Biodiesel Purification via Ultrasonic-Assisted Solvent-Aided Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Operation of the Solvent-Aided Crystallization System
2.2. Materials and Reagents
2.3. Biodiesel Production
2.4. Biodiesel Purification
2.5. Biodiesel Characterization
2.6. Statistical Analysis
3. Results
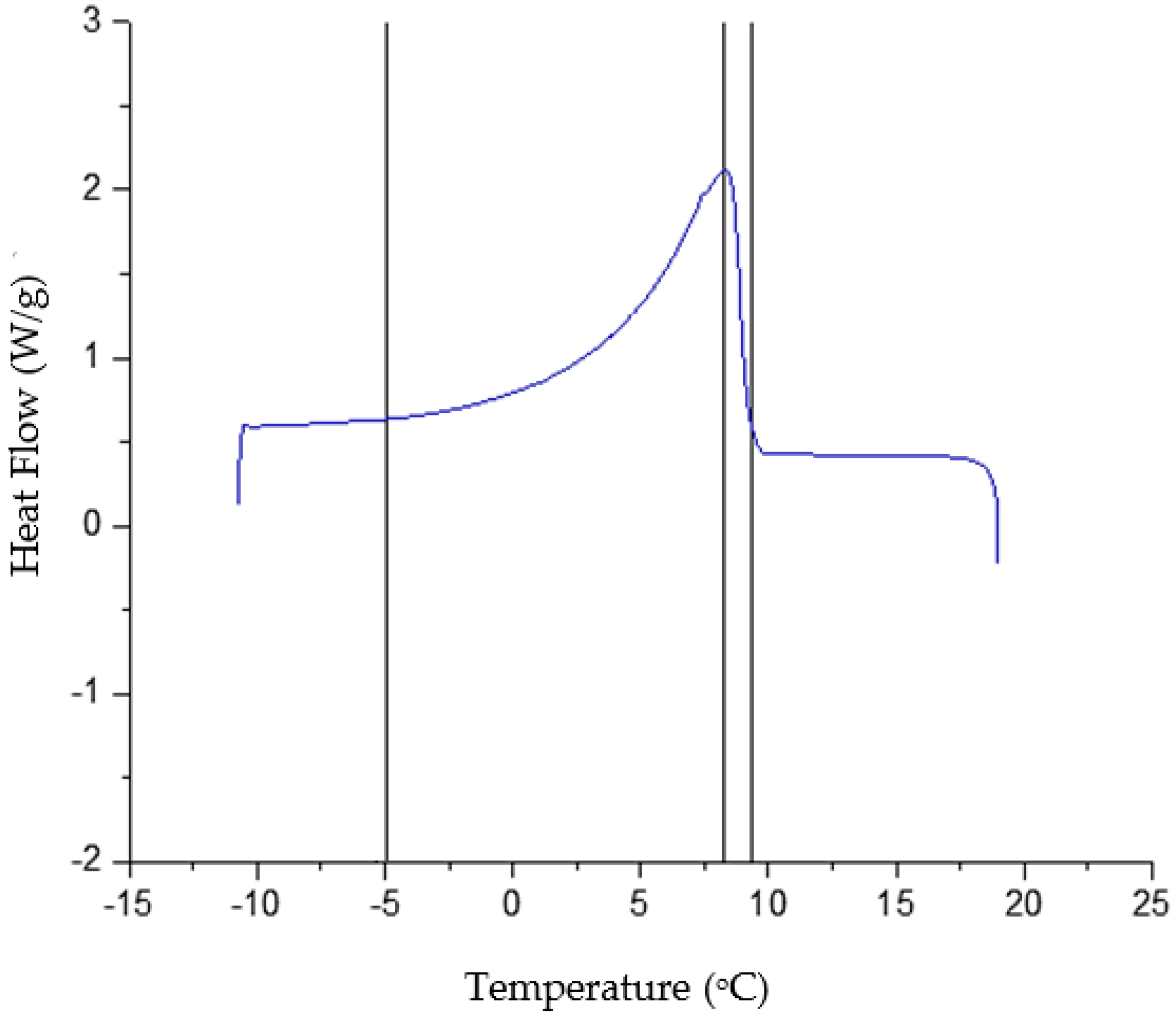
3.1. Biodiesel Characterization
3.2. Solvent-Aided Crystallization
3.2.1. Effect of 1-Butanol Concentration on SAC
3.2.2. Effect of Coolant Temperature on SAC
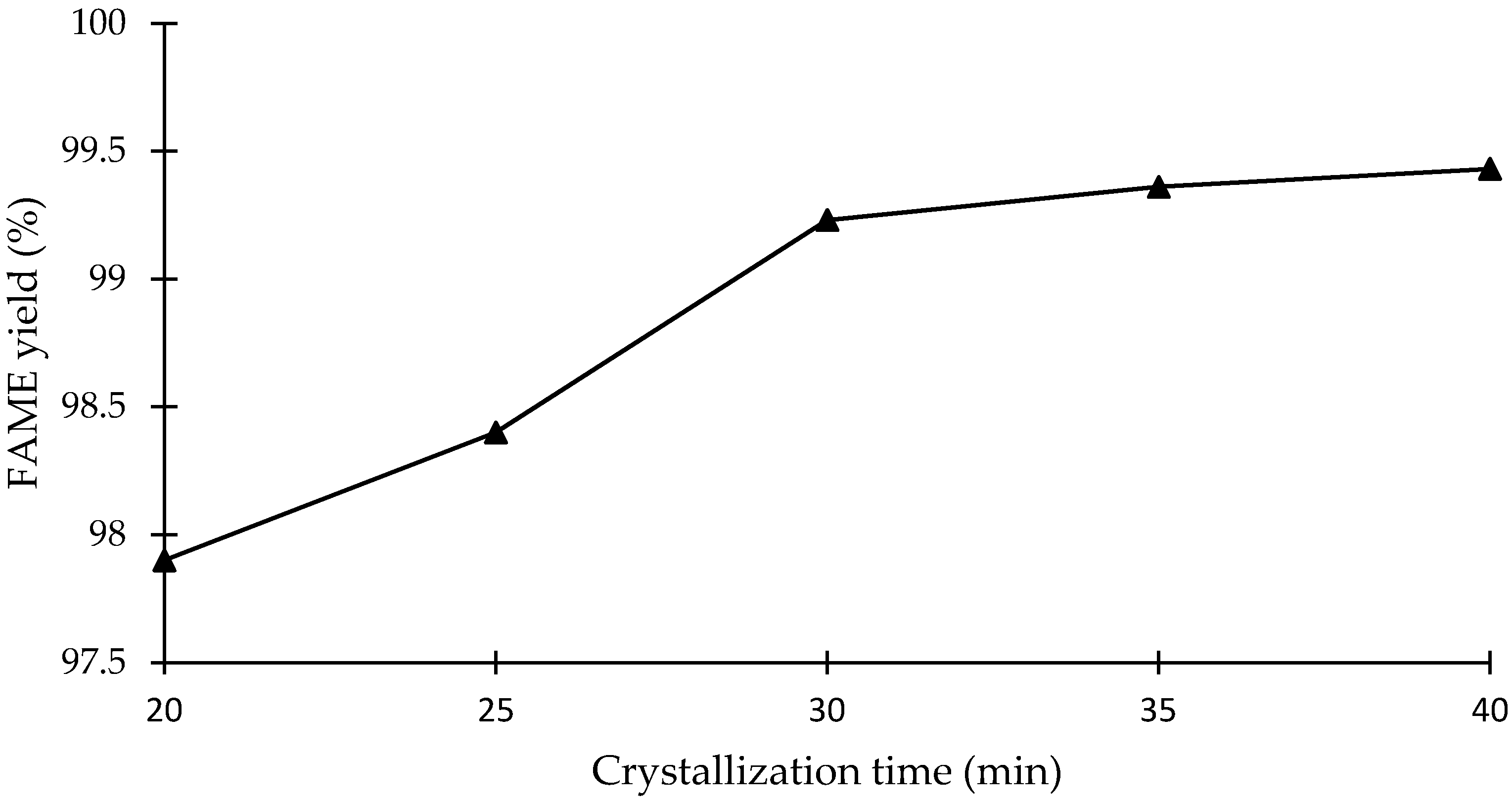
3.2.3. Effect of Crystallization Time on SAC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catarino, M.; Ferreira, E.; Dias, A.P.S.; Gomes, J. Dry washing biodiesel purification using fumed silica sorbent. Chem. Eng. J. 2020, 386, 123930. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Ge, J.C.; Ho, Y.K.; Sam, K.Y.; Nag, J.C. Optimization of palm oil biodiesel blends and engine operating parameters to improve performance and PM morphology in a common rail direct injection diesel engine. Fuel 2020, 260, 116326. [Google Scholar] [CrossRef]
- Ge, J.C.; Ho, Y.K.; Sam, K.Y.; Nag, J.C. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Rajalingam, A.; Jani, S.P.; Kumar, A.S.; Khan, M.A. Production methods of biodiesel. J. Chem. Pharm. Res. 2016, 8, 170–173. [Google Scholar]
- Salmanizade, F.; Ghazanfari Moghaddam, A.; Mohebbi, A. Improvement hydrocyclone separation of biodiesel impurities prepared from waste cooking oil using CFD simulation. Sep. Sci. Technol. 2020, 1–16. [Google Scholar] [CrossRef]
- Limmun, W.; Sansiribhan, S. Water-spray washing technique as a purification process in the production of biodiesel. E3S Web Conf. 2020, 187, 03006. [Google Scholar] [CrossRef]
- Tanattı, P.; Şengil, İ.A.; Özdemir, A. Treatment of biodiesel wastewater by solvent extraction: Evaluation of kinetic and thermodynamic data. Environ. Eng. Manag. J. 2018, 17, 2657–2665. [Google Scholar]
- Suthar, K.; Dwivedi, A.; Joshipura, M. A review on separation and purification techniques for biodiesel production with special emphasis on Jatropha oil as a feedstock. Asia-Pac. J. Chem. Eng. 2019, 14, e2361. [Google Scholar] [CrossRef]
