methane selectivity. Appl. Catal. A Gen. 2014, 470, 250–260. [Google Scholar] [CrossRef]
- Borg, Ø.; Yu, Z.; Chen, D.; Blekkan, E.A.; Rytter, E.; Holmen, A. The effect of water on the activity and selectivity for carbon nanofiber supported cobalt Fischer–tropsch catalysts. Top. Catal. 2014, 57, 491–499. [Google Scholar] [CrossRef]
- Sirikulbodee, P.; Ratana, T.; Sornchamni, T.; Phongaksorn, M.; Tungkamani, S. Catalytic performance of Iron-based catalyst in Fischer–Tropsch synthesis using CO2 containing syngas. Energy Procedia 2017, 138, 998–1003. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, X.; Hildebrandt, D.; Glasser, D. The effect of CO2 on a cobalt-based catalyst for low temperature Fischer–Tropsch synthesis. Chem. Eng. J. 2012, 193, 318–327. [Google Scholar] [CrossRef]
- James, O.O.; Mesubi, A.M.; Ako, T.C.; Maity, S. Increasing carbon utilization in Fischer–Tropsch synthesis using H2-deficient or CO2-rich syngas feeds. Fuel Process. Technol. 2010, 91, 136–144. [Google Scholar] [CrossRef]
- Kim, S.M.; Bae, J.W.; Lee, Y.J.; Jun, K.W. Effect of CO2 in the feed stream on the deactivation of Co/γ-Al2O3 Fischer–Tropsch catalyst. Catal. Commun. 2008, 9, 2269–2273. [Google Scholar] [CrossRef]
- Yao, Y.; Hildebrandt, D.; Glasser, D.; Liu, X. Fischer− Tropsch synthesis using H2/CO/CO2 syngas mixtures over a cobalt catalyst. Ind. Eng. Chem. Res. 2010, 49, 11061–11066. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Sparks, D.E.; Pendyala, V.R.R.; Hopps, S.G.; Thomas, G.A.; Davis, B.H. Fischer–Tropsch synthesis: Effect of ammonia in syngas on the Fischer–Tropsch synthesis performance of a precipitated iron catalyst. J. Catal. 2015, 326, 149–160. [Google Scholar] [CrossRef]
- Pendyala, V.R.R.; Gnanamani, M.K.; Jacobs, G.; Ma, W.; Shafer, W.D.; Davis, B.H. Fischer–Tropsch synthesis: Effect of ammonia impurities in syngas feed over a cobalt/alumina catalyst. Appl. Catal. A Gen. 2013, 468, 38–43. [Google Scholar] [CrossRef]
- Sango, T.; Fischer, N.; Henkel, R.; Roessner, F.; van Steen, E.; Claeys, M. Formation of nitrogen containing compounds from ammonia co-fed to the Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2015, 502, 150–156. [Google Scholar] [CrossRef]
- Steynberg, A.P.; Deshmukh, S.R.; Robota, H.J. Fischer-Tropsch catalyst deactivation in commercial microchannel reactor operation. Catal. Today 2018, 299, 10–13. [Google Scholar] [CrossRef]
- Díaz, J.A.; de la Osa, A.R.; Sánchez, P.; Romero, A.; Valverde, J.L. Influence of CO2 co-feeding on Fischer–Tropsch fuels production over carbon nanofibers supported cobalt catalyst. Catal. Commun. 2014, 44, 57–61. [Google Scholar] [CrossRef]
- Liao, P.Y.; Zhang, C.; Zhang, L.J.; Yang, Y.Z.; Zhong, L.S.; Wang, H.; Sun, Y.H. Effect of promoter and CO2 content in the feed on the performance of CuFeZr catalyst in the synthesis of higher alcohol from syngas. J. Fuel Chem. Technol. 2017, 45, 547–555. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.H.; Wang, Y.; Li, Y.; Hao, X.; Bai, L.; Li, Y.W. Effect of co-feeding carbon dioxide on Fischer–Tropsch synthesis over an iron–manganese catalyst in a spinning basket reactor. Fuel Process. Technol. 2008, 89, 234–241. [Google Scholar] [CrossRef]
