Low-Carbon Sustainable Composites from Waste Phosphogypsum and Their Environmental Impacts
Abstract
:1. Introduction
2. Purification
2.1. Chemical Methods
2.2. Physical Methods
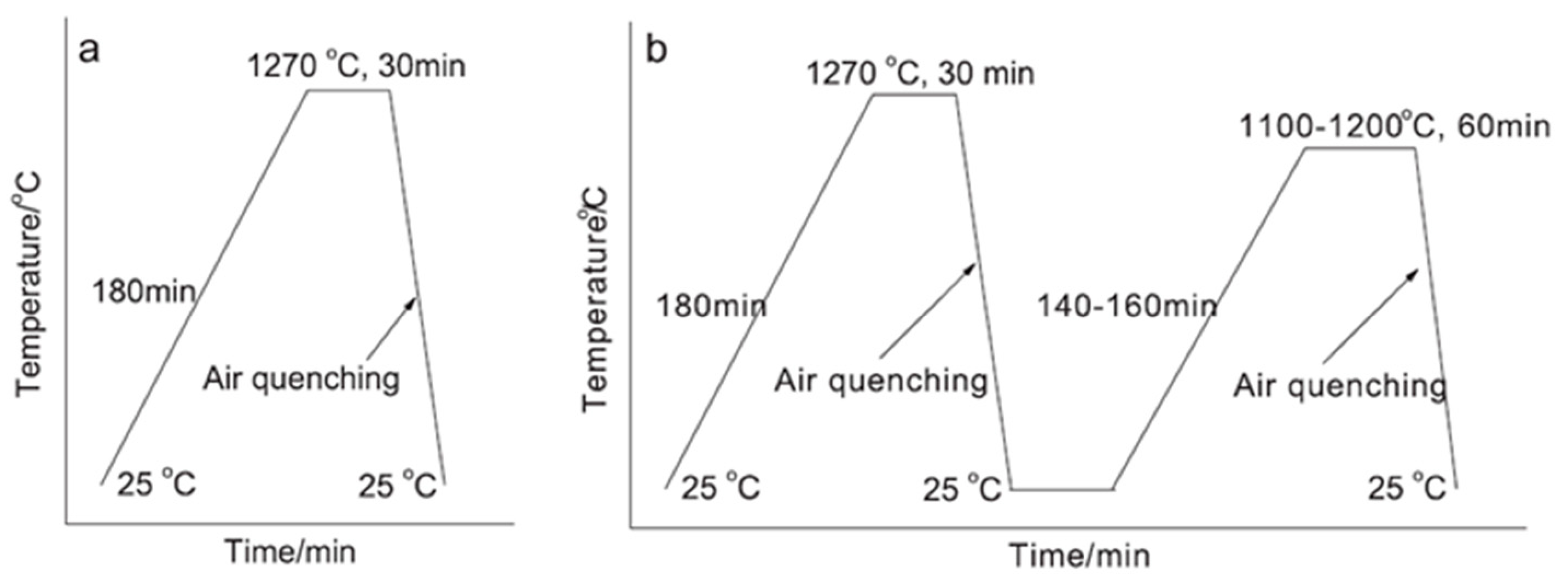
2.3. Calcination Methods
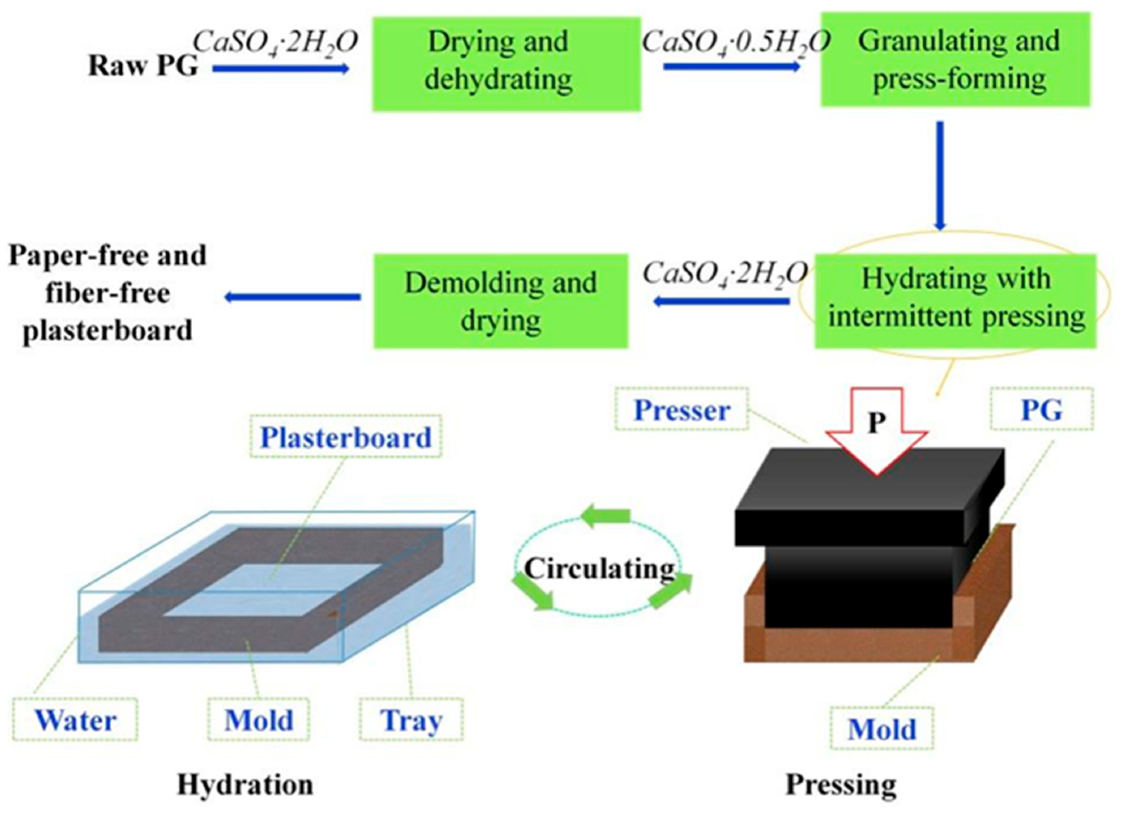
3. Building Materials
3.1. Raw Material for Cement Production
3.2. Component in Cementitious Materials
3.3. Cemented Paste Backfill
3.4. Additives in Pavement Materials
4. Useful Chemicals
4.1. Fertilizer
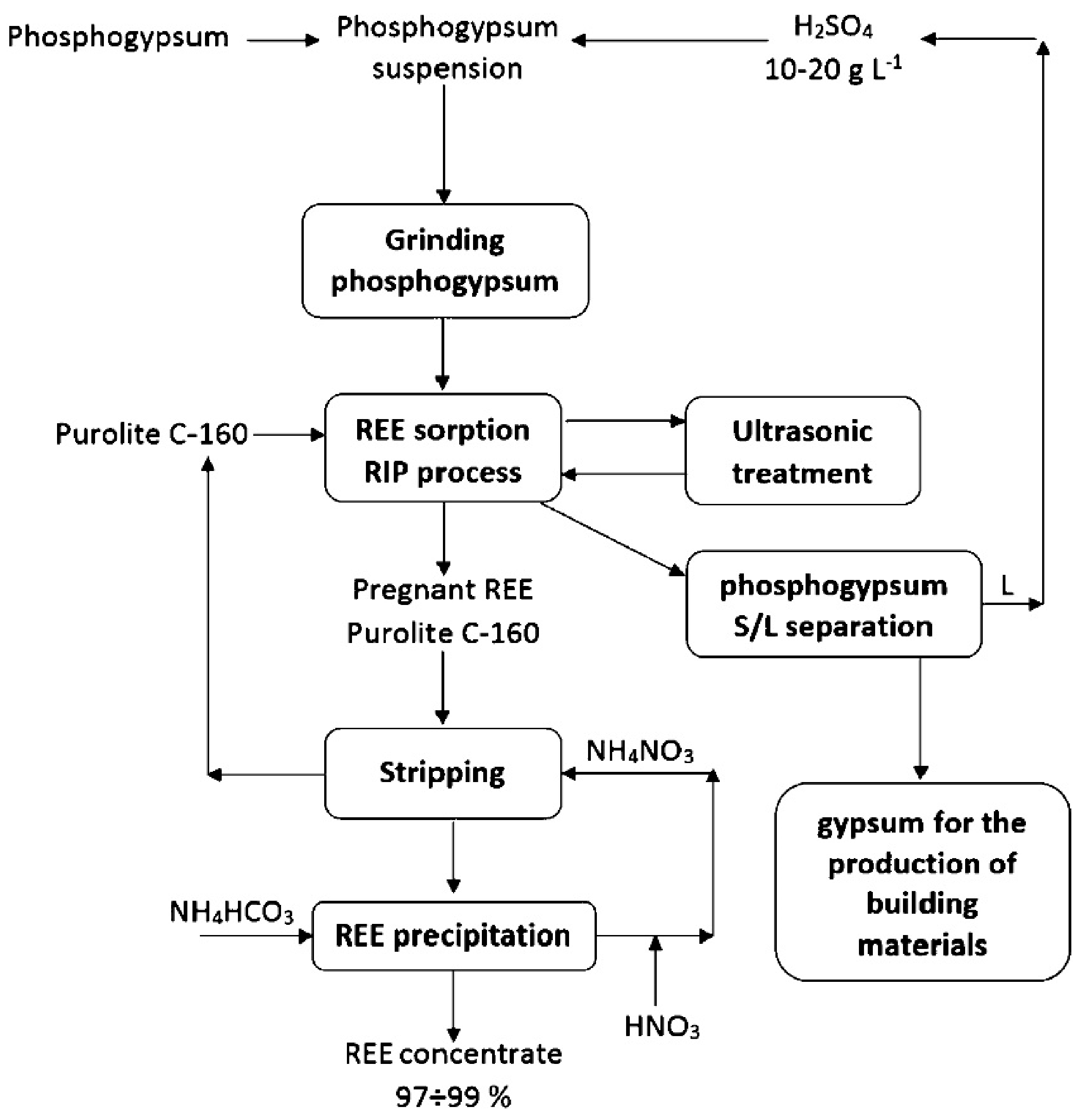
4.2. Rare Earth Elements Recovery
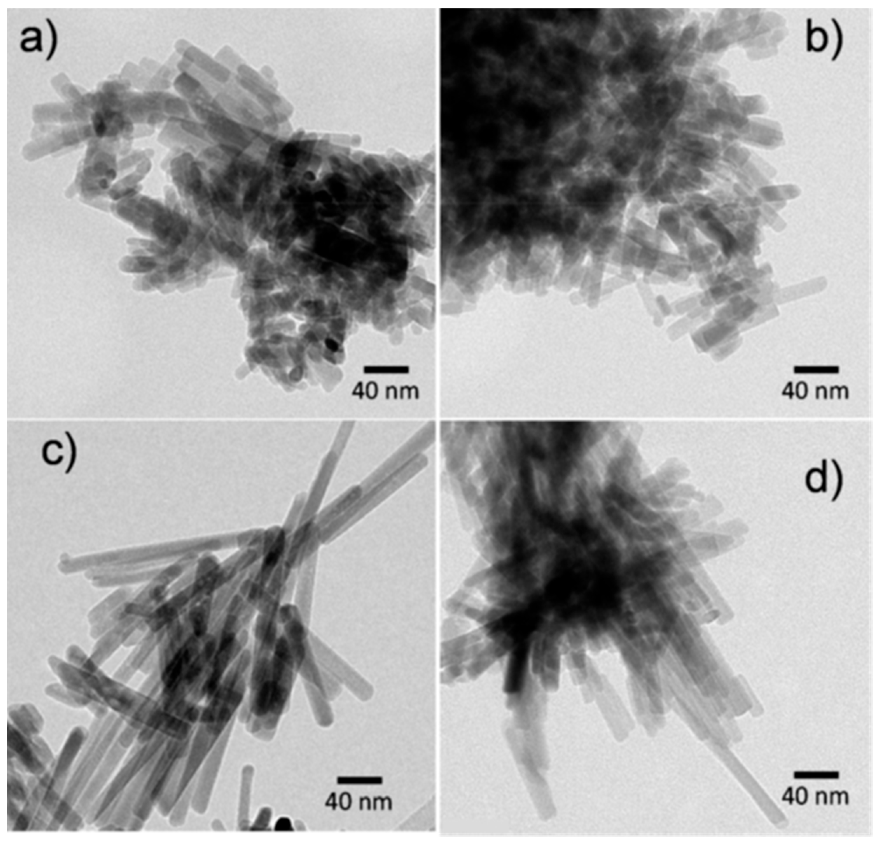
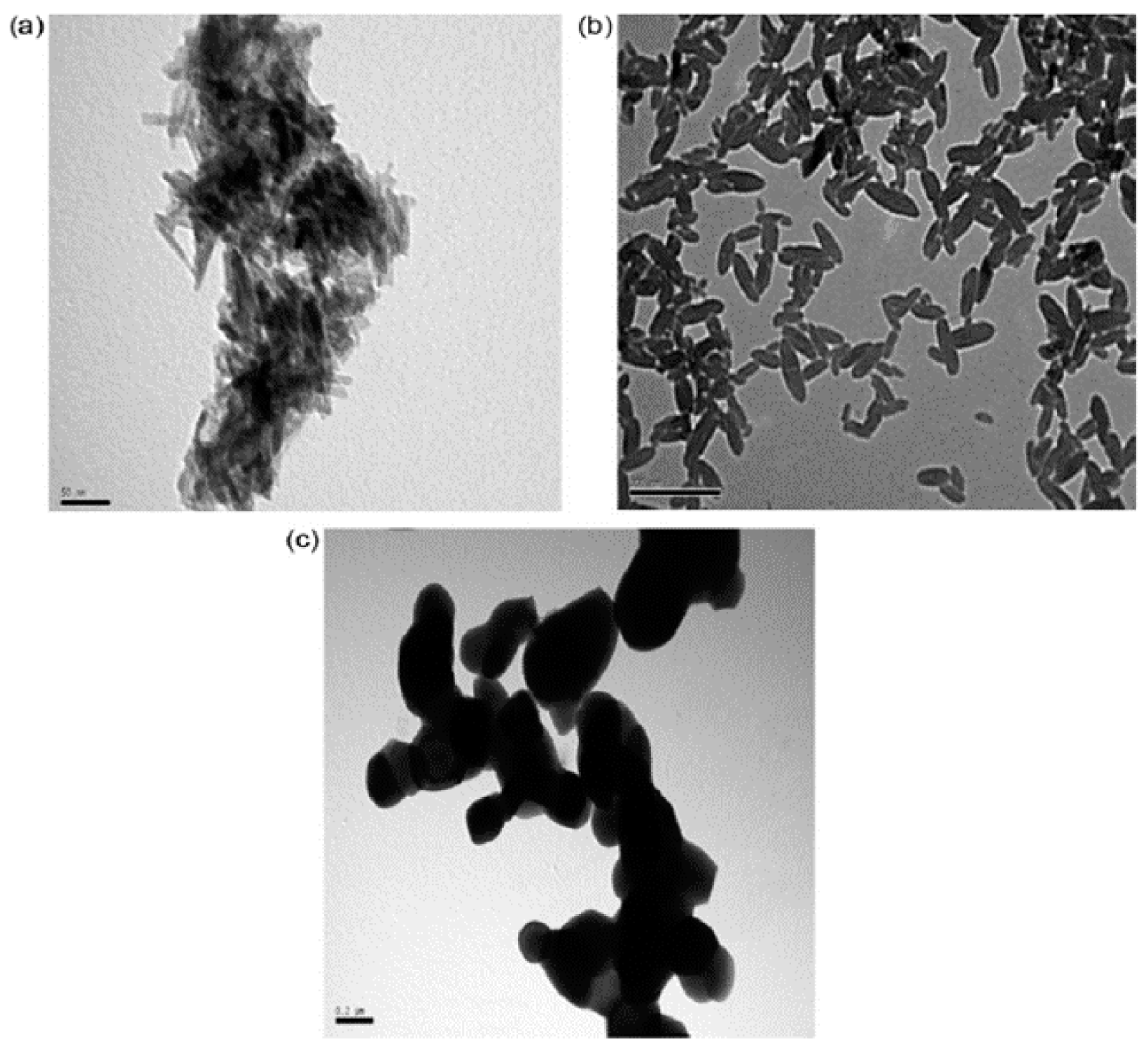
4.3. Nanocrystalline Materials
5. Sustainable Composites
6. Environmental Impacts
6.1. Soil
6.2. Radioactivity
7. Concluding Remarks and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tayibi, H.; Choura, M.; Lopez, F.A.; Alguacil, F.J.; Lopez-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, R.C.; Ribeiro, F.C.A.; da Costa Lauria, D.; Bernedo, A.V.B. Radioactive characterization of phosphogypsum from Imbituba, Brazil. J. Environ. Radioact. 2013, 126, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-L.; Bian, X.; Zhao, L.; Wang, Y.-J.; Hong, Z.-S. Effect of phosphogypsum on physiochemical and mechanical behaviour of cement stabilized dredged soil from Fuzhou, China. Geomech. Energy Environ. 2021, 25, 100195. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Wang, X.; Yang, L.; Zhong, B.; Liu, J. Thermodynamic study of phosphogypsum decomposition by sulfur. J. Chem. Thermodyn. 2013, 57, 39–45. [Google Scholar] [CrossRef]
- Elloumi, N.; Zouari, M.; Chaari, L.; Abdallah, F.B.; Woodward, S.; Kallel, M. Effect of phosphogypsum on growth, physiology, and the antioxidative defense system in sunflower seedlings. Environ. Sci. Pollut. Res. 2015, 22, 14829–14840. [Google Scholar] [CrossRef]
- Wang, J. Utilization effects and environmental risks of phosphogypsum in agriculture: A review. J. Clean. Prod. 2020, 276, 123337. [Google Scholar] [CrossRef]
- Singh, M. Role of phosphogypsum impurities on strength and microstructure of selenite plaster. Constr. Build. Mater. 2005, 19, 480–486. [Google Scholar] [CrossRef]
- Mashifana, T.P. Chemical treatment of phosphogypsum and its potential application for building and construction. Procedia Manuf. 2019, 35, 641–648. [Google Scholar] [CrossRef]
