Termination Control of (001) and (110) NdGaO3 Single-Crystal Substrates by Selective Chemical Etching
Abstract
:1. Introduction
2. Materials and Methods
3. Results
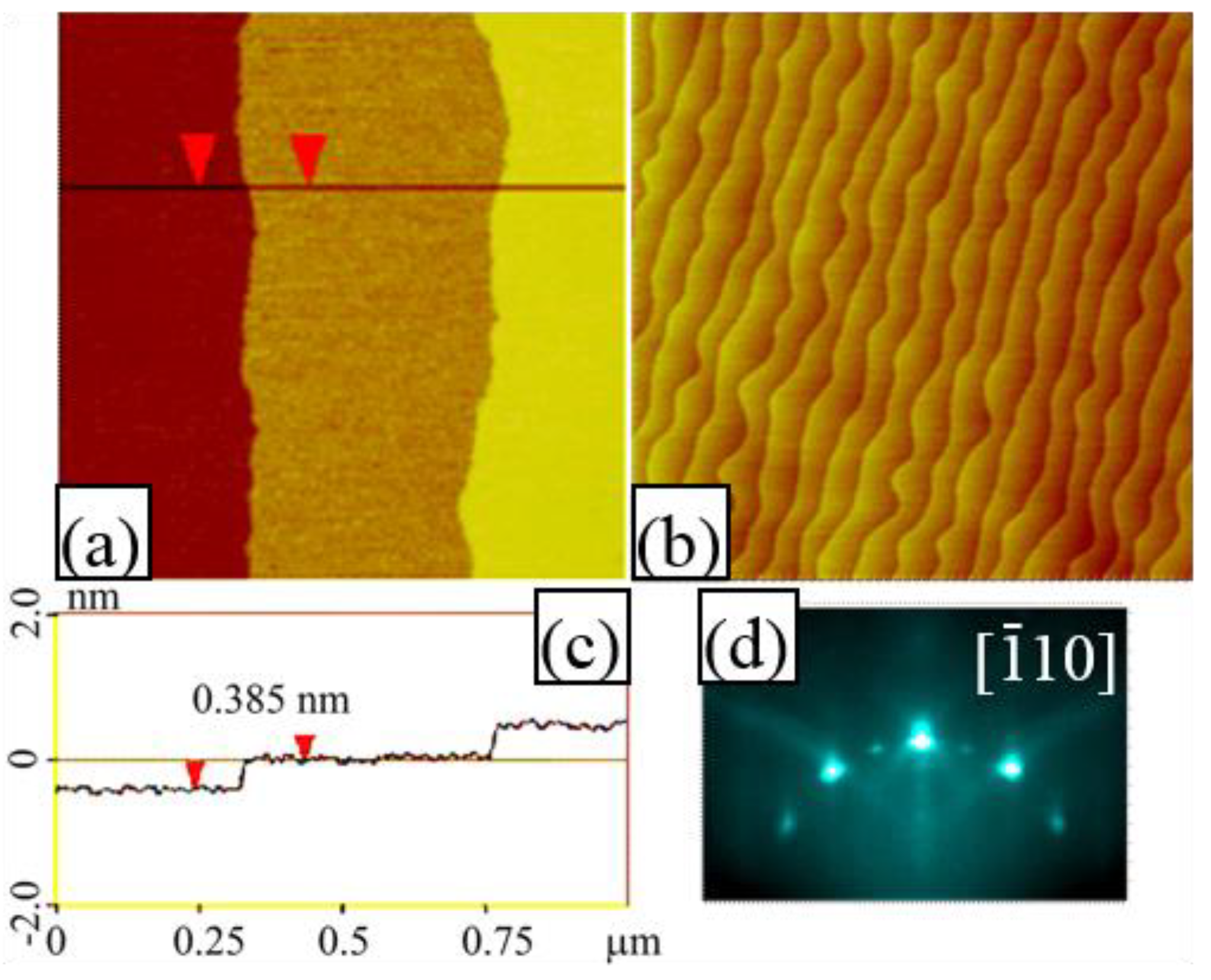
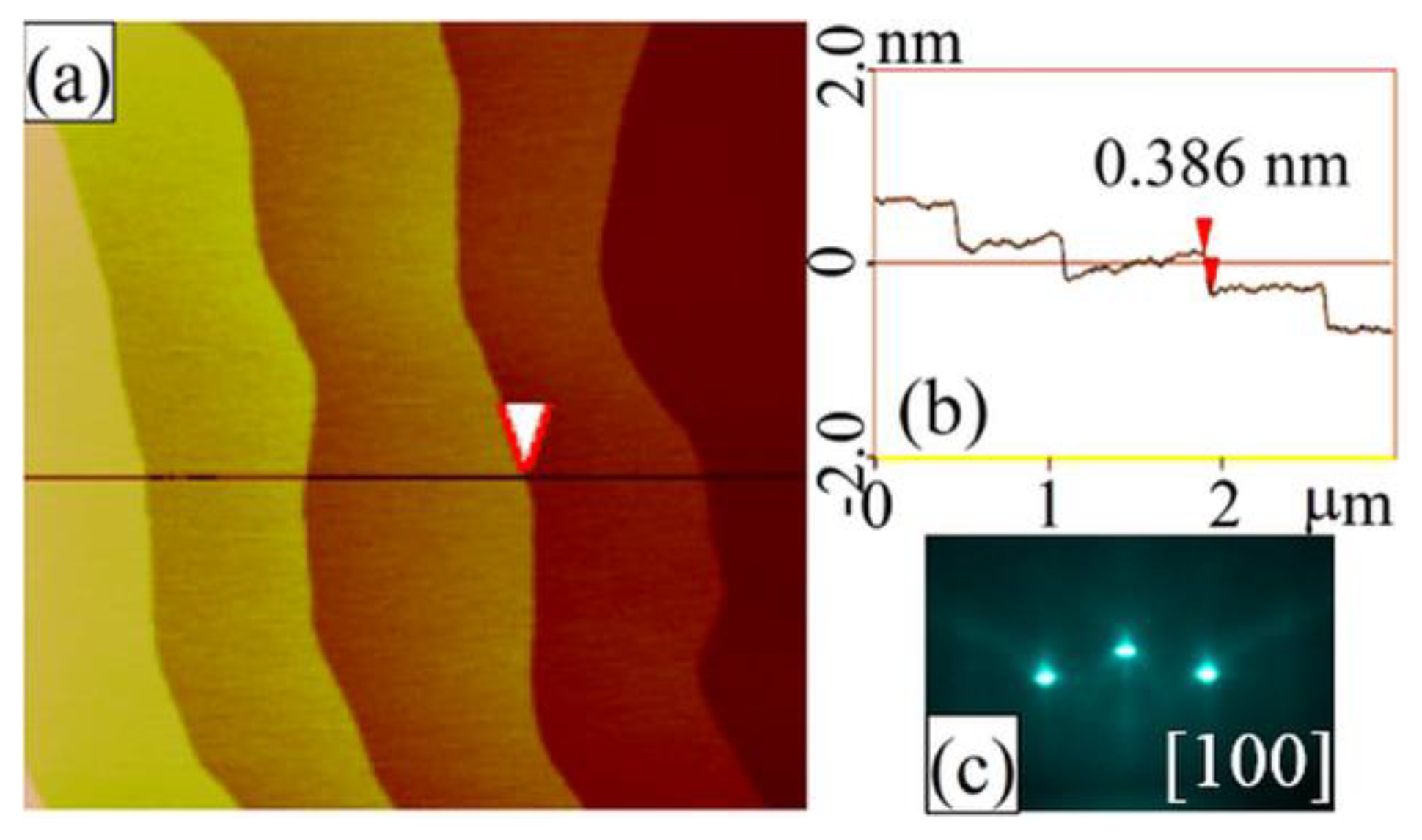
3.1. Chemical-Etching Treatment
3.2. Composition of the Terminating Layer
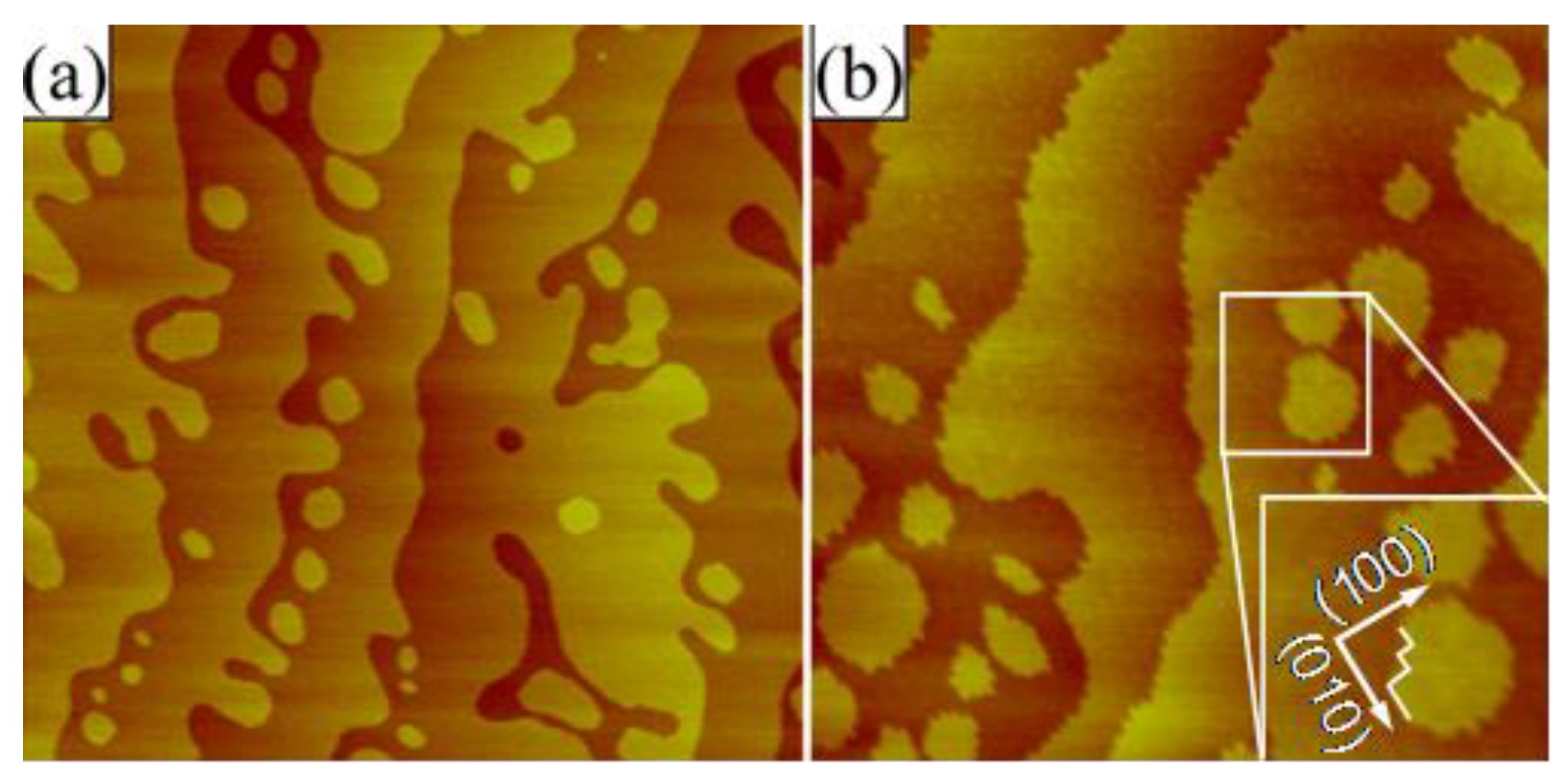
- Two slices were cut from one (001) NdGaO3 wafer (characterized by a Δ angle of ~45°) and then cleaned using chloroform, acetone and ethanol, as described in the previous section. One slice was then chemically etched in m-BHF solution for 2 min, but without undergoing annealing. The resulting etched slice and the as-received one were afterwards annealed simultaneously in air for 2 h at 1000 °C. Contact-mode AFM topographic micrographs (Figure 5) revealed surfaces characterized by terraces with two types of edges. For the chemically etched sample in Figure 5a, the terraces’ edges bore a rounded appearance, i.e., the edge energy was minimized. This contrasts with the morphology of the annealed surface Figure 5b, which featured saw-tooth-like step edges, aligned along the [100] and [010] crystallographic directions, as indicated by the zoomed-in area from Figure 5b. Therefore, it may be concluded that the surface of the chemically etched substrate is characterized by a lower surface energy and features a different chemical composition than that of the annealed one. Similar differences in the type of terraces edges and the chemistry of the termination layer were observed between the (only) annealed and the chemically etched (001) SrTiO3 substrates [1,26,59].