- Lu, Y.; Lee, T. Influence of the feed gas composition on the Fischer-Tropsch synthesis in commercial operations. J. Nat. Gas Chem. 2007, 16, 329–341. [Google Scholar] [CrossRef]
- Daramola, M.O.; Matamela, K.; Sadare, O.O. Effect of CO2 co-feeding on the conversion of syngas derived from waste to liquid fuel over a bi-functional Co/H-ZSM-5 catalyst. J. Environ. Chem. Eng. 2017, 5, 54–62. [Google Scholar] [CrossRef]
- Muleja, A.A.; Yao, Y.; Glasser, D.; Hildebrandt, D. Effect of feeding nitrogen to a fixed bed Fischer–Tropsch reactor while keeping the partial pressures of reactants the same. Chem. Eng. J. 2016, 293, 151–160. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, J.; Han, J.; Yu, F. Synthesis of gasoline-range hydrocarbons from nitrogen-rich syngas over a Mo/HZSM-5 bi-functional catalyst. J. Energy Inst. 2016, 89, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Muleja, A.A.; Yao, Y.; Glasser, D.; Hildebrandt, D. Variation of the short-chain paraffin and olefin formation rates with time for a cobalt Fischer–Tropsch catalyst. Ind. Eng. Chem. Res. 2017, 56, 469–478. [Google Scholar] [CrossRef]
- Snel, R.; Espinoza, R.L. Fischer-tropsch synthesis on iron-based catalysts: The effect of co-feeding small oxygenates. J. Mol. Catal. 1989, 54, 213–223. [Google Scholar] [CrossRef]
- Sarup, B.; Wojciechowski, B.W. The effect of time on stream and feed composition on the selectivity of a cobalt fischer—Tropsch catalyst. Can. J. Chem. Eng. 1984, 62, 249–256. [Google Scholar] [CrossRef]
- Dalai, A.K.; Davis, B.H. Fischer–Tropsch synthesis: A review of water effects on the performances of unsupported and supported Co catalysts. Appl. Catal. A Gen. 2008, 348, 1–15. [Google Scholar] [CrossRef]
- Bertole, C.J.; Mims, C.A.; Kiss, G. The effect of water on the cobalt-catalyzed Fischer–Tropsch synthesis. J. Catal. 2002, 210, 84–96. [Google Scholar] [CrossRef]
- Rothaemel, M.; Hanssen, K.F.; Blekkan, E.A.; Schanke, D.; Holmen, A. The effect of water on cobalt Fischer-Tropsch catalysts studied by steady-state isotopic transient kinetic analysis (SSITKA). Catal. Today 1997, 38, 79–84. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Shafer, W.D.; Sparks, D.E.; Davis, B.H. Fischer–Tropsch synthesis: Effect of CO2 containing syngas over Pt promoted Co/γ-Al2O3 and K-promoted Fe catalysts. Catal. Commun. 2011, 12, 936–939. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, X.; Hildebrandt, D.; Glasser, D. Fischer–Tropsch synthesis using H2/CO/CO2 syngas mixtures: A comparison of paraffin to olefin ratios for iron and cobalt based catalysts. Appl. Catal. A Gen. 2012, 433, 58–68. [Google Scholar] [CrossRef]
- Zhang, R.; Hao, X.; Li, Y. Investigation of acetylene addition to Fischer–Tropsch Synthesis. Catal. Commun. 2011, 12, 1146–1148. [Google Scholar] [CrossRef]
- Kuipers, E.W.; Vinkenburg, I.H.; Oosterbeek, H. Chain length dependence of α-olefin readsorption in Fischer-Tropsch synthesis. J. Catal. 1995, 152, 137–146. [Google Scholar] [CrossRef]
- Iglesia, E.; Reyes, S.C.; Madon, R.J. Transport-enhanced α-olefin readsorption pathways in Ru-catalyzed hydrocarbon synthesis. J. Catal. 1991, 129, 238–256. [Google Scholar] [CrossRef]
