Fumarate Based Metal–Organic Framework: An Effective Catalyst for the Transesterification of Used Vegetable Oil
Abstract
:1. Introduction
2. Materials and Methods
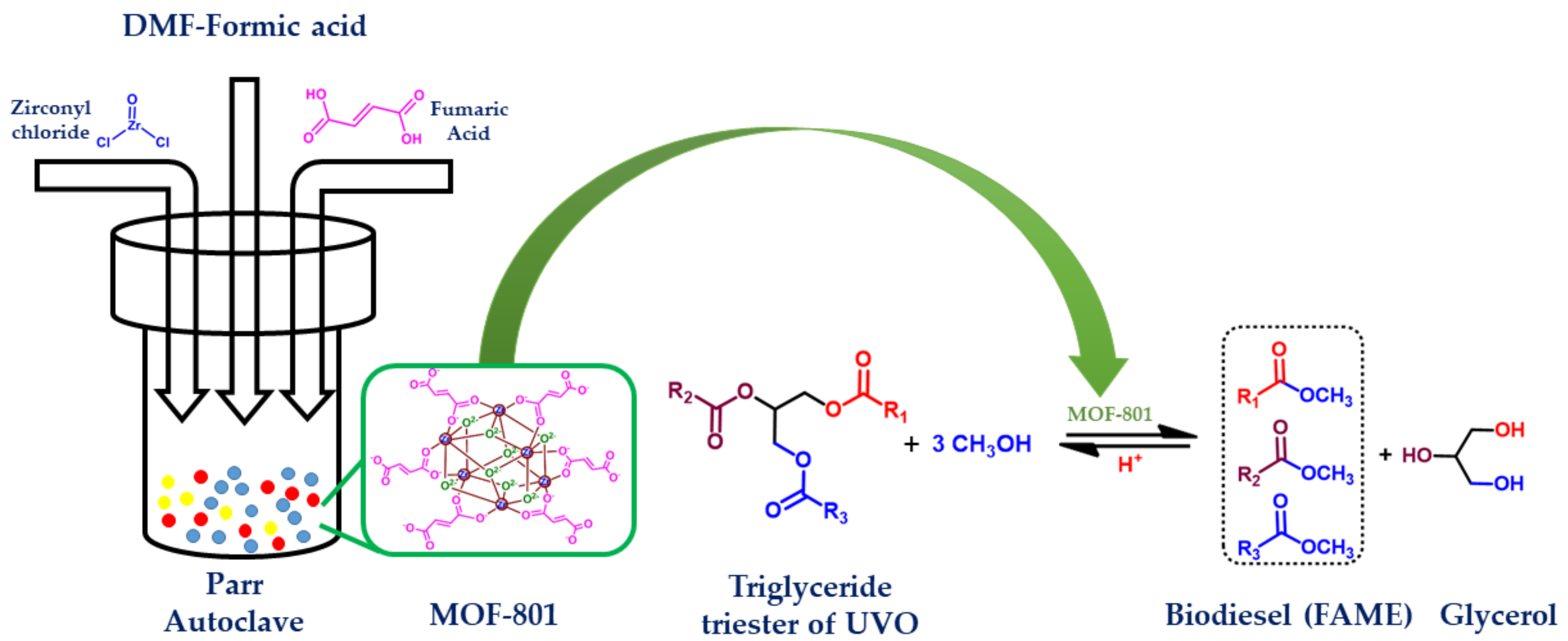
2.1. Preparation of MOF-801
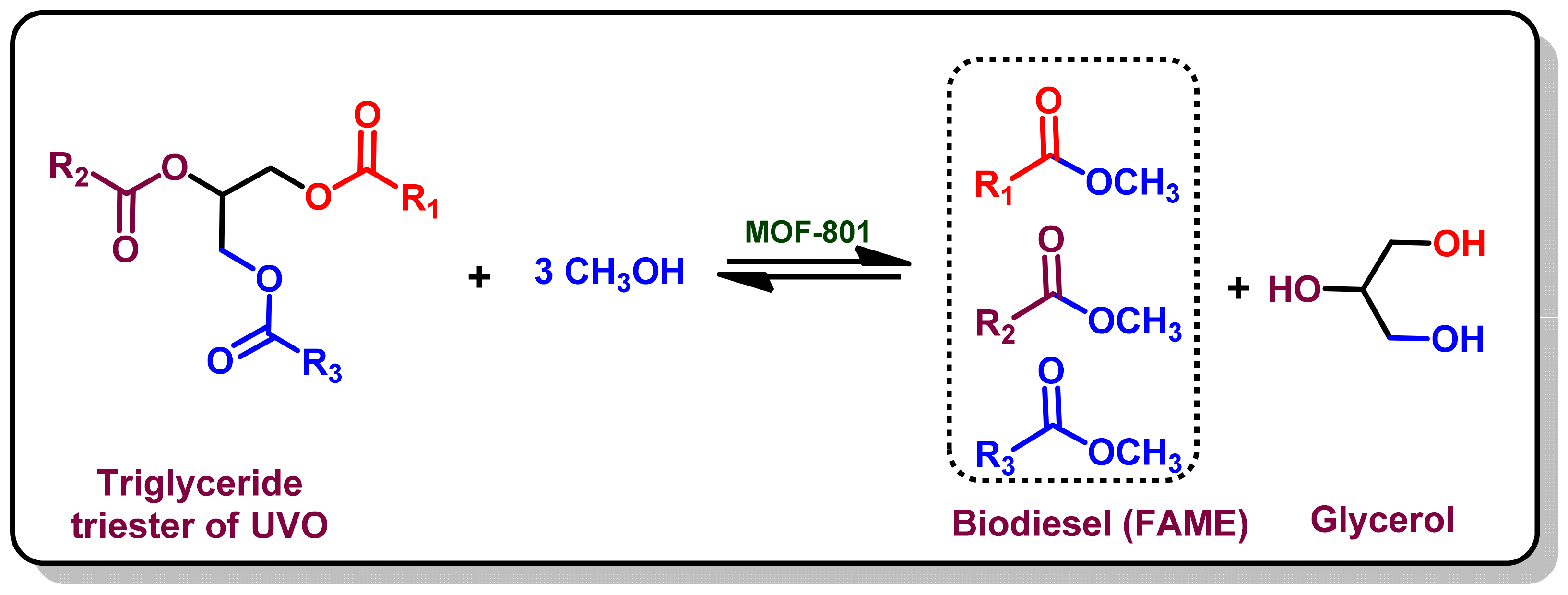
2.2. Transesterification of Used Vegetables Oil (UVO)
3. Results and Discussions