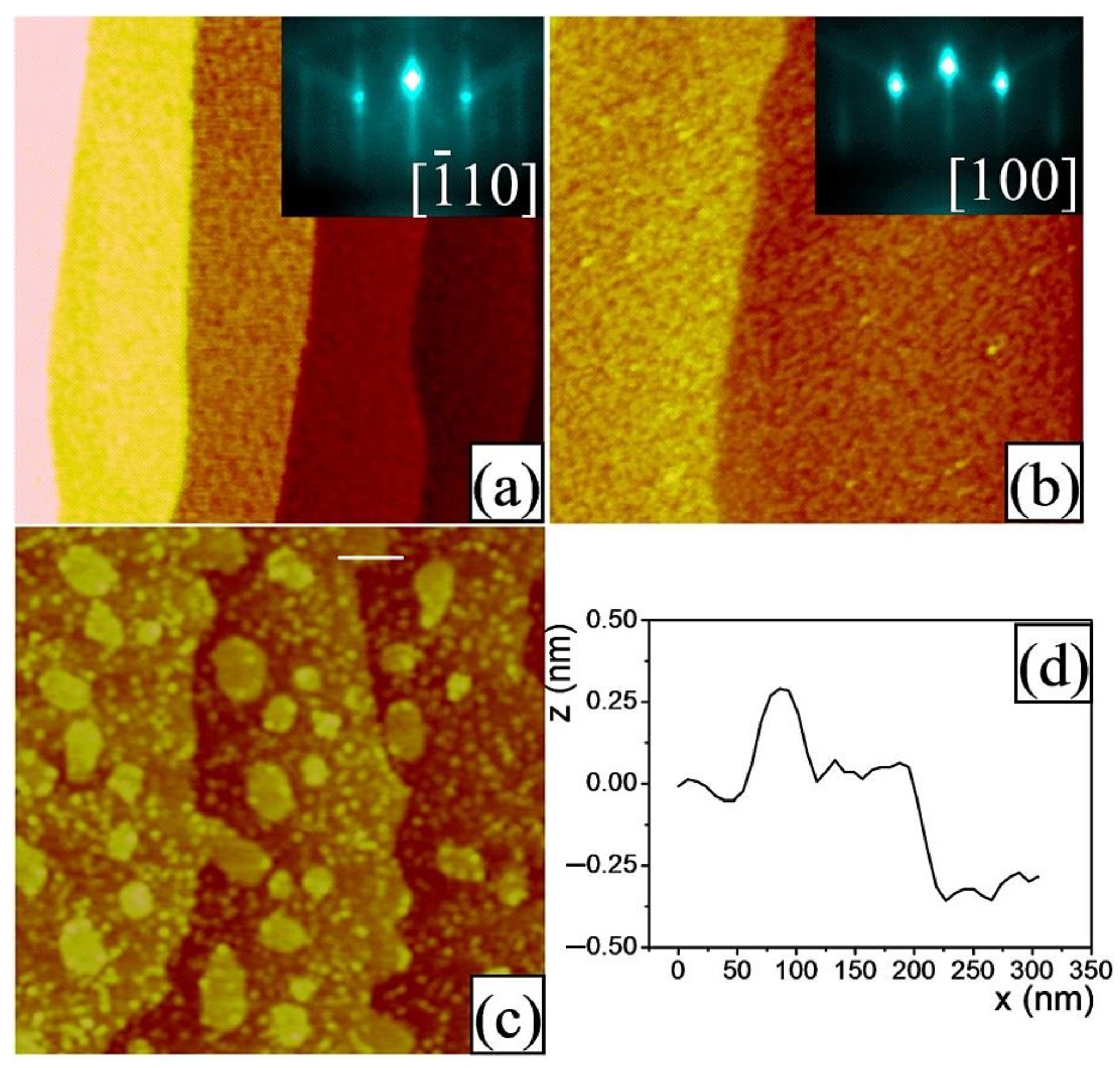
- The wetting of the substrate’s surface with either NdO1+x or GaO2−x termination was determined by hetero-epitaxial growth of one-unit-cell-thick TiO2, SrO or BaO layers by means of PLD on the annealed or chemically treated substrates. The amount of deposited material was less than (but close to) one monolayer in order to prevent the formation of precipitates [2,53]. The deposition conditions for each oxide are provided in Table 2. All targets were single crystals. First, TiO2 was deposited on an annealed (110) NdGaO3 surface. Figure 6a illustrates the AFM and RHEED data of the resulting film surface. Regularly spaced terraces separated by ~0.4nm-height steps are visible. It can be concluded that TiO2 is wetting the NdO1+x on the surface forming an almost closed layer. The corresponding RHEED pattern indicates a highly ordered surface. Since a B-site oxide (e.g., TiO2) does not wet a B-site-terminated ABO3 surface [1,2,26,60] (e.g., a GaO2−x terminated NdGaO3), it can be concluded that the annealed NdGaO3 substrate features a NdO1+x termination, thereby confirming the results from Ref. [36].
