Green Synthesis of Zinc Oxide Nanocrystals Utilizing Origanum majorana Leaf Extract and Their Synergistic Patterns with Colistin against Multidrug-Resistant Bacterial Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Aqueous Extract of O. majorana Leaves
2.2. Green Formulation of ZnO NPs
2.3. Characterization of the Bioformulated ZnO NPs
2.4. Screening of the Antimicrobial Proficiency of the Bioformulated ZnO NPs
2.5. Detection of the Synergistic Patterns of the Bioformulated ZnO NPs with Colistin
2.6. Statistical Analysis
3. Results and Discussion
3.1. Green Bioformulation of ZnO NPs
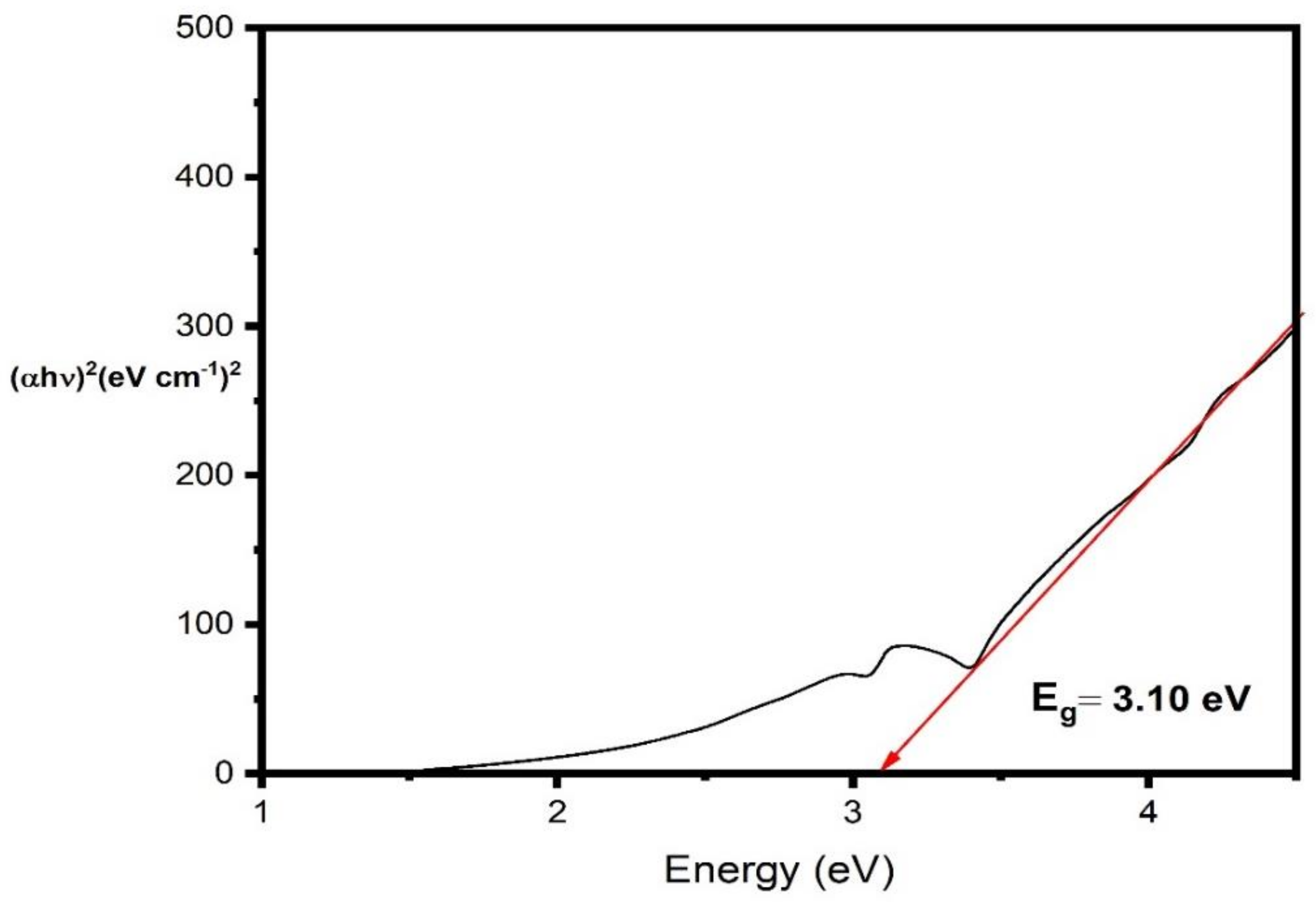
3.2. UV Spectral Analysis
3.3. Transmission Electron Microscope (TEM) Analysis
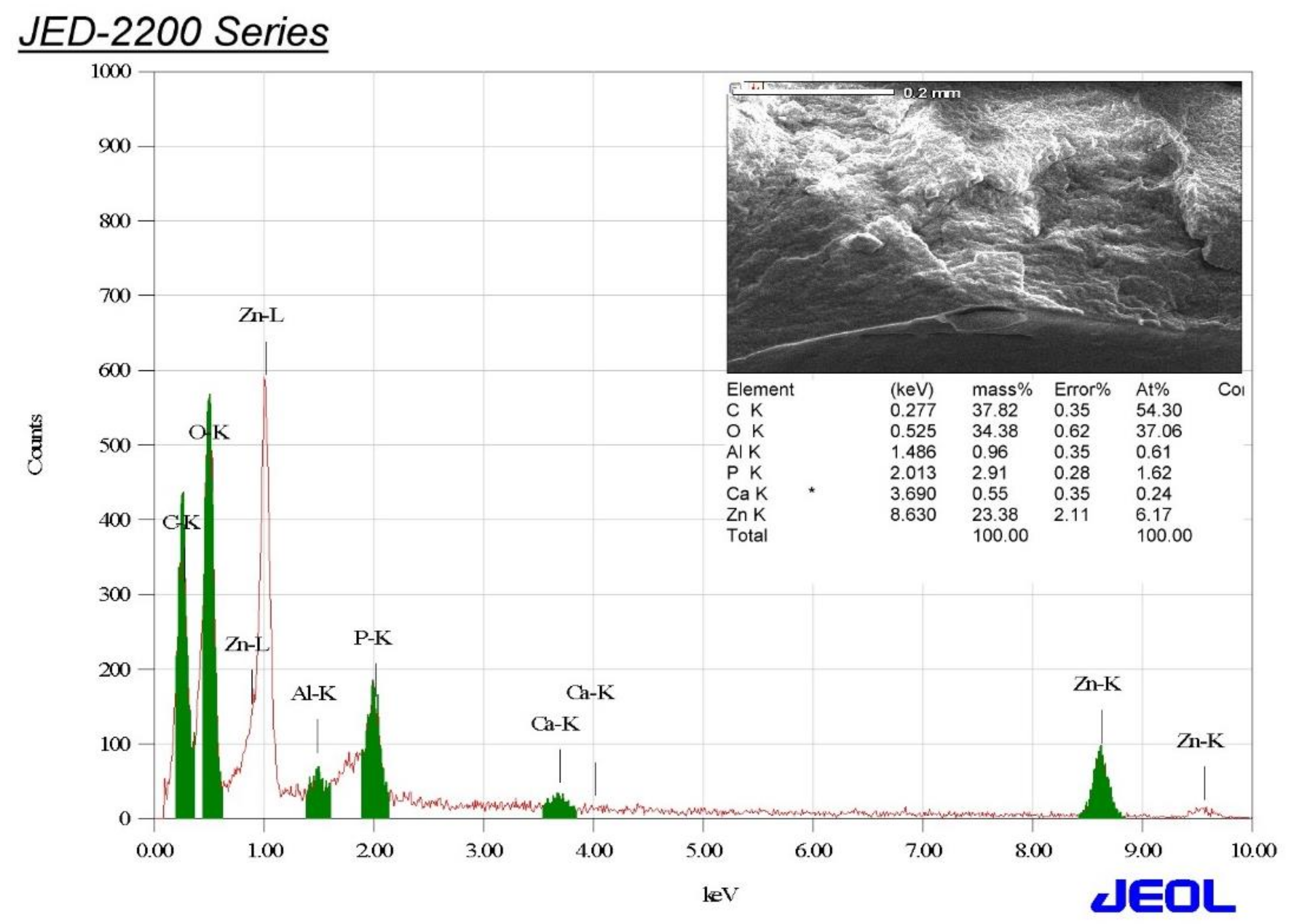
3.4. Energy-Dispersive X-ray (EDX) Analysis
3.5. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
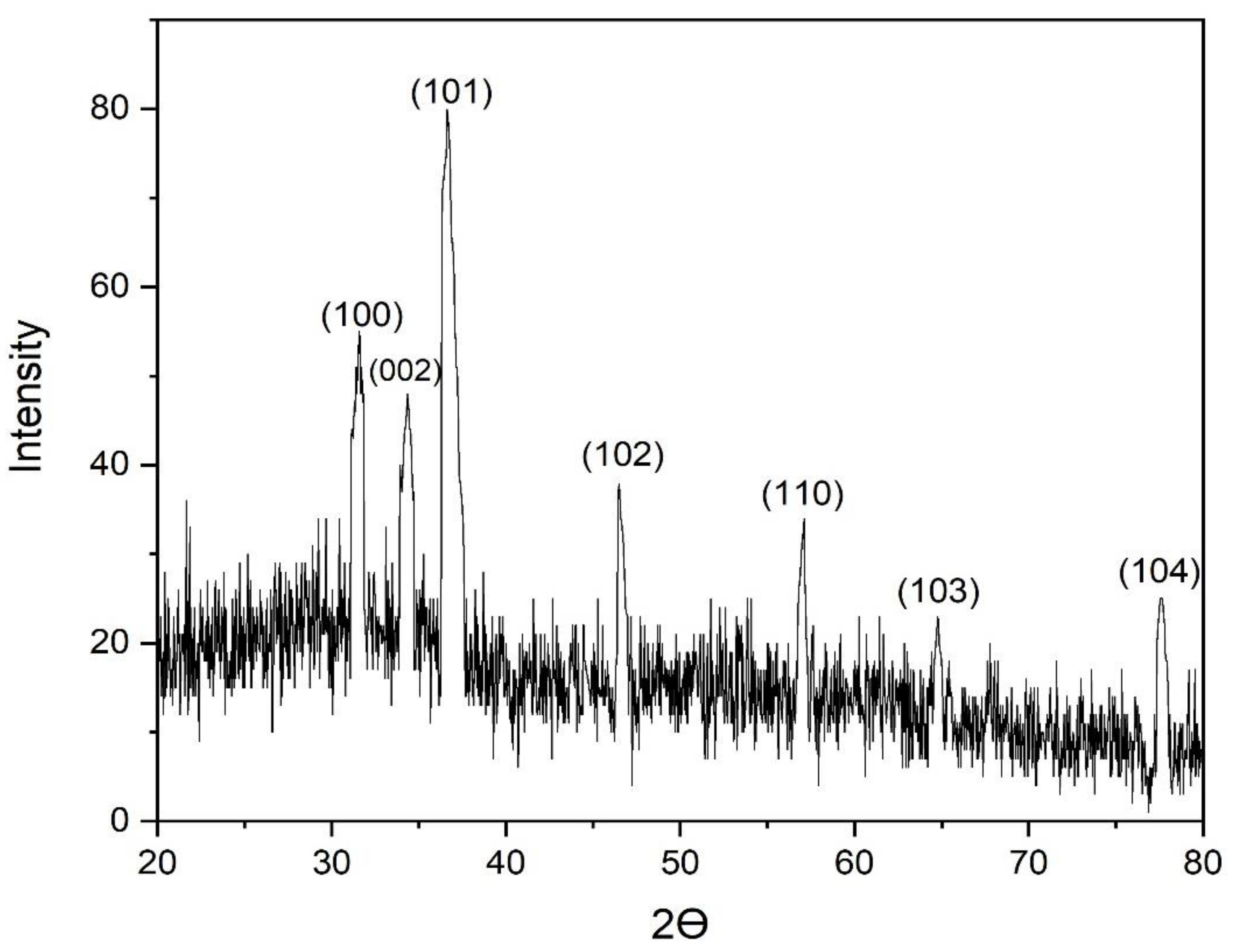
3.6. X-ray Diffraction (XRD) Analysis of the Biofabricated ZnO NPs
