Electrorheological Effect of Gold Nanoparticles Coated with Fluorescent Mesogenic Groups Dispersed in Nematic Liquid Crystal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Rheological Measurements
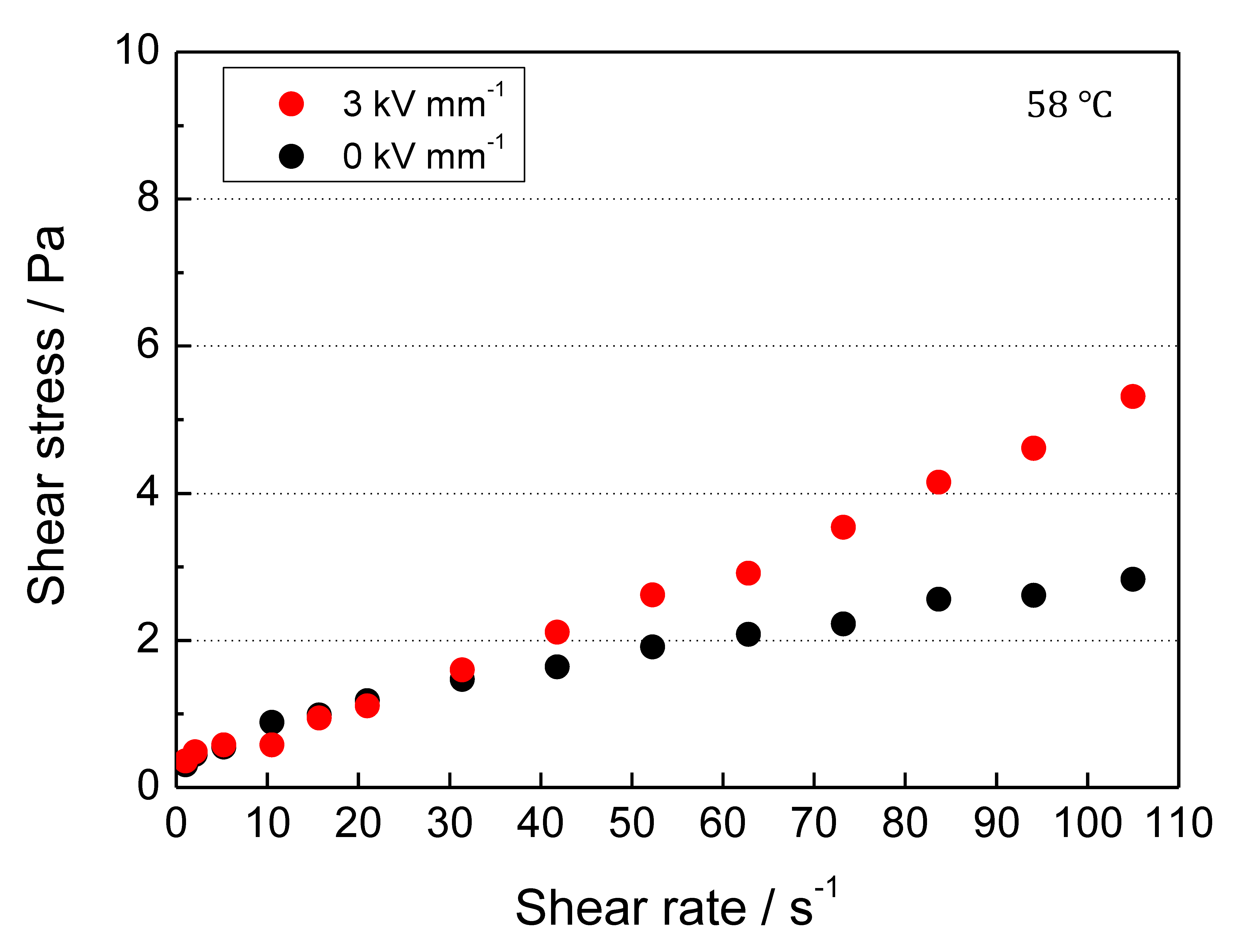
3. Results and Discussion
3.1. Phase Behavior
3.2. Characterization of the GNPs
3.3. Phase Behavior of the Composites
3.4. Electrorheological Property
3.5. POM Observation under an Applied Electric Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baginski, M.; Tupikowska, M.; Gonzalez-Rubio, G.; Wojcik, M.; Lewandowski, W. Shaping Liquid Crystals with Gold Nanoparticles: Helical Assemblies with Tunable and Hierarchical Structures via Thin-Film Cooperative Interactions. Adv. Mater. 2020, 32, 1904581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, A.; Zhang, Z.; Yin, R.; Gao, Y.; Feng, L.; Yang, S. Repeatable and Reprogrammable Shape Morphing from Photoresponsive Gold Nanorod/Liquid Crystal Elastomers. Adv. Mater. 2020, 32, 2004270. [Google Scholar] [CrossRef]
- Shivaraja, S.J.; Gupta, R.K.; Kumar, S.; Manjuladevi, V. Enhanced electro-optical response of nematic liquid crystal doped with functionalised silver nanoparticles in twisted nematic configuration. Liq. Cryst. 2020, 47, 1678–1690. [Google Scholar]
- Hu, J.; Kuang, Z.-Y.; Tao, L.; Huang, Y.-F.; Wang, Q.; Xie, H.-L.; Yin, J.-R.; Chen, E.-Q. Programmable 3D Shape-Change Liquid Crystalline Elastomer Based on a Vertically Aligned Monodomain with Cross-link Gradient. ACS Appl. Mater. Interfaces 2019, 11, 48393–48401. [Google Scholar] [CrossRef]
- Lesiak, P.; Bednarska, K.; Lewandowski, W.; Wojcik, M.; Polakiewicz, S.; Baginski, M.; Osuch, T.; Markowski, K.; Orzechowski, K.; Makowski, M.; et al. Self-organized, one-dimensional periodic structures in gold nanoparticle-doped nematic liquid crystal composite. ACS Nano 2019, 13, 10154–10160. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, S.; Yang, W.; Qin, B.; Wang, X.; Wang, Y.; Cao, M.; Gao, Y.; Li, C.; Dong, Y. Photo actuation of liquid crystalline elastomer nanocomposites incorporated with gold nanoparticles based on surface plasmon resonance. Soft Matter 2019, 15, 6116–6126. [Google Scholar] [CrossRef] [PubMed]
- Mangaiyarkarasi, R.; Sivaranjini, B.; Umadevi, S. Facile synthesis of gold nanoparticles capped with an ammonium-based chiral ionic liquid crystal. Liq. Cryst. 2019, 46, 584–593. [Google Scholar] [CrossRef]
- Das, N.; Borah, D.; Acharya, H.; Choudhury, S.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Grafting a mesomorphic Schiff base onto gold nanoparticle via ester link—Photoluminescence, mesomorphism, electrical conductivity and antioxidant activity. Liq. Cryst. 2019, 46, 609–617. [Google Scholar] [CrossRef]