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koster, G.; Rijnders, G.; Blank, D.H.A.; Rogalla, H. Surface morphology determined by (001) single-crystal SrTiO3 termination. Phys. C Supercond. 2000, 339, 215–230. [Google Scholar] [CrossRef]
- Huijbregtse, J.M.; Rector, J.H.; Dam, B. Effect of the two (100) SrTiO3 substrate terminations on the nucleation and growth of YBa2Cu3O7−δ thin films. Phys. C Supercond. 2001, 351, 183–199. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ohsawa, T.; Takahashi, R.; Koinuma, H. Surface termination effect on the photocatalysis on atomically controlled SrTiO3(001) surface. Thin Solid Films 2005, 486, 11–14. [Google Scholar] [CrossRef]
- Leca, V.; Blank, D.; Rijnders, G.; Bals, S.; van Tendeloo, G. Superconducting single-phase Sr1−xLaxCuO2 thin films with improved crystallinity grown by pulsed laser deposition. Appl. Phys. Lett. 2006, 89, 092504. [Google Scholar] [CrossRef]
- Guo, R.; Bhalla, A.S.; Cross, L.E.; Roy, R. Surface crystallographic structure compatibility between substrates and high Tc (YBCO) thin films. J. Mater. Res. 1994, 9, 1644–1656. [Google Scholar] [CrossRef]
- Koster, G.; Kropman, B.L.; Rijnders, G.J.H.M.; Blank, D.H.A.; Rogalla, H. Influence of the surface treatment on the homoepitaxial growth of SrTiO3. Mater. Sci. Eng. B 1998, 56, 209–212. [Google Scholar] [CrossRef]
- Phillips, J.M. Substrate selection for high-temperature superconducting thin films. J. Appl. Phys. 1996, 79, 1829–1848. [Google Scholar] [CrossRef]
- Vailionis, A.; Boschker, H.; Siemons, W.; Houwman, E.P.; Blank, D.H.A.; Rijnders, G.; Koster, G. Misfit strain accommodation in epitaxial ABO3 perovskites: Lattice rotations and lattice modulations. Phys. Rev. B 2011, 83, 64101. [Google Scholar] [CrossRef] [Green Version]
- Chakhalian, J.; Freeland, J.W.; Habermeier, H.-U.; Cristiani, G.; Khaliullin, G.; van Veenendaal, M.; Keimer, B. Orbital reconstruction and covalent bonding at an oxide interface. Science 2007, 318, 1114–1117. [Google Scholar] [CrossRef] [Green Version]
- Huijben, M.; Rijnders, G.; Blank, D.H.A.; Bals, S.; Van Aert, S.; Verbeeck, J.; Van Tendeloo, G.; Brinkman, A.; Hilgenkamp, H. Electronically coupled complementary interfaces between perovskite band insulators. Nat. Mater. 2006, 5, 556–560. [Google Scholar] [CrossRef]
- Koren, G.; Gupta, A.; Giess, E.; Segmuller, A.; Laibowitz, R. Epitaxial films of YBa2Cu3O7-δ on NdGaO3, LaGaO3, and SrTiO3 substrates deposited by laser ablation. Appl. Phys. Lett. 1989, 54, 1054–1056. [Google Scholar] [CrossRef]
- Balestrino, G.; Desfeux, R.; Martellucci, S.; Paoletti, A.; Petrocelli, G.; Tebano, A.; Mercey, B.; Hervieu, M. Growth of CaCuO2 and SrxCa1-xCuO2 epitaxial films on NdGaO3 substrates by pulsed laser deposition. J. Mater. Chem. 1995, 5, 1879–1883. [Google Scholar] [CrossRef]
- Li, K.; Qi, Z.; Li, X.; Zhu, J.; Zhang, Y. Growth, structural characteristics and magnetoresistance in LaCaMnO thin films prepared by dc magnetron sputtering. Thin Solid Films 1997, 304, 386–391. [Google Scholar] [CrossRef]
- Okazaki, H.; Arakawa, A.; Asahi, T.; Oda, O.; Aiki, K. GaN epitaxial growth on neodium gallate substrates. Solid. State. Electron. 1997, 41, 263–266. [Google Scholar] [CrossRef]
- Madhavan, S.; Mitchell, J.A.; Nemoto, T.; Wozniak, S.; Liu, Y.; Schlom, D.G.; Dabkowski, A.; Dabkowska, H.A. Growth of epitaxial Sr2RuO4 films and YBa2Cu3O7−δSr2RuO4 heterostructures. J. Cryst. Growth 1997, 174, 417–423. [Google Scholar] [CrossRef]
- Cao, L.; Zegenhagen, J.; Sozontov, E. The effect of epitaxial strain on RBa2Cu3O7 thin films and the perovskite substrate. Phys. C 2000, 337, 24–30. [Google Scholar] [CrossRef]