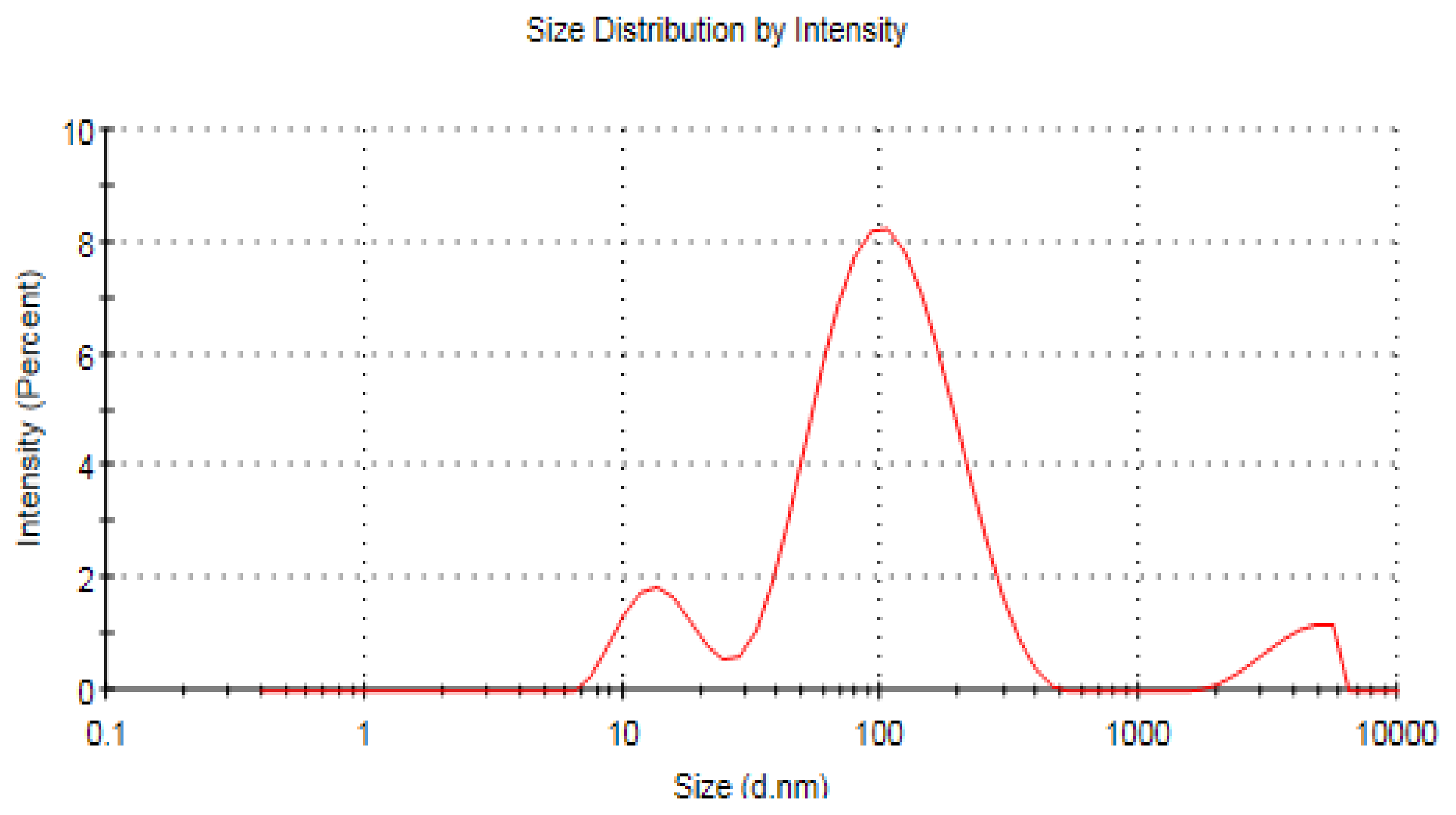
3.7. Zeta Analysis of ZnO NPs
3.8. Screening of the Antimicrobial Efficiency of the Biosynthesized ZnO NPs
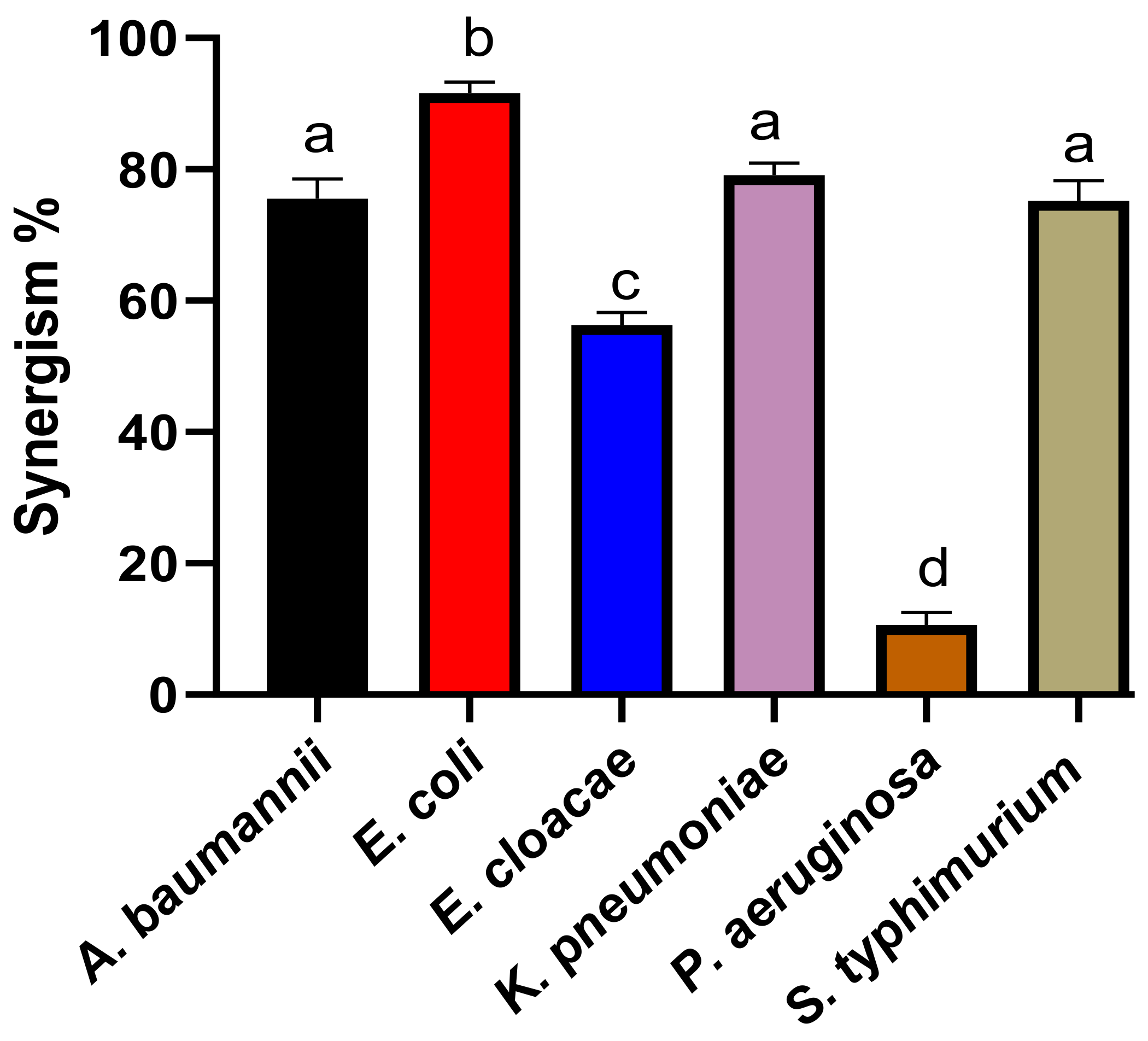
3.9. Detection of the Synergistic Potency of the Biosynthesized ZnO NPs with Colistin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial resistance in the context of the sustainable development goals: A brief review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. 2014. Available online: https://www.who.int/publications/i/item/9789241564748 (accessed on 26 June 2019).
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, Z.; Folic, M.M.; Zivic, Z.; Markovic, V.; Jankovic, S.M. Nosocomial urinary tract infections caused by Pseudomonas aeruginosa and Acinetobacter species: Sensitivity to antibiotics and risk factors. Am. J. Infect. Control 2013, 41, 1182–1187. [Google Scholar] [CrossRef]