- Snel, R.; Espinoza, R.L. Secondary reactions of primary products of the Fischer-Tropsch synthesis: Part 1. The role of ethene. J. Mol. Catal. 1987, 43, 237–247. [Google Scholar] [CrossRef]
- Fan, L.; Fujimoto, K. Fischer–Tropsch synthesis in supercritical fluid: Characteristics and application. Appl. Catal. A Gen. 1999, 186, 343–354. [Google Scholar] [CrossRef]
- Snel, R.; Espinoza, R.L. Secondary reactions of primary products of the fischer-tropsch synthesis: Part II. The role of propene. J. Mol. Catal. 1989, 54, 103–117. [Google Scholar] [CrossRef]
- Henkel, R. The Influence of Ammonia on Fischer-Tropsch Synthesis and Formation of N-Containing Compounds. Ph.D. Thesis, Universität Oldenburg, Oldenburg, Germany, 2013. [Google Scholar]
- Gibson, E.J.; Hall, C.C. Fischer-tropsch synthesis with cobalt catalysts. II. The effect of nitrogen, carbon dioxide and methane in the synthesis gas. J. Appl. Chem. 1954, 4, 464–468. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Carvalho, A.; Legras, B.; Paul, S.; Virginie, M.; Sushkevich, V.L.; Khodakov, A.Y. Effects of co-feeding with nitrogen-containing compounds on the performance of supported cobalt and iron catalysts in Fischer–Tropsch synthesis. Catal. Today 2016, 275, 84–93. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Yoneyama, Y.; Vitidsant, T.; Tsubaki, N. Jet fuel synthesis via Fischer–Tropsch synthesis with varied 1-olefins as additives using Co/ZrO2–SiO2 bimodal catalyst. Fuel 2016, 171, 159–166. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, X.; Masuku, C.M.; Hildebrandt, D.; Glasser, D. A study of the Fischer–Tropsch synthesis in a batch reactor: Rate, phase of water, and catalyst oxidation. Energy Fuels 2017, 31, 7405–7412. [Google Scholar] [CrossRef]
- Seadira, T.; Sadanandam, G.; Ntho, T.A.; Lu, X.; Masuku, C.M.; Scurrell, M. Hydrogen production from glycerol reforming: Conventional and green production. Rev. Chem. Eng. 2018, 34, 695–726. [Google Scholar] [CrossRef]
- Masuku, C.M.; Biegler, L.T. Recent advances in gas-to-liquids process intensification with emphasis on reactive distillation. Curr. Opin. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Eze, P.C.; Masuku, C.M. Vapour–liquid equilibrium prediction for synthesis gas conversion using artificial neural networks. S. Afr. J. Chem. Eng. 2018, 26, 80–85. [Google Scholar] [CrossRef]
- Eze, P.C.; Masuku, C.M. Supporting plots and tables on vapour–liquid equilibrium prediction for synthesis gas conversion using artificial neural networks. Data Brief 2018, 21, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Masuku, C.M.; Biegler, L.T. Equation-oriented framework for optimal synthesis of integrated reactive distillation systems for Fischer–Tropsch processes. Energy Fuels 2018, 32, 7199–7209. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Moyo, M.; Liu, X.; Hildebrandt, D. A critical review of the impact of water on cobalt-based catalysts in Fischer-Tropsch synthesis. Fuel Process. Technol. 2019, 192, 105–129. [Google Scholar] [CrossRef]