- Macías, F.; Pérez-López, R.; Cánovas, C.R.; Carrero, S.; Cruz-Hernandez, P. Environmental Assessment and Management of Phosphogypsum According to European and United States of America Regulations. Procedia Earth Planet. Sci. 2017, 17, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Shi, Y.; Cheng, L.; Tao, M.; Tong, S.; Yao, X.; Liu, Y. Using modified quartz sand for phosphate pollution control in cemented phosphogypsum (PG) backfill. J. Clean. Prod. 2021, 283, 124652. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Zhou, Y.; Min, C.; Shi, Y. Effect of phosphorus on the properties of phosphogypsum-based cemented backfill. J. Hazard. Mater. 2020, 399, 122993. [Google Scholar] [CrossRef]
- Tafu, M.; Chohji, T. Reaction between calcium phosphate and fluoride in phosphogypsum. J. Eur. Ceram. Soc. 2006, 26, 767–770. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, H.; Zheng, L.; Wang, K.; Li, H.; Wang, Y.; Feng, H. Utilization of waste phosphogypsum to prepare hydroxyapatite nanoparticles and its application towards removal of fluoride from aqueous solution. J. Hazard. Mater. 2012, 241–242, 418–426. [Google Scholar] [CrossRef]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Chowdhury, F.H.; Raihan, M.T.; Amit, S.K.S.; Islam, M.R. Effect of Phosphogypsum on the Properties of Portland Cement. Procedia Eng. 2017, 171, 744–751. [Google Scholar] [CrossRef]
- Mashifana, T.; Ntuli, F.; Okonta, F. Leaching kinetics on the removal of phosphorus from waste phosphogypsum by application of shrinking core model. S. Afr. J. Chem. Eng. 2019, 27, 1–6. [Google Scholar] [CrossRef]
- Xue, S.; Li, M.; Jiang, J.; Millar, G.J.; Li, C.; Kong, X. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics. J. Environ. Sci. (China) 2019, 77, 1–10. [Google Scholar] [CrossRef]
- Min, Y.; Jueshi, Q.; Ying, P. Activation of fly ash–lime systems using calcined phosphogypsum. Constr. Build. Mater. 2008, 22, 1004–1008. [Google Scholar] [CrossRef]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.J.; Chae, J.S.; Oh, J.S.; Kwon, E.; Lim, J.; Lee, H.; Han, J.H.; Pham, M.K.; Nour, S.; et al. Preparation and evaluation of new reference materials for naturally occurring radioactive materials (NORM): Zirconium silicate, bauxite, and phosphogypsum. Appl. Radiat. Isot. 2021, 168, 109525. [Google Scholar] [CrossRef]
- Moalla, R.; Gargouri, M.; Khmiri, F.; Kamoun, L.; Zairi, M. Phosphogypsum purification for plaster production: A process optimization using full factorial design. Environ. Eng. Res. 2017, 23, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Garg, M.; Rehsi, S.S. Purifying phosphogypsum for cement manufacture. Constr. Build. Mater. 1993, 7, 3–7. [Google Scholar] [CrossRef]
- Ölmez, H.; Erdem, E. The effects of phosphogypsum on the setting and mechanical properties of Portland cement and trass cement. Cem. Concr. Res. 1989, 19, 377–384. [Google Scholar] [CrossRef]
- Van der Merwe, E.M.; Strydom, C.A. Purification of South African phosphogypsum for use as Portland cement retarder by a combined thermal and sulphuric acid treatment method: Research in action. S. Afr. J. Sci. 2004, 100, 411–414. [Google Scholar]