- Avinash, A.; Murugesan, A. Judicious recycling of biobased adsorbents for biodiesel purification: A critical review. Environ. Prog. Sustain. Energy 2019, 38, e13077. [Google Scholar] [CrossRef]
- Nadeem, F.; Shahzadi, A.; El Zerey-Belaskri, A.; Abbas, Z. Conventional and Advanced Purification Techniques for Crude Biodiesel–A Critical Review. Int. J. Chem. Biochem. Sci. 2017, 12, 113–121. [Google Scholar]
- Eisenbart, F.J.; Ulrich, J. Solvent-aided layer crystallization—Case study glycerol–water. Chem. Eng. Sci. 2015, 133, 24–29. [Google Scholar] [CrossRef]
- Samsuri, S.; Jian, N.L.; Jusoh, F.W.; Hernández Yáñez, E.; Yahya, N.Y. Solvent-Aided Crystallization for Biodiesel Purification. Chem. Eng. Technol. 2020, 43, 447–456. [Google Scholar] [CrossRef]
- Karunanithi, A.T.; Acquah, C.; Achenie, L.E.; Sithambaram, S.; Suib, S.L. Solvent design for crystallization of carboxylic acids. Comp. Chem. Eng. 2009, 33, 1014–1021. [Google Scholar] [CrossRef]
- Eisenbart, F.J.; Ulrich, J. Solvent-Aided Layer Crystallization of Glycerol–Post-Treatment and the Influence of Agitation. Chem. Eng. Technol. 2016, 39, 1251–1256. [Google Scholar] [CrossRef]
- Fujimoto, R.; Maruyama, M.; Okada, S.; Adachi, H.; Yoshikawa, H.Y.; Takano, K.; Imanishi, M.; Tsukamoto, K.; Yoshimura, M.; Mori, Y. Large-scale crystallization of acetaminophen trihydrate by a novel stirring technique. App. Phys. Exp. 2019, 12, 045503. [Google Scholar] [CrossRef]
- Ullah, I.; Ali, S.; Grøndahl, L. Evaluation of an ultrasonic-assisted mechanical stirring technique for the synthesis of an efficient nano-photocatalyst. Res. Chem. Intermed. 2018, 44, 4015–4028. [Google Scholar] [CrossRef]
- Ruecroft, G.; David, H.; Tuan, L.; Neil, M.; Peter, W.C. Sonocrystallization: The use of ultrasound for improved industrial crystallization. Org. Process Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Acquah, C.; Cagnetta, M.; Achenie, L.E.; Suib, S.L.; Karunanithi, A.T. Effect of solvent topography and steric hindrance on crystal morphology. Ind. Eng. Chem. Res. 2015, 54, 12108–12113. [Google Scholar] [CrossRef]