- Zhang, S.; Seeberger, P.H. Total Syntheses of Conjugation-Ready Repeating Units of Acinetobacter baumannii AB5075 for Glycoconjugate Vaccine Development. Chem. Eur. J. 2021, 27, 17444–17451. [Google Scholar] [CrossRef]
- Lupo, A.; Haenni, M.; Madec, J.Y. Antimicrobial resistance in Acinetobacter spp. and Pseudomonas spp. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Diggle, S.P.; Whiteley, M. Microbe Profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology 2020, 166, 30. [Google Scholar] [CrossRef]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef]
- Mohd Asri, N.A.; Ahmad, S.; Mohamud, R.; Mohd Hanafi, N.; Mohd Zaidi, N.F.; Irekeola, A.A.; Shueb, R.H.; Yee, L.Y.; Noor, N.H.; Mustafa, F.H.; et al. Global prevalence of nosocomial multidrug-resistant Klebsiella pneumoniae: A systematic review and meta-analysis. Antibiotics 2021, 10, 1508. [Google Scholar] [CrossRef]
- Lou, T.; Du, X.; Zhang, P.; Shi, Q.; Han, X.; Lan, P.; Yan, R.; Hu, H.; Wang, Y.; Wu, X.; et al. Risk factors for infection and mortality caused by carbapenem-resistant Klebsiella pneumoniae: A large multicentre case–control and cohort study. J. Infect. 2022, 84, 637–647. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Pagès, J.M. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anju, V.T.; Siddhardha, B.; Dyavaiah, M. Enterobacter infections and antimicrobial drug resistance. In Model Organisms for Microbial Pathogenesis, Biofilm Formation and Antimicrobial Drug Discovery; Springer: Singapore, 2020; pp. 175–194. [Google Scholar]
- Öztürk, R.; Murt, A. Epidemiology of urological infections: A global burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care. AURA 2019: Third Australian Report on Antimicrobial Use and Resistance in Human Health. 2019. Available online: https://www.safetyandquality.gov.au/sites/default/files/2019-06/AURA-2019-Report.pdf (accessed on 10 November 2019).