- Potgieter, J.H.; Potgieter, S.S.; McCrindle, R.I.; Strydom, C.A. An investigation into the effect of various chemical and physical treatments of a South African phosphogypsum to render it suitable as a set retarder for cement. Cem. Concr. Res. 2003, 33, 1223–1227. [Google Scholar] [CrossRef]
- Al-Hwaiti, M.; Ibrahim, K.A.; Harrara, M. Removal of heavy metals from waste phosphogypsum materials using polyethylene glycol and polyvinyl alcohol polymers. Arab. J. Chem. 2015, 12, 3141–3150. [Google Scholar] [CrossRef] [Green Version]
- Shu, J.; Chen, M.; Wu, H.; Li, B.; Wang, B.; Li, B.; Liu, R.; Liu, Z. An innovative method for synergistic stabilization/solidification of Mn2+, NH4+-N, PO43− and F− in electrolytic manganese residue and phosphogypsum. J. Hazard. Mater. 2019, 376, 212–222. [Google Scholar] [CrossRef]
- Nizevičienė, D.; Vaičiukynienė, D.; Vaitkevičius, V.; Rudžionis, Ž. Effects of waste fluid catalytic cracking on the properties of semi-hydrate phosphogypsum. J. Clean. Prod. 2016, 137, 150–156. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Kielė, A.; Janavičius, E.; Pupeikis, D. Effect of phosphogypsum on the stability upon firing treatment of alkali-activated slag. Constr. Build. Mater. 2018, 184, 485–491. [Google Scholar] [CrossRef]
- Savich, V.I.; Artikova, H.T.; Nafetdinov, S.S.; Salimova, K.H. Optimization Of Plant Development In Case Of Soil Salinization. Am. J. Agric. Biomed. Eng. 2021, 3, 24–29. [Google Scholar] [CrossRef]
- Fornés, I.V.; Vaičiukynienė, D.; Nizevičienė, D.; Doroševas, V.; Dvořák, K. A method to prepare a high-strength building material from press-formed phosphogypsum purified with waste zeolite. J. Build. Eng. 2021, 34, 101919. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Chen, Q.; Qi, C.; Su, Z.; Huang, Z. Utilisation of Water-Washing Pre-Treated Phosphogypsum for Cemented Paste Backfill. Minerals 2019, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Garg, M.; Verma, C.L.; Handa, S.K.; Kumar, R. An improved process for the purification of phosphogypsum. Constr. Build. Mater. 1996, 10, 597–600. [Google Scholar] [CrossRef]
- Singh, M.; Garg, M. Activation of gypsum anhydrite-slag mixtures. Cem. Concr. Res. 1995, 25, 332–338. [Google Scholar] [CrossRef]
- Taher, M.A. Influence of thermally treated phosphogypsum on the properties of Portland slag cement. Resour. Conserv. Recycl. 2007, 52, 28–38. [Google Scholar] [CrossRef]
- Yang, M.; Qian, J. Activation of anhydrate phosphogypsum by K2SO4 and hemihydrate gypsum. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 1103–1107. [Google Scholar] [CrossRef]