- Wördenweber, R.; Schwarzkopf, J.; Hollmann, E.; Duk, A.; Cai, B.; Schmidbauer, M. Impact of compressive in-plane strain on the ferroelectric properties of epitaxial NaNbO3 films on (110) NdGaO3. Appl. Phys. Lett. 2013, 103, 132908. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, B.; Zheng, Y. Ultrathin Ferroelectric Films: Growth, Characterization, Physics and Applications. Materials 2014, 7, 6377–6485. [Google Scholar] [CrossRef]
- Biegalski, M.D.; Qiao, L.; Gu, Y.; Mehta, A.; He, Q.; Takamura, Y.; Borisevich, A.; Chen, L.-Q. Impact of symmetry on the ferroelectric properties of CaTiO3 thin films. Appl. Phys. Lett. 2015, 106, 162904. [Google Scholar] [CrossRef] [Green Version]
- Schlom, D.G.; Chen, L.-Q.; Eom, C.-B.; Rabe, K.M.; Streiffer, S.K.; Triscone, J.-M. Strain Tuning of Ferroelectric Thin Films. Annu. Rev. Mater. Res. 2007, 37, 589–626. [Google Scholar] [CrossRef]
- Schwarzkopf, J.; Braun, D.; Hanke, M.; Uecker, R.; Schmidbauer, M. Strain Engineering of Ferroelectric Domains in KxNa1−xNbO3 Epitaxial Layers. Front. Mater. 2017, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Sharma, Y.; Agarwal, R.; Phatak, C.; Kim, B.; Jeon, S.; Katiyar, R.S.; Hong, S. Long-range Stripe Nanodomains in Epitaxial (110) BiFeO3 Thin Films on (100) NdGaO3 Substrate. Sci. Rep. 2017, 7, 4857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Pryds, N.; Park, D.-S.; Gauquelin, N.; Santucci, S.; Christensen, D.V.; Jannis, D.; Chezganov, D.; Rata, D.A.; Insinga, A.R.; et al. Atomically engineered interfaces yield extraordinary electrostriction. Nature 2022, 609, 695–700. [Google Scholar] [CrossRef]
- Li, F.; Jin, L.; Xu, Z.; Zhang, S. Electrostrictive effect in ferroelectrics: An alternative approach to improve piezoelectricity. Appl. Phys. Rev. 2014, 1, 11103. [Google Scholar] [CrossRef] [Green Version]
- Rogdakis, K.; Seo, J.W.; Viskadourakis, Z.; Wang, Y.; Ah Qune, L.F.N.; Choi, E.; Burton, J.D.; Tsymbal, E.Y.; Lee, J.; Panagopoulos, C. Tunable ferroelectricity in artificial tri-layer superlattices comprised of non-ferroic components. Nat. Commun. 2012, 3, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koster, G. Artificially Layered Oxides by Pulsed Laser Deposition; University of Twente: Enschede, The Netherlands, 1999. [Google Scholar]
- Utke, I.; Klemenz, C.; Scheel, H.J.; Sasaura, M.; Miyazawa, S. Misfit problems in epitaxy of high-Tc superconductors. J. Cryst. Growth 1997, 174, 806–812. [Google Scholar] [CrossRef]
- Vasylechko, L.; Matkovskii, A.; Savytskii, D.; Suchocki, A.; Wallrafen, F. Crystal structure of GdFeO3-type rare earth gallates and aluminates. J. Alloys Compd. 1999, 291, 57–65. [Google Scholar] [CrossRef]
- Marti, W.; Fischer, P.; Altorfer, F.; Scheel, H.J.; Tadin, M. Crystal structures and phase transitions of orthorhombic and rhombohedral RGaO3(R=La,Pr,Nd) investigated by neutron powder diffraction. J. Phys. Condens. Matter 1994, 6, 127–135. [Google Scholar] [CrossRef]
- Vasylechko, L.; Akselrud, L.; Morgenroth, W.; Bismayer, U.; Matkovskii, A.; Savytskii, D. The crystal structure of NdGaO3 at 100 K and 293 K based on synchrotron data. J. Alloys Compd. 2000, 297, 46–52. [Google Scholar] [CrossRef]
- Gunkel, F.; Skaja, K.; Shkabko, A.; Dittmann, R.; Hoffmann-Eifert, S.; Waser, R. Stoichiometry dependence and thermal stability of conducting NdGaO3/SrTiO3 heterointerfaces. Appl. Phys. Lett. 2013, 102, 71601. [Google Scholar] [CrossRef]
- Wade, K.; Banister, A.J. Comprehensive Inorganic Chemistry; Oxford Pergamon Press: London, UK, 1973. [Google Scholar]
- Gao, G.; Yin, Z.; Huang, Z.; Jin, S.; Wu, W. The thickness evolution of orthorhombic lattice distortions in heteroepitaxial La0.67Ca0.33MnO3/NdGaO3(110)Or observed by X-ray reciprocal space mapping. J. Phys. D Appl. Phys. 2008, 41, 152001. [Google Scholar] [CrossRef]