- Samsuri, S.; Amran, N.A.; Yahya, N.; Jusoh, M. Review on progressive freeze concentration designs. Chem. Eng. Commun. 2016, 203, 345–363. [Google Scholar] [CrossRef]
- Tariq, M.; Ali, S.; Ahmad, F.; Ahmad, M.; Zafar, M.; Khalid, N.; Khan, M.A. Identification, FT-IR, NMR (1H and 13C) and GC/MS studies of fatty acid methyl esters in biodiesel from rocket seed oil. Fuel Process. Technol. 2011, 92, 336–341. [Google Scholar] [CrossRef]
- Du, L.; Li, Z.; Ding, S.; Chen, C.; Qu, S.; Yi, W.; Ding, J. Synthesis and characterization of carbon-based MgO catalysts for biodiesel production from castor oil. Fuel 2019, 258, 116122. [Google Scholar] [CrossRef]
- Leoneti, A.B.; Aragão-Leoneti, V.; de Oliveira, S.V.W.B. Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unrefined glycerol. Renew. Energy 2012, 45, 138–145. [Google Scholar] [CrossRef]
- Cermak, S.C.; Roque, L.E.; James, A.K. Distillation of Natural Fatty Acids and Their Chemical Derivatives. In Distillation-Advances from Modeling to Applications; Zereshki, S., Ed.; InTech: London, UK, 2012. [Google Scholar]
- Abdullah, A.Z.; Salamatinia, B.; Mootabadi, H.; Bhatia, S. Status and policies on biodiesel industry in Malaysia as the world’s leading producer of palm oil. Energy Policy 2009, 37, 5440–5448. [Google Scholar] [CrossRef]
- Amran, N.A.; Samsuri, S.; Safiei, N.Z.; Zakaria, Z.Y.; Jusoh, M. Parametric study on the performance of progressive cryoconcentration system. Chem. Eng. Commun. 2016, 203, 957–975. [Google Scholar] [CrossRef]
- Eisenbart, F.J.; Angermeier, N.; Ulrich, J. Production of highly dry glycerol by solvent-aided melt layer crystallization. J. Cryst. Growth 2017, 469, 191–196. [Google Scholar] [CrossRef]
- Ab Hamid, F.H.; Salim, S.A.; Mat-Shayuti, M.S. Optimization of progressive freeze concentration on stormwater purification via response surface methodology. Asia-Pac. J. Chem. Eng. 2020, 15. [Google Scholar] [CrossRef]
- Samsuri, S.; Mohd Bakri, M.M. Optimization of fractional crystallization on crude biodiesel purification via response surface methodology. Sep. Sci. Technol. 2018, 53, 567–572. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, Y.; de Lourdes Mendoza, M.; Zhai, S.; Feng, W.; Wang, Y.; Gu, M.; Cai, L.; Zhang, L. Progressive freezing and suspension crystallization methods for tetrahydrofuran recovery from Grignard reagent wastewater. J. Clean. Prod. 2017, 144, 180–186. [Google Scholar] [CrossRef]