- Leskevicienė, V.; Nizevičienė, D. Influence of the setting activators on the physical mechanical properties of phosphoanhydrite. Chem. Ind. Chem. Eng. Q. 2014, 20, 233–240. [Google Scholar] [CrossRef]
- Liu, S.; Ouyang, J.; Ren, J. Mechanism of calcination modification of phosphogypsum and its effect on the hydration properties of phosphogypsum-based supersulfated cement. Constr. Build. Mater. 2020, 243, 118226. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Kang, X.; Yu, J.; Fan, Y.; Dang, Y.; Zhang, W.; Wang, S. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Chai, J.; Fan, Y. Calcium sulphoaluminate cements made with phosphogypsum: Production issues and material properties. Cem. Concr. Compos. 2014, 48, 67–74. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Huang, Y.; Yang, D. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem. Concr. Compos. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Değirmenci, N. Utilization of phosphogypsum as raw and calcined material in manufacturing of building products. Constr. Build. Mater. 2008, 22, 1857–1862. [Google Scholar] [CrossRef]
- Mun, K.J.; Hyoung, W.K.; Lee, C.W.; So, S.Y.; Soh, Y.S. Basic properties of non-sintering cement using phosphogypsum and waste lime as activator. Constr. Build. Mater. 2007, 21, 1342–1350. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z. Investigation on phosphogypsum–steel slag–granulated blast-furnace slag–limestone cement. Constr. Build. Mater. 2010, 24, 1296–1301. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-activated binders based on ground granulated blast furnace slag and phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, X.; Bie, S.; Yang, C. Hardening Performance of Phosphogypsum-Slag-Based Material. Procedia Environ. Sci. 2016, 31, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.; Wang, K.; Yao, X. Developing high performance phosphogypsum-based cementitious materials for oil well cementing through a step-by-step optimization method. Cem. Concr. Compos. 2016, 72, 299–308. [Google Scholar] [CrossRef]
- Min, C.; Li, X.; He, S.; Zhou, S.; Zhou, Y.; Yang, S.; Shi, Y. Effect of mixing time on the properties of phosphogypsum-based cemented backfill. Constr. Build. Mater. 2019, 210, 564–573. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Fourie, A.; Xin, C. Utilization of phosphogypsum and phosphate tailings for cemented paste backfill. J. Environ. Manag. 2017, 201, 19–27. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Qi, C.; Fourie, A.; Xiao, C. Recycling phosphogypsum and construction demolition waste for cemented paste backfill and its environmental impact. J. Clean. Prod. 2018, 186, 418–429. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Immobilisation of radioactive wastes in bitumen. In An Introduction to Nuclear Waste Immobilisation; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2005; pp. 201–212. [Google Scholar]