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Health Res. 2018, 15, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.M.; Roy, A.; Wahab, M.; Ahmed, M.; Othman-Qadir, G.; Elesawy, B.H.; Khandaker, M.U.; Islam, M.N.; Emran, T.B. Applications of nanomaterials in agrifood and pharmaceutical industry. J. Nanomater. 2021, 2021, 1472096. [Google Scholar] [CrossRef]
- Dutta, G.; Sugumaran, A. Bioengineered zinc oxide nanoparticles: Chemical, green, biological fabrication methods and its potential biomedical applications. J. Drug Deliv. Sci. Technol. 2021, 66, 102853. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Ahmad, W.; Kalra, D. Green synthesis, characterization and anti microbial activities of ZnO nanoparticles using Euphorbia hirta leaf extract. J. King Saud Univ. Sci. 2020, 32, 2358–2364. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef]
- Remya, V.R.; Abitha, V.K.; Rajput, P.S.; Rane, A.V.; Dutta, A. Silver nanoparticles green synthesis: A mini review. Chem. Int. 2017, 3, 165–171. [Google Scholar]
- Salunke, B.K.; Sathiyamoorthi, E.; Tran, T.K.; Kim, B.S. Phyto-synthesized silver nanoparticles for biological applications. Korean J. Chem. Eng. 2017, 34, 943–951. [Google Scholar] [CrossRef]
- El Shafey, A.M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Alrajhi, A.H.; Ahmed, N.M.; Al Shafouri, M.; Almessiere, M.A. Green synthesis of zinc oxide nanoparticles using salvia officials extract. Mater. Sci. Semicond. Process. 2021, 125, 105641. [Google Scholar] [CrossRef]
- Fagier, M.A. Plant-mediated biosynthesis and photocatalysis activities of zinc oxide nanoparticles: A prospect towards dyes mineralization. J. Nanotechnol. 2021, 2021, 6629180. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent advances in zinc oxide nanoparticles (Zno nps) for cancer diagnosis, target drug delivery, and treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Aldeen, T.S.; Mohamed, H.E.A.; Maaza, M. ZnO nanoparticles prepared via a green synthesis approach: Physical properties, photocatalytic and antibacterial activity. J. Phys. Chem. Solids 2022, 160, 110313. [Google Scholar] [CrossRef]
- Archana, P.; Janarthanan, B.; Bhuvana, S.; Rajiv, P.; Sharmila, S. Concert of zinc oxide nanoparticles synthesized using Cucumis melo by green synthesis and the antibacterial activity on pathogenic bacteria. Inorg. Chem. Commun. 2022, 137, 109255. [Google Scholar]
- Azimpanah, R.; Solati, Z.; Hashemi, M. Synthesis of ZnO nanoparticles with Antibacterial properties using terminalia catappa leaf extract. Chem. Eng. Technol. 2022, 45, 658–666. [Google Scholar] [CrossRef]
- Alshameri, A.W.; Owais, M. Antibacterial and cytotoxic potency of the plant-mediated synthesis of metallic nanoparticles Ag NPs and ZnO NPs: A Review. OpenNano 2022, 8, 100077. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Prasanna, M.; Subathra, S. Synergistic antibacterial activity of zinc oxide nanoparticles with antibiotics against the human pathogenic bacteria. J. Chem. Pharm. Res. 2015, 7, 133–138. [Google Scholar]
- Farzana, R.; Iqra, P.; Shafaq, F.; Sumaira, S.; Zakia, K.; Hunaiza, T.; Husna, M. Antimicrobial behavior of zinc oxide nanoparticles and β-lactam antibiotics against pathogenic Bacteria. Arch. Clin. Microbiol. 2017, 8, 57. [Google Scholar]
- Paudel, P.N.; Satyal, P.; Satyal, R.; Setzer, W.N.; Gyawali, R. Chemical Composition, Enantiomeric Distribution, Antimicrobial and Antioxidant Activities of Origanum majorana L. Essential Oil from Nepal. Molecules 2022, 27, 6136. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef] [PubMed]
- Fadwa, A.O.; Alkoblan, D.K.; Mateen, A.; Albarag, A.M. Synergistic effects of zinc oxide nanoparticles and various antibiotics combination against Pseudomonas aeruginosa clinically isolated bacterial strains. Saudi J. Biol. Sci. 2021, 28, 928–935. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic antibacterial activity of green synthesized silver nanomaterials with colistin antibiotic against multidrug-resistant bacterial pathogens. Crystals 2022, 12, 1057. [Google Scholar] [CrossRef]
- Lopez-Carrizales, M.; Pérez-Díaz, M.A.; Mendoza-Mendoza, E.; Peralta-Rodríguez, R.D.; Ojeda-Galván, H.J.; Portales-Pérez, D.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Martinez-Gutierrez, F. Green, novel, and one-step synthesis of silver oxide nanoparticles: Antimicrobial activity, synergism with antibiotics, and cytotoxic studies. New J. Chem. 2022, 46, 17841–17853. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antifungal Efficiency of Biogenic Silver Nanoparticles with Itraconazole against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 816. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Sayed, S.R. In vitro antimicrobial activity of Thymus vulgaris extracts against some nosocomial and food poisoning bacterial strains. Process. Biochem 2022, 115, 152–159. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A. In vitro anticandidal potency of Syzygium aromaticum (clove) extracts against vaginal candidiasis. BMC Complement. Altern. Med. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yassin, M.T.; Mostafa, A.A.; Al-Askar, A.A. Anticandidal and anti-carcinogenic activities of Mentha longifolia (Wild Mint) extracts in vitro. J. King Saud Univ. Sci. 2020, 32, 2046–2052. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Alkhelaif, A.S. In vitro antimicrobial potency of Elettaria cardamomum ethanolic extract against multidrug resistant of food poisoning bacterial strains. J. King Saud Univ. Sci. 2022, 34, 102167. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile Green Synthesis of Zinc Oxide Nanoparticles with Potential Synergistic Activity with Common Antifungal Agents against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 774. [Google Scholar] [CrossRef]