- Fischer, N.; Clapham, B.; Feltes, T.; Claeys, M. Cobalt-based Fischer–Tropsch activity and selectivity as a function of crystallite size and water partial pressure. ACS Catal. 2014, 5, 113–121. [Google Scholar] [CrossRef]
- Masuku, C.M.; Lu, X.; Hildebrandt, D.; Glasser, D. Reactive distillation in conventional Fischer–Tropsch reactors. Fuel Process. Technol. 2015, 130, 54–61. [Google Scholar] [CrossRef]
- Iglesia, E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl. Catal. A Gen. 1997, 161, 59–78. [Google Scholar] [CrossRef]
- Li, J.; Jacobs, G.; Das, T.; Davis, B.H. Fischer–Tropsch synthesis: Effect of water on the catalytic properties of a ruthenium promoted Co/TiO2 catalyst. Appl. Catal. A Gen. 2002, 233, 255–262. [Google Scholar] [CrossRef]
- Li, J.; Jacobs, G.; Das, T.; Zhang, Y.; Davis, B. Fischer–Tropsch synthesis: Effect of water on the catalytic properties of a Co/SiO2 catalyst. Appl. Catal. A Gen. 2002, 236, 67–76. [Google Scholar] [CrossRef]
- Yan, Q.; Yu, F.; Cai, Z.; Zhang, J. Catalytic upgrading nitrogen-riched wood syngas to liquid hydrocarbon mixture over a Fe–Pd/ZSM-5 catalyst. Biomass Bioenergy 2012, 47, 469–473. [Google Scholar] [CrossRef]
- Visconti, C.G.; Mascellaro, M. Calculating the product yields and the vapor–liquid equilibrium in the low-temperature Fischer–Tropsch synthesis. Catal. Today 2013, 214, 61–73. [Google Scholar] [CrossRef]
- Jess, A.; Popp, R.; Hedden, K. Fischer–Tropsch-synthesis with nitrogen-rich syngas: fundamentals and reactor design aspects. Appl. Catal. A Gen. 1999, 186, 321–342. [Google Scholar] [CrossRef]
- Dai, X.; Yu, C. Characterization and catalytic performance of CeO2-Co/SiO2 catalyst for Fischer-Tropsch synthesis using nitrogen-diluted synthesis gas over a laboratory scale fixed-bed reactor. J. Nat. Gas Chem. 2008, 17, 17–23. [Google Scholar] [CrossRef]
- Jess, A.; Hedden, K.; Popp, R. Diesel Oil from Natural Gas by Fischer-Tropsch Synthesis Using Nitrogen-Rich Syngas. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2001, 24, 27–31. [Google Scholar] [CrossRef]
- Xu, D.; Duan, H.; Li, W.; Xu, H. Investigation on the Fischer−Tropsch Synthesis with nitrogen-containing syngas over CoPtZrO2/Al2O3 catalyst. Energy Fuels 2006, 20, 955–958. [Google Scholar] [CrossRef]
- Patzlaff, J.; Liu, Y.; Graffmann, C.; Gaube, J. Studies on product distributions of iron and cobalt catalyzed Fischer–Tropsch synthesis. Appl. Catal. A Gen. 1999, 186, 109–119. [Google Scholar] [CrossRef]
- Patzlaff, J.; Liu, Y.; Graffmann, C.; Gaube, J. Interpretation and kinetic modeling of product distributions of cobalt catalyzed Fischer–Tropsch synthesis. Catal. Today 2002, 71, 381–394. [Google Scholar] [CrossRef]
- Hanlon, R.T.; Satterfield, C.N. Reactions of selected 1-olefins and ethanol added during the Fischer-Tropsch synthesis. Energy Fuels 1988, 2, 196–204. [Google Scholar] [CrossRef]
- Biquiza, L.D.; Claeys, M.; Van Steen, E. Thermodynamic and experimental aspects of ‘supercritical’ Fischer–Tropsch synthesis. Fuel Process. Technol. 2010, 91, 1250–1255. [Google Scholar] [CrossRef]
- De Klerk, A. Fischer–Tropsch refining: Technology selection to match molecules. Green Chem. 2008, 10, 1249–1279. [Google Scholar] [CrossRef]