- Hirata, A.; Ando, A.; Saiki, K.; Koma, A. Characterization of surface defects formation in strontium titanate(100). Surf. Sci.–Surf. SCI 1994, 310, 89–94. [Google Scholar] [CrossRef]
- Ikeda, A.; Nishimura, T.; Morishita, T.; Kido, Y. Surface relaxation and rumpling of TiO2-terminated SrTiO3(001) determined by medium energy ion scattering. Surf. Sci.–Surf. SCI 1999, 433, 520–524. [Google Scholar] [CrossRef]
- Ohnishi, T.; Takahashi, K.; Nakamura, M.; Kawasaki, M.; Yoshimoto, M.; Koinuma, H. A-site layer terminated perovskite substrate: NdGaO3. Appl. Phys. Lett. 1999, 74, 2531–2533. [Google Scholar] [CrossRef]
- Kwon, C.; Li, Q.; Xi, X.; Bhattacharya, S.; Doughty, C.; Venkatesan, T.; Zhang, H.; Lynn, J.; Peng, J.; Li, Z.; et al. High critical current densities in ultrathin YBa2Cu3O7-δ films sandwiched between (PrxY1-x)Ba2Cu3O7-δ layers. Appl. Phys. Lett. 1993, 62, 1289–1291. [Google Scholar] [CrossRef]
- Sum, R.; Lang, H.P.; Güntherodt, H.-J. Scanning force microscopy study of single-crystal substrates used for thin-film growth of high-temperature superconductors. Phys. C Supercond. 1995, 242, 174–182. [Google Scholar] [CrossRef]
- Dirsyte, R.; Schwarzkopf, J.; Wagner, G.; Lienemann, J.; Busch, M.; Winter, H.; Fornari, R. Surface termination of the NdGaO3(110). Appl. Surf. Sci. 2009, 255, 8685–8687. [Google Scholar] [CrossRef]
- Cavallaro, A.; Harrington, G.F.; Skinner, S.J.; Kilner, J.A. Controlling the surface termination of NdGaO3(110): The role of the gas atmosphere. Nanoscale 2014, 6, 7263–7273. [Google Scholar] [CrossRef] [PubMed]
- Leca, V. Heteroepitaxial Growth of Copper Oxide Superconductors by Pulsed Laser Deposition; University of Twente: Enschede, The Netherlands, 2003. [Google Scholar]
- Leca, V.; Rijnders, G.; Koster, G.; Blank, D.H.A.; Rogalla, H. Wet Etching Methods for Perovskite Substrates. MRS Proc. 1999, 587, O3.6. [Google Scholar] [CrossRef]
- Leca, V.; Andronescu, E. Improved surface morphology of (110) NdGaO3 substrates by thermal and chemical treatments. Rom. J. Mater. 2011, 41, 127. [Google Scholar]
- Kleibeuker, J.E.; Koster, G.; Siemons, W.; Dubbink, D.; Kuiper, B.; Blok, J.L.; Yang, C.-H.; Ravichandran, J.; Ramesh, R.; ten Elshof, J.E.; et al. Atomically Defined Rare-Earth Scandate Crystal Surfaces. Adv. Funct. Mater. 2010, 20, 3490–3496. [Google Scholar] [CrossRef]
- Biswas, A.; Yang, C.-H.; Ramesh, R.; Jeong, Y.H. Atomically flat single terminated oxide substrate surfaces. Prog. Surf. Sci. 2017, 92, 117–141. [Google Scholar] [CrossRef] [Green Version]
- Polli, A.D.; Wagner, T.; Rühle, M. Effect of Ca impurities and wet chemical etching on the surface morphology of SrTiO3 substrates. Surf. Sci. 1999, 429, 237–245. [Google Scholar] [CrossRef]
- Kawasaki, M.; Takahashi, K.; Maeda, T.; Tsuchiya, R.; Shinohara, M.; Ishiyama, O.; Yonezawa, T.; Yoshimoto, M.; Koinuma, H. Atomic Control of the SrTiO3 Crystal Surface. Science 1994, 266, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Ohtomo, A.; Arakane, T.; Takahashi, K.; Yoshimoto, M.; Koinuma, H. Atomic control of SrTiO3 surface for perfect epitaxy of perovskite oxides. Appl. Surf. Sci. 1996, 107, 102–106. [Google Scholar] [CrossRef]
- Lippmaa, M.; Takahashi, K.; Ohtomo, A.; Ohashi, S.; Ohnishi, T.; Nakagawa, N.; Sato, T.; Iwatsuki, M.; Koinuma, H.; Kawasaki, M. Atom technology for Josephson tunnel junctions: SrTiO3 substrate surface. Mater. Sci. Eng. B 1998, 56, 111–116. [Google Scholar] [CrossRef]
- Koster, G.; Kropman, B.L.; Rijnders, G.J.H.M.; Blank, D.H.A.; Rogalla, H. Quasi-ideal strontium titanate crystal surfaces through formation of strontium hydroxide. Appl. Phys. Lett. 1998, 73, 2920–2922. [Google Scholar] [CrossRef] [Green Version]