- Cuadri, A.A.; Navarro, F.J.; García-Morales, M.; Bolívar, J.P. Valorization of phosphogypsum waste as asphaltic bitumen modifier. J. Hazard. Mater. 2014, 279, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Cuadri, A.A.; Navarro, F.J.; Partal, P. Synergistic ethylcellulose/polyphosphoric acid modification of bitumen for paving applications. Mater. Struct. 2020, 23, 1–13. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Pérez-Moreno, S.; Altamar, C.L.; Navarro, F.J.; Bolívar, J.P. Phosphogypsum as additive for foamed bitumen manufacturing used in asphalt paving. J. Clean. Prod. 2021, 283, 124661. [Google Scholar] [CrossRef]
- Kandil, A.-H.T.; Cheira, M.F.; Gado, H.S.; Soliman, M.H.; Akl, H.M. Ammonium sulfate preparation from phosphogypsum waste. J. Radiat. Res. Appl. Sci. 2019, 10, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Grabas, K.; Paweczyk, A.; Ostrowski, A.; Strk, S.; Nowak, P. Recovery of lanthanide from apatite phosphogypsum. In Proceedings of the COM 2014-Conference of Metallurgists Proceedings, Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Semenishchev, V.S.; Bunkov, G.M.; Botalov, M.S.; Smyshlyaev, D.V.; Malyshev, A.S. Recovery of rare earth elements from phosphogypsum. J. Clean. Prod. 2018, 196, 674–681. [Google Scholar] [CrossRef]
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Barca, D. Rare earths concentration from phosphogypsum waste by two-step leaching method. Int. J. Miner. Process. 2016, 149, 78–83. [Google Scholar] [CrossRef]
- Kulczycka, J.; Kowalski, Z.; Smol, M.; Wirth, H. Evaluation of the recovery of Rare Earth Elements (REE) from phosphogypsum waste—Case study of the WIZÓW Chemical Plant (Poland). J. Clean. Prod. 2016, 113, 345–354. [Google Scholar] [CrossRef]
- Bensalah, H.; Bekheet, M.F.; Younssi, S.A.; Ouammou, M.; Gurlo, A. Hydrothermal synthesis of nanocrystalline hydroxyapatite from phosphogypsum waste. J. Environ. Chem. Eng. 2018, 6, 1347–1352. [Google Scholar] [CrossRef]
- Mousa, S.; Hanna, A. Synthesis of nano-crystalline hydroxyapatite and ammonium sulfate from phosphogypsum waste. Mater. Res. Bull. 2013, 48, 823–828. [Google Scholar] [CrossRef]
- Mohamed, K.R.; Mousa, S.M.; El Bassyouni, G.T. Fabrication of nano structural biphasic materials from phosphogypsum waste and their in vitro applications. Mater. Res. Bull. 2014, 50, 432–439. [Google Scholar] [CrossRef]
- Zhou, L. Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment. Open Chem. 2018, 16, 864–868. [Google Scholar] [CrossRef]