- Amran, N.A.; Jusoh, M. Effect of coolant temperature and circulation flowrate on the performance of a vertical finned crystallizer. Procedia Eng. 2016, 148, 1408–1415. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.S.; Chen, W.W.; Han, W.F. Experimental study on factors affecting the quality of ice crystal during the freezing concentration for the brackish water. Desalination 2010, 260, 231–238. [Google Scholar] [CrossRef]
- Samsuri, S.; Amran, N.A.; Zheng, L.J.; Bakri, M.M.M. Effect of coolant temperature and cooling time on fractional crystallization of biodiesel and glycerol. Malays. J. Fundam. Appl. Sci. 2017, 13, 676–679. [Google Scholar] [CrossRef]
- Yahya, N.; Ismail, N.; Zakaria, Z.Y.; Ngadi, N.; Rahman, R.A.; Jusoh, M. The effect of coolant temperature and stirrer speed for concentration of sugarcane via progressive freeze concentration process. Chem. Eng. Trans. 2017, 56, 1147–1152. [Google Scholar]
- Kherici, S.; Benouali, D.; Benyetou, M.; Ghidossi, R.; Lacampagne, S.; Mietton-Peuchot, M. Study of potassium hydrogen tartrate unseeded batch crystallization for tracking optimum cooling mode. Orient. J. Chem. 2015, 31, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Moreno, F.L.; Raventos, E.; Hernandez, Y.R. Block freeze-concentration of coffee extract: Effect of freezing and thawing stages on solute recovery and bioactive compounds. J. Food Eng. 2014, 120, 158–166. [Google Scholar] [CrossRef]
- Williams, P.M.; Ahmad, M.; Connolly, B.S.; Oatley-Radcliffe, D.L. Technology for freeze concentration in the desalination industry. Desalination 2015, 356, 314–327. [Google Scholar] [CrossRef]
- Jusoh, M.; Nor, N.N.M.; Zakaria, Z.Y. Progressive freeze concentration of coconut water. J. Teknol. 2014, 67. [Google Scholar] [CrossRef] [Green Version]







| Peak Number | Retention Time (tR) | Library/ID (Systematic Name) | Trivial Name | Types of Fatty Acids | Composition of FAME% (Purity) |
|---|---|---|---|---|---|
| 3 | 12.875 | Dodecanoic acid | Lauric | Saturated | 0.47% |