- Nemati, A.; Shadpour, S.; Querciagrossa, L.; Li, L.; Mori, T.; Gao, M.; Zannoni, C.; Hegmann, T. Chirality amplification by desymmetrization of chiral ligand-capped nanoparticles to nanorods quantified in soft condensed matter. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cseh, L.; Mang, X.; Zeng, X.; Liu, F.; Mehl, G.H.; Ungar, G.; Siligardi, G. Helically Twisted Chiral Arrays of Gold Nanoparticles Coated with a Cholesterol Mesogen. J. Am. Chem. Soc. 2015, 137, 12736–12739. [Google Scholar] [CrossRef]
- Winslow, W.M. Induced Fibration of Suspensions. J. Appl. Phys. 1949, 20, 1137–1140. [Google Scholar] [CrossRef]
- Inoue, A.; Maniwa, S. Electrorheological effect of liquid crystalline polymers. J. Appl. Polym. Sci. 1995, 55, 113–118. [Google Scholar] [CrossRef]
- Inoue, A.; Ide, Y.; Oda, H. Influence of dilution by polydimethylsiloxane on electrorheological effect of side-chain liquid crystalline polysiloxane. J. Appl. Polym. Sci. 1997, 64, 1319–1328. [Google Scholar] [CrossRef]
- Inoue, A.; Maniwa, S.; Ide, Y. Relation between molecular structure and electrorheological effects in liquid crystalline polymers. J. Appl. Polym. Sci. 1997, 64, 303–310. [Google Scholar] [CrossRef]
- Tanaka, K.; Oiwa, Y.; Akiyama, R.; Kubono, A. Shear Thinning and Electro-Rheological Effect for Neat Liquid Crystalline Polysiloxane in the Vicinity of Isotropic-Liquid Crystalline Phase Transition. Polym. J. 1998, 30, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Orihara, H.; Kawasaki, T.; Doi, M.; Inoue, A. Immiscible polymer blend electrorheological fluids: Composition dependence. J. Appl. Polym. Sci. 2002, 86, 3673–3680. [Google Scholar] [CrossRef]
- Mimura, K.; Nishimoto, Y.; Orihara, H.; Moriya, M.; Sakamoto, W.; Yogo, T. Synthesis of Transparent and Field-Responsive BaTiO3 Particle/Organosiloxane Hybrid Fluid. Angew. Chem. Int. Ed. 2010, 49, 4902–4906. [Google Scholar] [CrossRef]
- Kaneko, K.; Kawai, T.; Nakamura, N. Electrorheological Effect of “Side-on” Liquid Crystalline Polysiloxane. ChemPhysChem 2008, 9, 2457–2460. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Mandai, A.; Heinrich, B.; Donnio, B.; Hanasaki, T. Electric Field-Induced Reversible Viscosity Change in Columnar Liquid Crystal. ChemPhysChem 2010, 11, 3596–3598. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, T.; Kamei, Y.; Mandai, A.; Uno, K.; Kaneko, K. Phase Transition Behavior and Electro-Rheological Effect of Liquid Crystalline Siloxane Dimers. Liq. Cryst. 2011, 38, 841–848. [Google Scholar] [CrossRef]