- Lu, S.Q.; Lan, P.Q.; Wu, S.F. Preparation of Nano-CaCO3 from Phosphogypsum by Gas–Liquid–Solid Reaction for CO2 Sorption. Ind. Eng. Chem. Res. 2016, 55, 10172–10177. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Zhang, L.; Xiao, B. Preparation of load-bearing building materials from autoclaved phosphogypsum. Constr. Build. Mater. 2009, 23, 687–693. [Google Scholar] [CrossRef]
- Dutta, R.K.; Khatri, V.N.; Panwar, V. Strength characteristics of fly ash stabilized with lime and modified with phosphogypsum. J. Build. Eng. 2017, 14, 32–40. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, H.; Shu, Z.; Wang, Y.; Yan, C. Utilization of waste phosphogypsum to prepare non-fired bricks by a novel Hydration–Recrystallization process. Constr. Build. Mater. 2012, 34, 114–119. [Google Scholar] [CrossRef]
- Zhou, J.; Sheng, Z.; Li, T.; Shu, Z.; Chen, Y.; Wang, Y. Preparation of hardened tiles from waste phosphogypsum by a new intermittent pressing hydration. Ceram. Int. 2016, 42, 7237–7245. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhou, J.; Shu, Z.; Yakubu, Y.; Chen, Y.; Wang, W.; Wang, Y. Calcium sulfate whisker reinforced non-fired ceramic tiles prepared from phosphogypsum. Boletín Soc. Española Cerámica Vidr. 2018, 57, 73–78. [Google Scholar] [CrossRef]
- Hua, S.; Wang, K.; Yao, X.; Xu, W.; He, Y. Effects of fibers on mechanical properties and freeze–thaw resistance of phosphogypsum-slag based cementitious materials. Constr. Build. Mater. 2016, 121, 290–299. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Zhao, Y.; Shu, Z.; Wang, Y.; Zhang, Y.; Shen, X. Preparation of paper-free and fiber-free plasterboard with high strength using phosphogypsum. Constr. Build. Mater. 2020, 243, 118091. [Google Scholar] [CrossRef]
- Ma, B.; Jin, Z.; Su, Y.; Lu, W.; Qi, H.; Hu, P. Utilization of hemihydrate phosphogypsum for the preparation of porous sound absorbing material. Constr. Build. Mater. 2020, 234, 117346. [Google Scholar] [CrossRef]
- Liang, J.; Karamanos, R.E.; Moir, M.E. The influence of brine contamination and phospho-gypsum amendments on soil chemical properties and plant response. Commun. Soil Sci. Plant Anal. 2008, 26, 1033–1057. [Google Scholar] [CrossRef]
- Nayak, A.K.; Mishra, V.K.; Sharma, D.K.; Jha, S.K.; Singh, C.S.; Shahabuddin, M.; Shahid, M. Efficiency of Phosphogypsum and Mined Gypsum in Reclamation and Productivity of Rice–Wheat Cropping System in Sodic Soil. Commun. Soil Sci. Plant Anal. 2013, 44, 909–921. [Google Scholar] [CrossRef]
- Domínguez, R.; Campillo, C.D.; Pena, F.; Delgado, A. Effect of Soil Properties and Reclamation Practices on Phosphorus Dynamics in Reclaimed Calcareous Marsh Soils from the Guadalquivir Valley, SW Spain. Arid. Land Res. Manag. 2001, 15, 203–221. [Google Scholar] [CrossRef]