- Kleibeuker, J.E.; Kuiper, B.; Harkema, S.; Blank, D.H.A.; Koster, G.; Rijnders, G.; Tinnemans, P.; Vlieg, E.; Rossen, P.B.; Siemons, W.; et al. Structure of singly terminated polar DyScO3 (110) surfaces. Phys. Rev. B 2012, 85, 165413. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Wang, H.; Makino, H.; Ko, H.-J.; Hanada, T.; Yao, T. Growth of PrSrMnO3-like thin films on NGO (110) substrates by plasma assisted MBE. J. Cryst. Growth 2001, 227–228, 960–965. [Google Scholar] [CrossRef]
- Gagnidze, T.; Ma, H.; Cancellieri, C.; Bona, G.-L.; Mattina, F. La Structural properties of ultrathin SrO film deposited on SrTiO3. Sci. Technol. Adv. Mater. 2019, 20, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Kikyuama, H.; Miki, N.; Saka, K.; Takano, J.; Kawanabe, I.; Miyashita, M.; Ohmi, T. Principles of wet chemical processing in ULSI microfabrication. IEEE Trans. Semicond. Manuf. 1991, 4, 26–35. [Google Scholar] [CrossRef]
- Kikuyama, H.; Waki, M.; Miyashita, M.; Yabune, T.; Miki, N.; Takano, J.; Ohmi, T. A Study of the Dissociation State and the SiO2 Etching Reaction for HF Solutions of Extremely Low Concentration. J. Electrochem. Soc. 1994, 141, 366. [Google Scholar] [CrossRef]
- Stäuble-Pümpin, B.; Ilge, B.; Matijasevic, V.C.; Scholte, P.M.L.O.; Steinfort, A.J.; Tuinstra, F. Atomic force microscopy study of (001) SrTiO3 surfaces. Surf. Sci. 1996, 369, 313–320. [Google Scholar] [CrossRef]
- Senapati, L.; Sharma, R.; Venkatesan, T.; Zhang, Z.; Chu, W.-K. Ion channeling studies of regrowth kinetics in crystalline metal oxides used with high temperature superconductors. Appl. Phys. Lett. 1996, 68, 123–125. [Google Scholar] [CrossRef]
- Rijnders, G.J.H.M.; Koster, G.; Blank, D.H.A.; Rogalla, H. In Situ monitoring during pulsed laser deposition of complex oxides using reflection high energy electron diffraction under high oxygen pressure. Appl. Phys. Lett. 1997, 70, 1888–1890. [Google Scholar] [CrossRef]
- Fompeyrine, J.; Berger, R.; Lang, H.P.; Perret, J.; Mächler, E.; Gerber, C.; Locquet, J.-P. Local determination of the stacking sequence of layered materials. Appl. Phys. Lett. 1998, 72, 1697–1699. [Google Scholar] [CrossRef]
- Tsuchiya, R.; Kawasaki, M.; Kubota, H.; Nishino, J.; Sato, H.; Akoh, H.; Koinuma, H. YBa2Cu3O7−δ trilayer junction with nm thick PrGaO3 barrier. Appl. Phys. Lett. 1997, 71, 1570–1572. [Google Scholar] [CrossRef]
- Baeumer, C.; Xu, C.; Gunkel, F.; Raab, N.; Heinen, R.A.; Koehl, A.; Dittmann, R. Surface Termination Conversion during SrTiO3 Thin Film Growth Revealed by X-ray Photoelectron Spectroscopy. Sci. Rep. 2015, 5, 11829. [Google Scholar] [CrossRef]


| Symmetry | Space Group | Unit Cell (Å) | mp (°C) | ε | ⍴ (g/cm3) | tan δ | α (K−1) |
|---|---|---|---|---|---|---|---|
| Orthorhombic, GdFeO3 type | Pbnm | ao = 5.428 bo = 5.498 co = 7.708 | 1605 | 20–25 (1 MHz) | 7.57 | 3 × 10−3 | 5.8 × 10−6 |
| Material | Td (°C) | Pd (Pa) | Ed (J/cm2) | f (Hz) | dst (mm) |
|---|---|---|---|---|---|
| TiO2 | 850 | 7.5 | 2 | 1 | 45 |
| SrO | 750 | 10 | 2 | 1 | 55 |
| BaO | 590 | 15 | 1.3 | 1 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leca, V. Termination Control of (001) and (110) NdGaO3 Single-Crystal Substrates by Selective Chemical Etching. Crystals 2022, 12, 1791. https://doi.org/10.3390/cryst12121791
Leca V. Termination Control of (001) and (110) NdGaO3 Single-Crystal Substrates by Selective Chemical Etching. Crystals. 2022; 12(12):1791. https://doi.org/10.3390/cryst12121791
Chicago/Turabian StyleLeca, Victor. 2022. "Termination Control of (001) and (110) NdGaO3 Single-Crystal Substrates by Selective Chemical Etching" Crystals 12, no. 12: 1791. https://doi.org/10.3390/cryst12121791