- Kaneko, K.; Oto, K.; Kawai, T.; Choi, H.; Kikuchi, H.; Nakamura, N. Electro-Rheological Effect and Electro-Optical Properties of Side-on Liquid Crystalline Polysiloxane in a Nematic Solvent. ChemPhysChem 2013, 14, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Ujihara, Y.; Oto, K.; Hashishin, T.; Hanasaki, T. Electric-Field-Induced Viscosity Change of a Nematic Liquid Crystal with Gold Nanoparticles. ChemPhysChem 2015, 16, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Cseh, L.; Mehl, G.H. The Design and Investigation of Room Temperature Thermotropic Nematic Gold Nanoparticles. J. Am. Chem. Soc. 2006, 128, 13376–13377. [Google Scholar] [CrossRef]
- Cseh, L.; Mehl, G.H. Structure-property relationships in nematic gold nanoparticles. J. Mater. Chem. 2007, 17, 311–315. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, F.; Fowler, A.G.; Ungar, G.; Cseh, L.; Mehl, G.H.; Macdonald, J.E. 3D Ordered Gold Strings by Coating Nanoparticles with Mesogens. Adv. Mater. 2009, 21, 1746–1750. [Google Scholar] [CrossRef]
- Kanayama, N.; Tsutsumi, O.; Kanazawa, A.; Ikeda, T. Distinct thermodynamic behaviour of a mesomorphic gold nanoparticle covered with a liquid-crystalline compound. Chem. Commun. 2001, 24, 2640–2641. [Google Scholar] [CrossRef]
- Qi, H.; Hegmann, T. Formation of periodic stripe patterns in nematic liquid crystals doped with functionalized gold nanoparticles. J. Mater. Chem. 2006, 16, 4197–4205. [Google Scholar] [CrossRef]
- Donnio, B.; García-Vázquez, P.; Gallani, J.-L.; Guillon, D.; Terazzi, E. Dendronized Ferromagnetic Gold Nanoparticles Self-Organized in a Thermotropic Cubic Phase. Adv. Mater. 2007, 19, 3534–3539. [Google Scholar] [CrossRef]
- Marx, V.M.; Girgis, H.; Heiney, P.A.; Hegmann, T. Bent-core liquid crystal (LC) decorated gold nanoclusters: Synthesis, self-assembly, and effects in mixtures with bent-core LC hosts. J. Mater. Chem. 2008, 18, 2983–2994. [Google Scholar] [CrossRef]
- Qi, H.; Kinkead, B.; Hegmann, T. Unprecedented Dual Alignment Mode and Freedericksz Transition in Planar Nematic Liquid Crystal Cells Doped with Gold Nanoclusters. Adv. Funct. Mater. 2008, 18, 212–221. [Google Scholar] [CrossRef]
- Qi, H.; Kinkead, B.; Marx, V.M.; Kinkead, B.; Zhang, H.R.; Kinkead, B.; Hegmann, T. Miscibility and Alignment Effects of Mixed Monolayer Cyanobiphenyl Liquid-Crystal-Capped Gold Nanoparticles in Nematic Cyanobiphenyl Liquid Crystal Hosts. ChemPhysChem 2009, 10, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Lewandowski, W.; Matraszek, J.; Mieczkowski, J.; Borysiuk, J.; Pociecha, D.; Gorecka, E. Liquid-Crystalline Phases Made of Gold Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 5167–5169. [Google Scholar] [CrossRef]