| 4 | 15.105 | Methyl tetradecanoate | Myristic | Saturated | 1.67% |
| 6 | 17.155 | Hexadecanoic acid | Palmitic | Saturated | 41.13% |
| 7 | 17.325 | 9-Hexadecenoic acid | Palmitoleic | Unsaturated | 0.27% |
| 11 | 19.004 | Methyl stearate | Stearic | Saturated | 4.13% |
| 12 | 19.200 | 9-Octadecenoic acid | Oleic | Unsaturated | 39.36% |
| 14 | 19.588 | 9,12-Octadecadienoic acid | Linoleic | Unsaturated | 9.84% |
| 16 | 20.080 | 9,12,15-Octadecatrienoic | Linolenic | Unsaturated | 0.56% |
| 19 | 20.710 | Eicosanoic acid | Arachidic | Saturated | 0.79% |
| Total | 98.22% | ||||
| Parameters | PORIM Specification (%) |
|---|---|
| Lauric | 0.0–0.4 |
| Myristic | 0.6–1.6 |
| Palmitic | 41–47 |
| Palmitoleic | 0–0.6 |
| Stearic | 3.7–5.6 |
| Oleic | 38.2–43.5 |
| Linoleic | 6.6–11.9 |
| Linolenic | 0.0–0.5 |
| Arachidic | 0.0–0.8 |
| Unsaturated Fatty Acid | 44.8–57.3 |
| Saturated Fatty Acid | 45.3–44.5 |
| Peak Number | Retention Time | Area |
|---|---|---|
| 1 | 7.532 | 38,752 |
| 2 | 10.381 | 44,401 |
| 3 | 12.875 | 547,914 |
| 4 | 15.105 | 1,952,011 |
| 5 | 16.140 | 81,944 |
| 6 | 17.154 | 48,143,358 |
| 7 | 17.325 | 316,072 |
| 8 | 17.387 | 234,127 |
| 9 | 18.080 | 163,889 |
| 10 | 18.292 | 51,066 |
| 11 | 19.004 | 3,324,613 |
| 12 | 19.200 | 46,075,665 |
| 13 | 19.245 | 1,767,664 |
| 14 | 19.588 | 11,519,082 |
| 15 | 19.687 | 353,028 |
| 16 | 20.080 | 538,494 |
| 17 | 20.137 | 386,310 |
| 18 | 20.250 | 73,585 |
| 19 | 20.712 | 926,727 |
| 20 | 20.887 | 337,839 |
| 21 | 22.509 | 105,357 |
| 22 | 24.993 | 81,944 |
| Total | 117,063,842 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.A.; Samsuri, S. Biodiesel Purification via Ultrasonic-Assisted Solvent-Aided Crystallization. Crystals 2021, 11, 212. https://doi.org/10.3390/cryst11020212
Ahmad MA, Samsuri S. Biodiesel Purification via Ultrasonic-Assisted Solvent-Aided Crystallization. Crystals. 2021; 11(2):212. https://doi.org/10.3390/cryst11020212
Chicago/Turabian StyleAhmad, Mohd. Afnan, and Shafirah Samsuri. 2021. "Biodiesel Purification via Ultrasonic-Assisted Solvent-Aided Crystallization" Crystals 11, no. 2: 212. https://doi.org/10.3390/cryst11020212