3.1. X-ray Powder Diffraction (XRD) Analysis
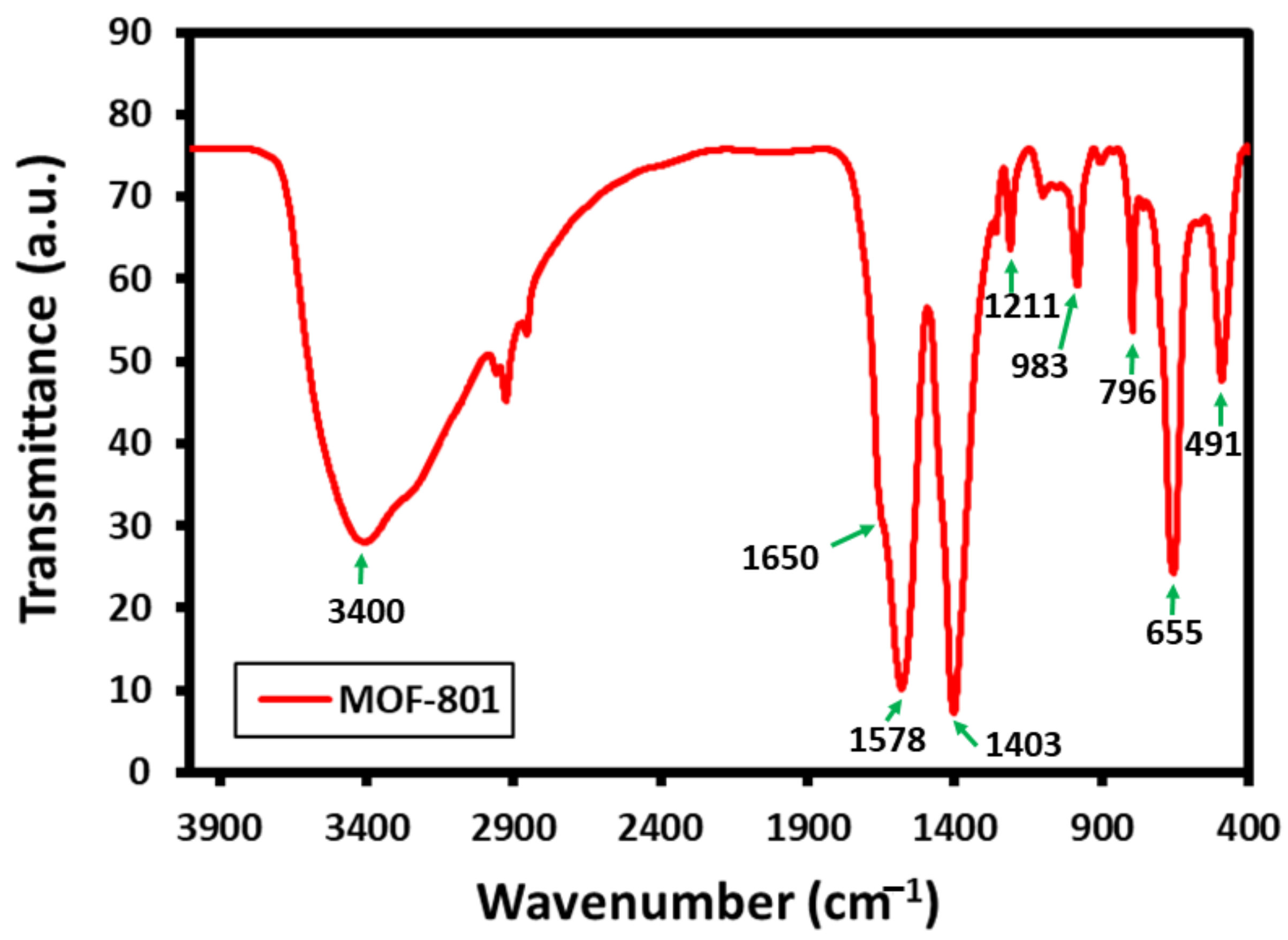
3.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
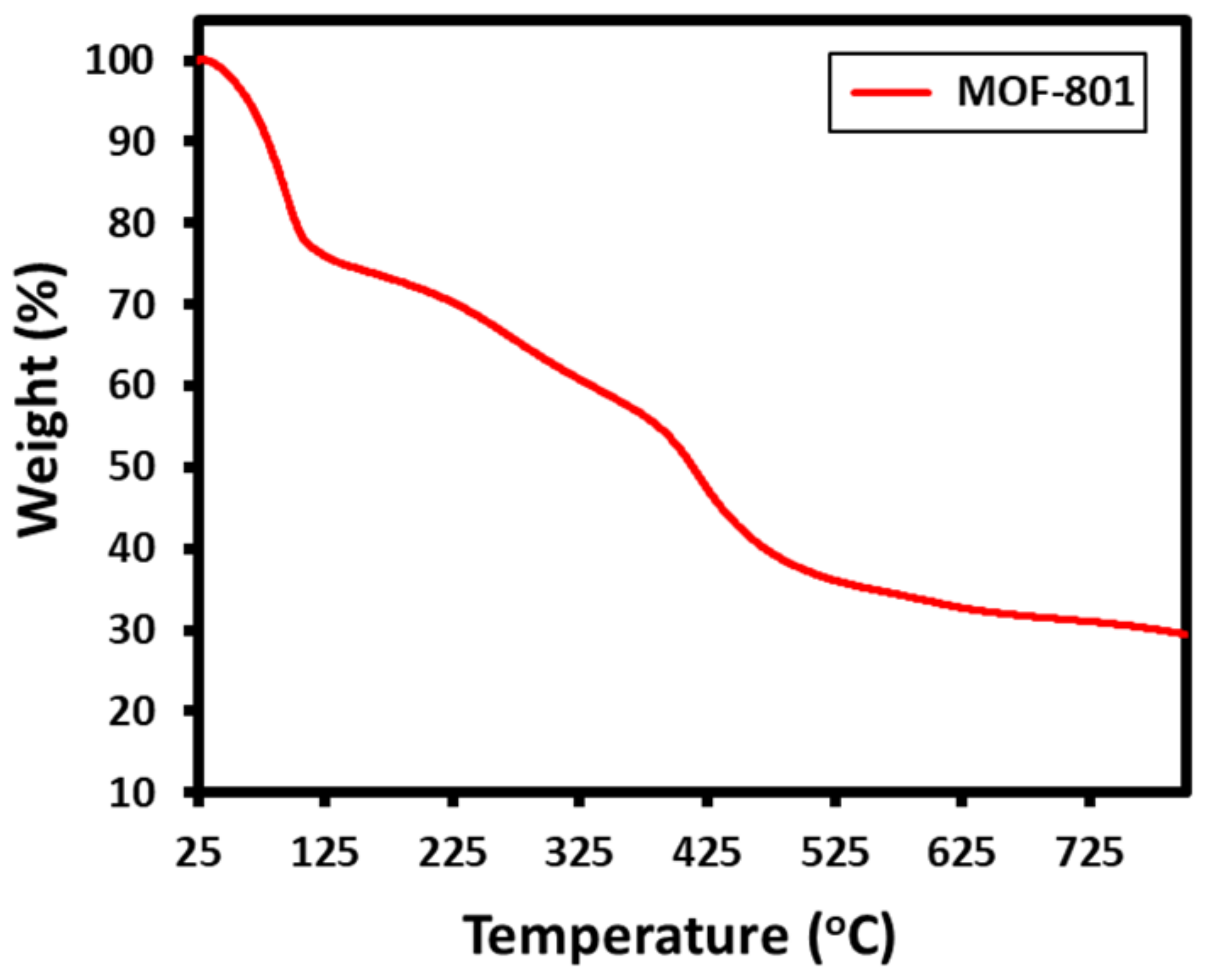