- Dry, M.E. The fischer–tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Jess, A.; Popp, R.; Hedden, K. From natural gas to liquid hydrocarbons. Pt. 4. Production of diesel oil and wax by Fischer-Tropsch-Synthesis using a nitrogen-rich synthesis gas-investigations on a semi-technical scale. Erdoel Erdgas Kohle 1997, 113, 531–540. [Google Scholar]
- Shi, H.X.; Li, Z.K.; Liu, K.F.; Xiao, H.C.; Kong, F.H.; Zhang, J.; Chen, J.G. Enhanced formation of α-olefins by the pulse process between Fischer-Tropsch synthesis and N2 purging. J. Fuel Chem. Technol. 2016, 44, 822–829. [Google Scholar] [CrossRef]
- Todic, B.; Ma, W.; Jacobs, G.; Davis, B.H.; Bukur, D.B. Effect of process conditions on the product distribution of Fischer–Tropsch synthesis over a Re-promoted cobalt-alumina catalyst using a stirred tank slurry reactor. J. Catal. 2014, 311, 325–338. [Google Scholar] [CrossRef]
- Van Steen, E.; Schulz, H. Polymerisation kinetics of the Fischer–Tropsch CO hydrogenation using iron and cobalt based catalysts. Appl. Catal. A Gen. 1999, 186, 309–320. [Google Scholar] [CrossRef]
- Muleja, A.A.; Yao, Y.; Glasser, D.; Hildebrandt, D. A study of Fischer-Tropsch synthesis: Product distribution of the light hydrocarbons. Appl. Catal. A Gen. 2016, 517, 217–226. [Google Scholar] [CrossRef]
- Kapfunde, N.; Masuku, C.M.; Hildebrandt, D. Optimization of the thermal efficiency of a fixed-bed gasifier using computational fluid dynamics. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44, pp. 1747–1752. [Google Scholar]
- He, N.; Hu, Y.; Masuku, C.M.; Biegler, L.T. 110th Anniversary: Fischer–Tropsch Synthesis for Multiphase Product Recovery through Reactive Distillation. Ind. Eng. Chem. Res. 2019, 58, 13249–13259. [Google Scholar] [CrossRef]
- Masuku, C.M.; Hildebrandt, D.; Glasser, D.; Davis, B.H. Steady-state attainment period for Fischer–Tropsch products. Top. Catal. 2014, 57, 582–587. [Google Scholar] [CrossRef]
- McNab, A.I.; McCue, A.J.; Dionisi, D.; Anderson, J.A. Combined quantitative FTIR and online GC study of Fischer-Tropsch synthesis involving co-fed ethylene. J. Catal. 2018, 362, 10–17. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Copperthwaite, R.G.; van der Riet, M. Low methane selectivity using Co/MnO catalysts for the Fischer-Tropsch reaction: Effect of increasing pressure and Co-feeding ethene. Top. Catal. 1995, 2, 163–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Masuku, C.M.; Biegler, L.T. An MPCC Reactive Distillation Optimization Model for Multi-Objective Fischer–Tropsch Synthesis. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 46, pp. 451–456. [Google Scholar]
- Ntelane, T.S.; Masuku, C.M.; Scurrell, M.S. TPSR study: Effect of microwave radiation on the physicochemical properties of Fe/ZSM-5 in the methanol to hydrocarbons (MTH) process. J. Porous Mater. 2019. [Google Scholar] [CrossRef]
- Yang, J.; Ledesma Rodriguez, C.; Holmen, A.; Chen, D. Ethylene co-feeding effect on Fischer–Tropsch synthesis to olefin over Co based catalysts. In Proceedings of the American Chemical Society National Meeting & Expo, San Diego, CA, USA, 25–29 August 2019. [Google Scholar]


| Nature of Co-Feed | Co-Feed | Catalyst Used | Reaction Conditions | Effect in FTS | Amount Used | Ref |