- Tang, Q.; Xia, H.; Liang, W.; Huo, X.; Wei, X. Synthesis and characterization of zinc oxide nanoparticles from Morus nigra and its anticancer activity of AGS gastric cancer cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111698. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard M2-A8; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2003. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.F.; Al Askar, A.A. In Vitro Evaluation of Biological Activities and Phytochemical Analysis of Different Solvent Extracts of Punica granatum L. (Pomegranate) Peels. Plants 2021, 10, 2742. [Google Scholar] [CrossRef]
- Punjabi, K.; Mehta, S.; Chavan, R.; Chitalia, V.; Deogharkar, D.; Deshpande, S. Efficiency of biosynthesized silver and zinc nanoparticles against multi-drug resistant pathogens. Front. Microbiol. 2018, 9, 2207. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. Synergistic antifungal activity of green synthesized silver nanoparticles and epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020, 9535432. [Google Scholar] [CrossRef] [Green Version]
- Lo, W.H.; Deng, F.S.; Chang, C.J.; Lin, C.H. Synergistic antifungal activity of chitosan with fluconazole against Candida albicans, Candida tropicalis, and fluconazole-resistant strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef]
- Moteriya, P.; Padalia, H.; Chanda, S. Characterization, synergistic antibacterial and free radical scavenging efficacy of silver nanoparticles synthesized using Cassia roxburghii leaf extract. J. Genet. Eng. Biotechnol. 2017, 15, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, P.; Saha, M. Green synthesis of zinc oxide nanoparticles using tomato (Lycopersicon esculentum) extract and its photovoltaic application. J. Exp. Nanosci. 2016, 11, 314–327. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, G.; Saroja, M.; Venkatachalam, M.; Evanjelene, V.K. Biological synthesis of zinc oxide nanoparticles from leaf extract of Curcuma neilgherrensis Wight. Int. J. Mater. Sci. 2017, 12, 73–86. [Google Scholar]
- Valduga, A.T.; Gonçalves, I.L.; Magri, E.; Finzer, J.R.D. Chemistry, pharmacology and new trends in traditional functional and medicinal beverages. Food Res. Int. 2019, 120, 478–503. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erenler, R.; Sen, O.; Aksit, H.; Demirtas, I.; Yaglioglu, A.S.; Elmastas, M.; Telci, I. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2016, 96, 822–836. [Google Scholar] [CrossRef]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile green synthesis of silver nanoparticles using aqueous leaf extract of Origanum majorana with potential bioactivity against multidrug resistant bacterial strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Mohammadian, M.; Es’haghi, Z.; Hooshmand, S. Green and chemical synthesis of zinc oxide nanoparticles and size evaluation by UV–vis spectroscopy. J. Nanomed. Res. 2018, 7, 00175. [Google Scholar]
- Babu, K.S.; Reddy, A.R.; Sujatha, C.; Reddy, K.V. Optimization of UV emission intensity of ZnO nanoparticles by changing the excitation wavelength. Mater. Lett. 2013, 99, 97–100. [Google Scholar] [CrossRef]
- Fuku, X.; Diallo, A.; Maaza, M. Nanoscaled electrocatalytic optically modulated ZnO nanoparticles through green process of Punica granatum L. and their antibacterial activities. Int. J. Electrochem. Sci. 2016, 2016, 4682967. [Google Scholar]
- Siva, N.; Sakthi, D.; Ragupathy, S.; Arun, V.; Kannadasan, N. Synthesis, structural, optical and photocatalytic behavior of Sn doped ZnO nanoparticles. Mater. Sci. Eng. B 2020, 253, 114497. [Google Scholar] [CrossRef]
- Fatimah, I.; Pradita, R.Y.; Nurfalinda, A. Plant extract mediated of ZnO nanoparticles by using ethanol extract of Mimosa pudica leaves and coffee powder. Procedia Eng. 2016, 148, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Ngoepe, N.M.; Mbita, Z.; Mathipa, M.; Mketo, N.; Ntsendwana, B.; Hintsho-Mbita, N.C. Biogenic synthesis of ZnO nanoparticles using Monsonia burkeana for use in photocatalytic, antibacterial and anticancer applications. Ceram. Int. 2018, 44, 16999–17006. [Google Scholar] [CrossRef]
- Falih, A.; Ahmed, N.M.; Rashid, M. Green synthesis of zinc oxide nanoparticles by fresh and dry alhagi plant. Mater. Today Proc. 2022, 49, 3624–3629. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Khamlich, S.; Maaza, M. Sageretia thea (Osbeck.) mediated synthesis of zinc oxide nanoparticles and its biological applications. Nanomedicine 2017, 12, 1767–1789. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Arthrospira platensis (Class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef]
- Sultana, K.A.; Islam, M.T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Vimala, K.; Sundarraj, S.; Paulpandi, M.; Vengatesan, S.; Kannan, S. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process. Biochem. 2014, 49, 160–172. [Google Scholar] [CrossRef]