- Wojcik, M.; Kolpaczynska, M.; Pociecha, D.; Mieczkowski, J.; Gorecka, E. Multidimensional structures made by gold nanoparticles with shape-adaptive grafting layers. Soft Matter 2010, 6, 5397–5400. [Google Scholar] [CrossRef]
- Draper, M.; Saez, I.M.; Cowling, S.J.; Gai, P.; Heinrich, B.; Donnio, B.; Guillon, D.; Goodby, J.W. Self-Assembly and Shape Morphology of Liquid Crystalline Gold Metamaterials. Adv. Funct. Mater. 2011, 21, 1260–1278. [Google Scholar] [CrossRef]
- Nealon, G.L.; Greget, R.; Dominguez, C.; Nagy, Z.T.; Guillon, D.; Gallani, J.-L.; Donnio, B. Liquid-crystalline nanoparticles: Hybrid design and mesophase structures. Beilstein J. Org. Chem. 2012, 8, 349–370. [Google Scholar] [CrossRef] [Green Version]
- Mischler, S.; Guerra, S.; Deschenaux, R. Design of liquid-crystalline gold nanoparticles by click chemistry. Chem. Commun. 2012, 48, 2183–2185. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Mitsuda, A.; Miyano, K.; Tanaka, T.; Morita, M.; Agou, T.; Kubota, T.; Konno, T. Development of novel solid-state light-emitting materials based on pentafluorinated tolane fluorophores. ACS Omega 2018, 3, 9105–9113. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Morita, M.; Agou, T.; Kubota, T.; Ichikawa, T.; Konno, T. Thermoresponsive luminescence properties of polyfluorinated bistolane-type light-emitting liquid crystals. Org. Biomol. Chem. 2018, 16, 5609–5617. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Miyano, K.; Konno, T.; Agou, T.; Kubota, T.; Hosokai, T. Fluorine-containing bistolanes as light-emitting liquid crystalline molecules. Org. Biomol. Chem. 2017, 15, 5949–5958. [Google Scholar] [CrossRef]
- Yamada, S.; Morita, M.; Konno, T. Multi-color photoluminescence induced by electron-density distribution of fluorinated bistolane derivatives. J. Fluorine Chem. 2017, 202, 54–64. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneko, K.; Yamashita, K.; Fujioka, D.; Kaneko, K.; Fuchigami, K.; Hashishin, T.; Hanasaki, T. Electrorheological Effect of Gold Nanoparticles Coated with Fluorescent Mesogenic Groups Dispersed in Nematic Liquid Crystal. Crystals 2021, 11, 192. https://doi.org/10.3390/cryst11020192
Kaneko K, Yamashita K, Fujioka D, Kaneko K, Fuchigami K, Hashishin T, Hanasaki T. Electrorheological Effect of Gold Nanoparticles Coated with Fluorescent Mesogenic Groups Dispersed in Nematic Liquid Crystal. Crystals. 2021; 11(2):192. https://doi.org/10.3390/cryst11020192
Chicago/Turabian StyleKaneko, Kosuke, Kosuke Yamashita, Daiki Fujioka, Kimiyoshi Kaneko, Kiyomi Fuchigami, Takeshi Hashishin, and Tomonori Hanasaki. 2021. "Electrorheological Effect of Gold Nanoparticles Coated with Fluorescent Mesogenic Groups Dispersed in Nematic Liquid Crystal" Crystals 11, no. 2: 192. https://doi.org/10.3390/cryst11020192