|---|---|---|---|---|---|---|
| Inorganic Co-feeds | Water | Pt (0.5%)-15%Co/A12O3 | 2.93 MPa, and H2/CO was 2.0. | Decreased CO conversion, permanent deactivation of the catalyst. | 3–25 vol% | [6] |
| Water | 0.27%Ru-25%Co/Al2O3 25%Co/γ-Al2O3 | 205−230 °C, 1.4−2.5 MPa, H2/CO = 1.0−2.5, and 3−16 | Reduction in the CH4 rate by 12% catalyst deactivation observed during the addition of water. | 10 vol% | [10] | |
| CO2 | Co/γ-Al2O3 | (H2/CO = 2), fixed-bed reactor; 220 °C, 20 bar, and SV (L/kg cat/h) = 2000. | Decrease in CO conversion and C5+ selectivity partial oxidation of surface cobalt metal | 20 vol% | [30] | |
| Nitrogen | Co/TiO2 | 220 °C; 60 (NTP)mL/min to 75 (NTP) mL/min; 20.85 bar abs 25.85 bar abs | Reduced selectivity to the undesired light hydrocarbons (mainly CH4) | 28% N2 | [41] | |
| Ammonia | 100Fe/5.1Si/2.0Cu/3.0K | 220–270 °C and 1.3 MPa using a 1-L slurry phase reactor. | Rapidly deactivation of catalyst simultaneously changed the product selectivities. | 0.1–400 ppm | [32] | |
| Ammonia | iron-catalyst | 250 °C, 75 mL NTP/min, H2:CO = 2 and 5 bar, respectively. | The selectivities toward nitrogen-containing compounds enhanced with increasing NH3 content. Rates of formation of alcohols, aldehydes, and organic acids decreased | 0–10 vol% | [59] | |
| Organic co-feeds | Ethene | 62 wt% cobalt oxide and was supported on kieselguhr | 473 K and 110 kPa pressure. | The selectivity of the higher hydrocarbons was improved. | 1% to 2% | [16] |
| Ethanol addition | 10% Co/TiO2 catalyst | T = 220 °C, P = 8 bar, H2/CO = 2) | The selectivity to light products increased, as well as the olefin to paraffin ratio. A significant decrease in the catalyst activity. | 2 vol% and 6 vol%) | [19] | |
| Small oxygenates | Iron catalysts. gas with a mol ratio H2:C0 = 0.5 | 2.0 MPa; 543 K flow (VHSV = 1000) | Aldehydes suppress and entirely change normal synthesis behavior. | 10 mol% dimethyl ether (DME). Diethyl ether (DEE) is 3.3 mol%. Acetaldehyde is 3 mol% | [44] | |
| 1-olefins as additives | Co/ZrO2–SiO2 bimodal catalyst | 513 K, 1.0 MPa, W/F Syngas = 10 g − cat h/mol. | Resulted in an anti-Anderson–Schulz–Flory (anti-ASF) product distribution. 1-decene and 1-tetradecene mixed with the volume ratio of 1:1, showed the highest selectivity to jet-fuel-like hydrocarbons. Formation rates of CH4 and CO2, as well as light hydrocarbons (C2–C4), suppressed | 20 mol% | [60] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muleja, A.A.; Gorimbo, J.; Masuku, C.M. Effect of Co-Feeding Inorganic and Organic Molecules in the Fe and Co Catalyzed Fischer–Tropsch Synthesis: A Review. Catalysts 2019, 9, 746. https://doi.org/10.3390/catal9090746
Muleja AA, Gorimbo J, Masuku CM. Effect of Co-Feeding Inorganic and Organic Molecules in the Fe and Co Catalyzed Fischer–Tropsch Synthesis: A Review. Catalysts. 2019; 9(9):746. https://doi.org/10.3390/catal9090746
Chicago/Turabian StyleMuleja, Adolph Anga, Joshua Gorimbo, and Cornelius Mduduzi Masuku. 2019. "Effect of Co-Feeding Inorganic and Organic Molecules in the Fe and Co Catalyzed Fischer–Tropsch Synthesis: A Review" Catalysts 9, no. 9: 746. https://doi.org/10.3390/catal9090746