- El-Hawwary, S.S.; Abd Almaksoud, H.M.; Saber, F.R.; Elimam, H.; Sayed, A.M.; El Raey, M.A.; Abdelmohsen, U.R. Green-synthesized zinc oxide nanoparticles, anti-Alzheimer potential and the metabolic profiling of Sabal blackburniana grown in Egypt supported by molecular modelling. RSC Adv. 2021, 11, 18009–18025. [Google Scholar] [CrossRef]
- Gharagozlou, M.; Naghibi, S. Sensitization of ZnO nanoparticle by vitamin B12: Investigation of microstructure, FTIR and optical properties. Mater. Res. Bull. 2016, 84, 71–78. [Google Scholar] [CrossRef]
- Acharya, T.R.; Lamichhane, P.; Wahab, R.; Chaudhary, D.K.; Shrestha, B.; Joshi, L.P.; Kaushik, N.K.; Choi, E.H. Study on the Synthesis of ZnO Nanoparticles Using Azadirachta indica Extracts for the Fabrication of a Gas Sensor. Molecules 2021, 26, 7685. [Google Scholar] [CrossRef]
- Ghaffari, S.B.; Sarrafzadeh, M.H.; Fakhroueian, Z.; Shahriari, S.; Khorramizadeh, M.R. Functionalization of ZnO nanoparticles by 3-mercaptopropionic acid for aqueous curcumin delivery: Synthesis, characterization, and anticancer assessment. Mater. Sci. Eng. C 2017, 79, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalek, S.; Eldala, A.; Awad, D.; Balbaa, M. Ameliorative effect of curcumin and zinc oxide nanoparticles on multiple mechanisms in obese rats with induced type 2 diabetes. Sci. Rep 2021, 11, 20677. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaushik, M. Physicochemical investigations of zinc oxide nanoparticles synthesized from Azadirachta Indica (Neem) leaf extract and their interaction with Calf-Thymus DNA. Results Phys. 2019, 13, 102168. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Khan, M.S.; Dhavan, P.P.; Jadhav, B.L.; Shimpi, N.G. Ultrasound-Assisted Green Synthesis of Ag-Decorated ZnO Nanoparticles Using Excoecaria agallocha Leaf Extract and Evaluation of Their Photocatalytic and Biological Activity. Chem. Sel. 2020, 5, 12660–12671. [Google Scholar]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green Synthesis of Zinc Oxide Nanoparticles Using Pomegranate Fruit Peel and Solid Coffee Grounds vs. Chemical Method of Synthesis, with Their Biocompatibility and Antibacterial Properties Investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef]
- Ifeanyichukwu, U.L.; Fayemi, O.E.; Ateba, C.N. Green synthesis of zinc oxide nanoparticles from pomegranate (Punica granatum) extracts and characterization of their antibacterial activity. Molecules 2020, 25, 4521. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Divya, M.; Vaseeharan, B.; Ranjan, S.; Kalaiselvi, V.; Dasgupta, N.; Chen, J.; Durán-Lara, E.F. Biogenic preparation and characterization of ZnO nanoparticles from natural polysaccharide Azadirachta indica. L. (neem gum) and its clinical implications. J. Clust. Sci. 2021, 32, 983–993. [Google Scholar] [CrossRef]
- Gawade, V.V.; Gavade, N.L.; Shinde, H.M.; Babar, S.B.; Kadam, A.N.; Garadkar, K.M. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017, 28, 14033–14039. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vinoj, G.; Malaikozhundan, B.; Shanthi, S.; Vaseeharan, B. Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.M.; Aboelfetoh, E.F.; Kimura, T.; Mohamed, T.M.; El-Keiy, M.M. Fenugreek-mediated synthesis of zinc oxide nanoparticles and evaluation of its in vitro and in vivo antitumor potency. Biomed. Res. Ther. 2021, 8, 4483–4496. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Kaur, T.; Bala, M.; Kumar, G.; Vyas, A. Biosynthesis of zinc oxide nanoparticles via endophyte Trichoderma viride and evaluation of their antimicrobial and antioxidant properties. Arch. Microbiol. 2022, 204, 620. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef] [Green Version]
- Al Sharie, A.H.; El-Elimat, T.; Darweesh, R.S.; Swedan, S.; Shubair, Z.; Al-Qiam, R.; Albarqi, H. Green synthesis of zinc oxide nanoflowers using Hypericum triquetrifolium extract: Characterization, antibacterial activity and cytotoxicity against lung cancer A549 cells. Appl. Organomet. Chem. 2020, 34, e5667. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.C.; Rusch, P.; Scheper, T.; Walter, J.G. Hypericum perforatum L.-mediated green synthesis of silver nanoparticles exhibiting antioxidant and anticancer activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef]
- Ramanarayanan, R.; Bhabhina, N.M.; Dharsana, M.V.; Nivedita, C.V.; Sindhu, S. Green synthesis of zinc oxide nanoparticles using extract of Averrhoa bilimbi (L) and their photoelectrode applications. Mater. Today Proc. 2018, 5, 16472–16477. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Hameed, S.; Munir, A.; Kanwal, S. Green synthesis and characterizations of Nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl. Organomet. Chem. 2019, 33, e4950. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; El-Mageed, A.; Taia, A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Velsankar, K.; Sudhahar, S.; Maheshwaran, G. Effect of biosynthesis of ZnO nanoparticles via Cucurbita seed extract on Culex tritaeniorhynchus mosquito larvae with its biological applications. J. Photochem. Photobiol. B Biol. 2019, 200, 111650. [Google Scholar]