- Carvalho, M.d.C.S.; Nascente, A.S. Application of lime, phosphogypsum and fertilization rates affect soil fertility and common bean development in no-tillage system in a Cerrado Oxisol. Acta Scientiarum. Agron. 2018, 40, e39322. [Google Scholar] [CrossRef]
- Dijkstra, J.J.; Meeussen, J.C.; Comans, R.N. Leaching of heavy metals from contaminated soils: An experimental and modeling study. Environ. Sci. Technol. 2004, 38, 4390–4395. [Google Scholar] [CrossRef]
- Mahmoud, E.; El-Kader, N.A. Heavy Metal Immobilization in Contaminated Soils using Phosphogypsum and Rice Straw Compost. Land Degrad. Dev. 2015, 26, 819–824. [Google Scholar] [CrossRef]
- Campbell, C.G.; Garrido, F.; Illera, V.; García-González, M.T. Transport of Cd, Cu and Pb in an acid soil amended with phosphogypsum, sugar foam and phosphoric rock. Appl. Geochem. 2006, 21, 1030–1043. [Google Scholar] [CrossRef]
- Hassoune, H.; Lachehab, A.; El Hajjaji, K.; Mertah, O.; Kherbeche, A. Dynamic of Heavy Metals and Environmental Impact of Waste Phosphogypsum. In Fate and Transport of Subsurface Pollutants; Springer: Singapore, 2021; pp. 57–77. [Google Scholar]
- Campos, M.P.; Costa, L.J.P.; Nisti, M.B.; Mazzilli, B.P. Phosphogypsum recycling in the building materials industry: Assessment of the radon exhalation rate. J. Environ. Radioact. 2017, 172, 232–236. [Google Scholar] [CrossRef]
- Kuzmanović, P.; Todorović, N.; Forkapić, S.; Petrović, L.F.; Knežević, J.; Nikolov, J.; Miljević, B. Radiological characterization of phosphogypsum produced in Serbia. Radiat. Phys. Chem. 2020, 166, 108463. [Google Scholar] [CrossRef]
- Attallah, M.F.; Metwally, S.S.; Moussa, S.I.; Soliman, M.A. Environmental impact assessment of phosphate fertilizers and phosphogypsum waste: Elemental and radiological effects. Microchem. J. 2019, 146, 789–797. [Google Scholar] [CrossRef]
- El-Bahi, S.M.; Sroor, A.; Mohamed, G.Y.; El-Gendy, N.S. Radiological impact of natural radioactivity in Egyptian phosphate rocks, phosphogypsum and phosphate fertilizers. Appl. Radiat. Isot. 2017, 123, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J. Radioecological study of an estuarine system located in the south of Spain. Water Res. 2000, 34, 2941–2950. [Google Scholar] [CrossRef]
- Federal Register. National emission standard for hazardous air pollutants; national emission standards for radon emissions from phosphogypsum stacks. Dly. J. United States Gov. 1999, 64, 5574–5580. [Google Scholar]
- Code of Federal Regulations, Title 40, Vol. 7, Parts 61.202 and 61.204 (40CFR61.202 and 40CFR 61.204). 1998; The United States Environmental Protection Agency. Available online: https://www.govinfo.gov/content/pkg/CFR-2011-title40-vol8/xml/CFR-2011-title40-vol8-part61.xml (accessed on 13 May 2021).