- Subramanian, H.; Krishnan, M.; Mahalingam, A. Photocatalytic dye degradation and photoexcited anti-microbial activities of green zinc oxide nanoparticles synthesized via Sargassum muticum extracts. RSC Adv. 2022, 12, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Zinc oxide and zinc oxide-based nanostructures: Biogenic and phytogenic synthesis, properties and applications. Bioprocess Biosyst. Eng. 2021, 44, 1333–1372. [Google Scholar] [CrossRef]
- Okeke, I.S.; Agwu, K.K.; Ubachukwu, A.A.; Madiba, I.G.; Maaza, M.; Whyte, G.M.; Ezema, F.I. Impact of particle size and surface defects on antibacterial and photocatalytic activities of undoped and Mg-doped ZnO nanoparticles, biosynthesized using one-step simple process. Vacuum 2020, 187, 110110. [Google Scholar] [CrossRef]
- Onyszko, M.; Zywicka, A.; Wenelska, K.; Mijowska, E. Revealing the Influence of the Shape, Size, and Aspect Ratio of ZnO Nanoparticles on Antibacterial and Mechanical Performance of Cellulose Fibers Based Paper. Part. Part. Syst. Charact 2022, 39, 2200014. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Cai, L.; Wu, Y.; Ji, M.; Jiang, H.; Chen, J. Dissection of the antibacterial mechanism of zinc oxide nanoparticles with manipulable nanoscale morphologies. J. Hazard. Mater. 2022, 430, 128436. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Forde, B.M.; Kidd, T.J.; Harris, P.N.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Abo-Shama, U.H.; el-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F.; Abdeen, E.E. Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef]
- Reyes-Torres, M.A.; Mendoza-Mendoza, E.; Miranda-Hernández, Á.M.; Pérez-Díaz, M.A.; López-Carrizales, M.; Peralta-Rodríguez, R.D.; Martinez-Gutierrez, F. Synthesis of CuO and ZnO nanoparticles by a novel green route: Antimicrobial activity, cytotoxic effects and their synergism with ampicillin. Ceram. Int. 2019, 45, 24461–24468. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic effects between metal nanoparticles and commercial antimicrobial agents: A Review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Aarestrup, F.M.; Hansen, E.B. The role of outer membrane proteins and lipopolysaccharides for the sensitivity of Escherichia coli to antimicrobial peptides. Front. Microbiol. 2018, 9, 2153. [Google Scholar] [CrossRef] [Green Version]
- Ledger, E.V.; Sabnis, A.; Edwards, A.M. Polymyxin and lipopeptide antibiotics: Membrane-targeting drugs of last resort. Microbiology 2022, 168, 001136. [Google Scholar] [CrossRef]
- Ahmadi Shadmehri, A.; Namvar, F. A Review on Green Synthesis, Cytotoxicity Mechanism and Antibacterial Activity of Zno-NPs. J. Res. Appl. Basic Med. Res. 2020, 6, 23–31. [Google Scholar]
- Singh, A.; Singh, N.Á.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]








| No. | Absorption Peak (cm−1) | Appearance | Functional Groups | Molecular Motion |
|---|---|---|---|---|
| 1 | 3434.50 | Strong, broad | Phenols | O-H stretching |
| 2 | 1628.74 | Medium | Cyclic alkene | C=C stretching |
| 3 | 1509.02 | Weak | Alkenes | C=C stretching |
| 4 | 1396.52 | Weak | Carboxylic acids | C=O stretching |
| 5 | 1263.40 | Weak | Amines | C–N stretching |
| 6 | 1048.02 | Medium | Alcohols | C−C stretching |
| 7 | 580.03 | Medium | Metal oxide bonds | Zn-O stretching |
| The Bacterial Strains | Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|
| ZnO NPs (50 µg/Disk) | ZnO NPs (100 µg/Disk) | Colistin (10 µg/Disk) | −ve Control | |
| A. baumannii | 17.14 ± 0.27 | 19.31 ± 0.56 | 11.13 ± 0.19 | 0.00 ± 0.00 |
| E. coli | 32.16 ± 0.49 | 38.16 ± 0.18 | 27.13 ± 0.45 | 0.00 ± 0.00 |
| E. cloacae | 21.65 ± 0.52 | 24.12 ± 0.23 | 14.08 ± 0.14 | 0.00 ± 0.00 |
| K. pneumoniae | 23.87 ± 0.16 | 26.47 ± 0.38 | 18.98 ± 0.15 | 0.00 ± 0.00 |
| P. aeruginosa | 13.18 ± 0.11 | 16.42 ± 0.31 | 15.93 ± 0.29 | 0.00 ± 0.00 |
| S. typhimurium | 20.13 ± 0.24 | 22.86 ± 0.18 | 17.15 ± 0.36 | 0.00 ± 0.00 |
| The Tested Strains | Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|
| Colistin (10 µg/Disk) | ZnO NPs (15 µg/Disk) | Colistin (10 µg/Disk) + ZnO NPs (15 µg/Disk) | −ve Control | |
| A. baumannii | 11.78 ± 0.23 | 10.35 ± 0.11 | 20.62 ± 0.14 | 0.00 ± 0.00 |
| E. coli | 17.89 ± 0.18 | 16.95 ± 0.38 | 34.18 ± 0.25 | 0.00 ± 0.00 |
| E. cloacae | 14.16 ± 0.56 | 15.18 ± 0.49 | 22.13 ± 0.32 | 0.00 ± 0.00 |
| K. pneumoniae | 15.29 ± 0.09 | 16.84 ± 0.24 | 27.38 ± 0.41 | 0.00 ± 0.00 |
| P. aeruginosa | 14.89 ± 0.16 | 8.98 ± 0.12 | 16.42 ± 0.46 | 0.00 ± 0.00 |
| S. typhimurium | 13.78 ± 0.51 | 14.97± 0.43 | 24.15 ± 0.17 | 0.00 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassin, M.T.; Al-Askar, A.A.; Maniah, K.; Al-Otibi, F.O. Green Synthesis of Zinc Oxide Nanocrystals Utilizing Origanum majorana Leaf Extract and Their Synergistic Patterns with Colistin against Multidrug-Resistant Bacterial Strains. Crystals 2022, 12, 1513. https://doi.org/10.3390/cryst12111513
Yassin MT, Al-Askar AA, Maniah K, Al-Otibi FO. Green Synthesis of Zinc Oxide Nanocrystals Utilizing Origanum majorana Leaf Extract and Their Synergistic Patterns with Colistin against Multidrug-Resistant Bacterial Strains. Crystals. 2022; 12(11):1513. https://doi.org/10.3390/cryst12111513
Chicago/Turabian StyleYassin, Mohamed Taha, Abdulaziz Abdulrahman Al-Askar, Khalid Maniah, and Fatimah O. Al-Otibi. 2022. "Green Synthesis of Zinc Oxide Nanocrystals Utilizing Origanum majorana Leaf Extract and Their Synergistic Patterns with Colistin against Multidrug-Resistant Bacterial Strains" Crystals 12, no. 11: 1513. https://doi.org/10.3390/cryst12111513