- Gijbels, K.; Nguyen, H.; Kinnunen, P.; Samyn, P.; Schroeyers, W.; Pontikes, Y.; Schreurs, S.; Illikainen, M. Radiological and leaching assessment of an ettringite-based mortar from ladle slag and phosphogypsum. Cem. Concr. Res. 2020, 128, 10594. [Google Scholar] [CrossRef]
- Kuzmanovic, P.; Todorovic, N.; Mrda, D.; Forkapic, S.; Petrovic, L.F.; Miljevic, B.; Hansman, J.; Knezevic, J. The possibility of the phosphogypsum use in the production of brick: Radiological and structural characterization. J. Hazard. Mater. 2021, 413, 125343. [Google Scholar] [CrossRef]

| Impurity Type | Solubility | Main Existing Forms | Effects |
|---|---|---|---|
| Phosphate [7,15,20] | Soluble | PO43−, HPO42−, H2PO4−, H3PO4 | Corrosive to equipment, prolonging the setting time of building gypsum and weakening its strength. |
| Eutectic morphology | CaHPO4·2H2O | Prolonging setting time and weakening the strength of gypsum hardened body. | |
| Insoluble | Phosphate complexes (with Al, Fe, alkali metals, etc.), decomposed apatite | The impact was small. | |
| Fluoride | soluble | F− | Promote coagulation, Reduce the strength of the gypsum. |
| Insoluble | Na3AlF6, CaSiF6, CaF | The impact was small. | |
| Organic matter | Insoluble | Plant roots mixed in phosphate rock, Defoamer, scale remover, and crystal transformation agent added in the production process | The water requirement of standard consistency increases. The porosity of the hardened body increases and the strength of PG decreases. |
| Other impurities [21] | soluble | K+, Na+ | Result in powdering and frosting on the surface of PG products. |
| Insoluble | Quartz, Oxides of Sr, Fe, and Mg or sulfate complexes of Sr and Fe, Ra226, Pb210, Po210, U238, and U234 | The effect of oxide was not palpable, radioactive elements do great harm to organisms. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, K.; Cui, N.; Zhao, S.; Zheng, K.; Ji, X.; Feng, L.; Cheng, X.; Xie, N. Low-Carbon Sustainable Composites from Waste Phosphogypsum and Their Environmental Impacts. Crystals 2021, 11, 719. https://doi.org/10.3390/cryst11070719
Ren K, Cui N, Zhao S, Zheng K, Ji X, Feng L, Cheng X, Xie N. Low-Carbon Sustainable Composites from Waste Phosphogypsum and Their Environmental Impacts. Crystals. 2021; 11(7):719. https://doi.org/10.3390/cryst11070719
Chicago/Turabian StyleRen, Kai, Na Cui, Shuyuan Zhao, Kai Zheng, Xia Ji, Lichao Feng, Xin Cheng, and Ning Xie. 2021. "Low-Carbon Sustainable Composites from Waste Phosphogypsum and Their Environmental Impacts" Crystals 11, no. 7: 719. https://doi.org/10.3390/cryst11070719