3.3. Thermogravimetric Analysis (TGA)
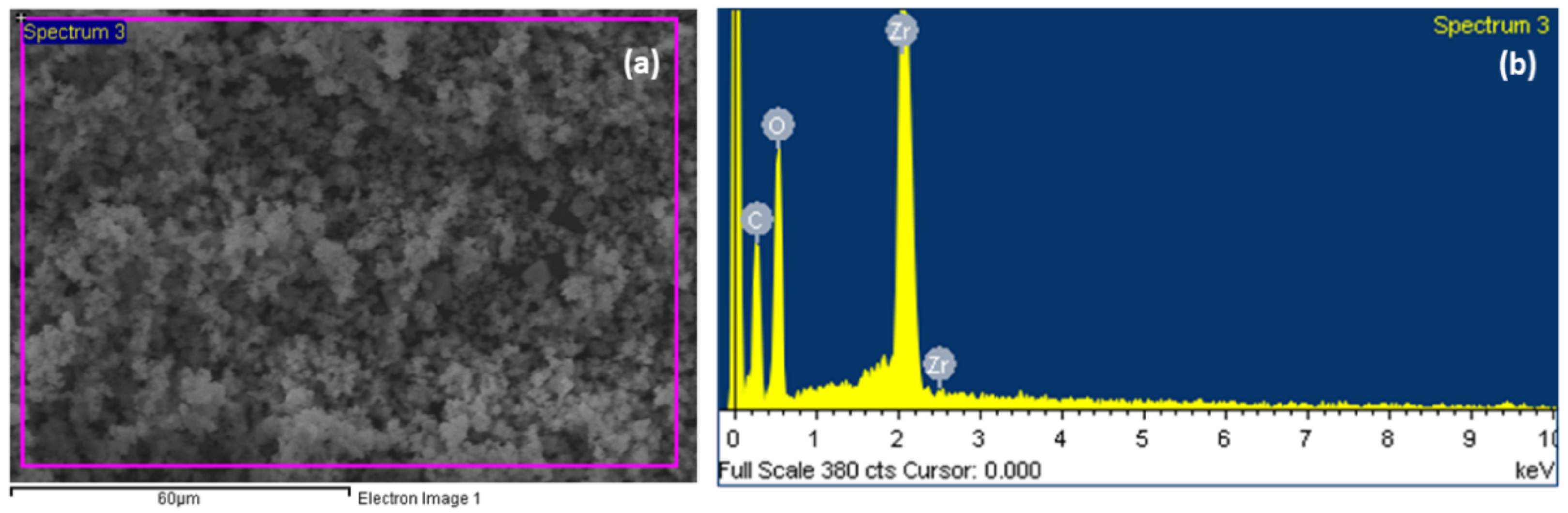
3.4. Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX) Analysis
3.5. N2 Isotherm Analysis
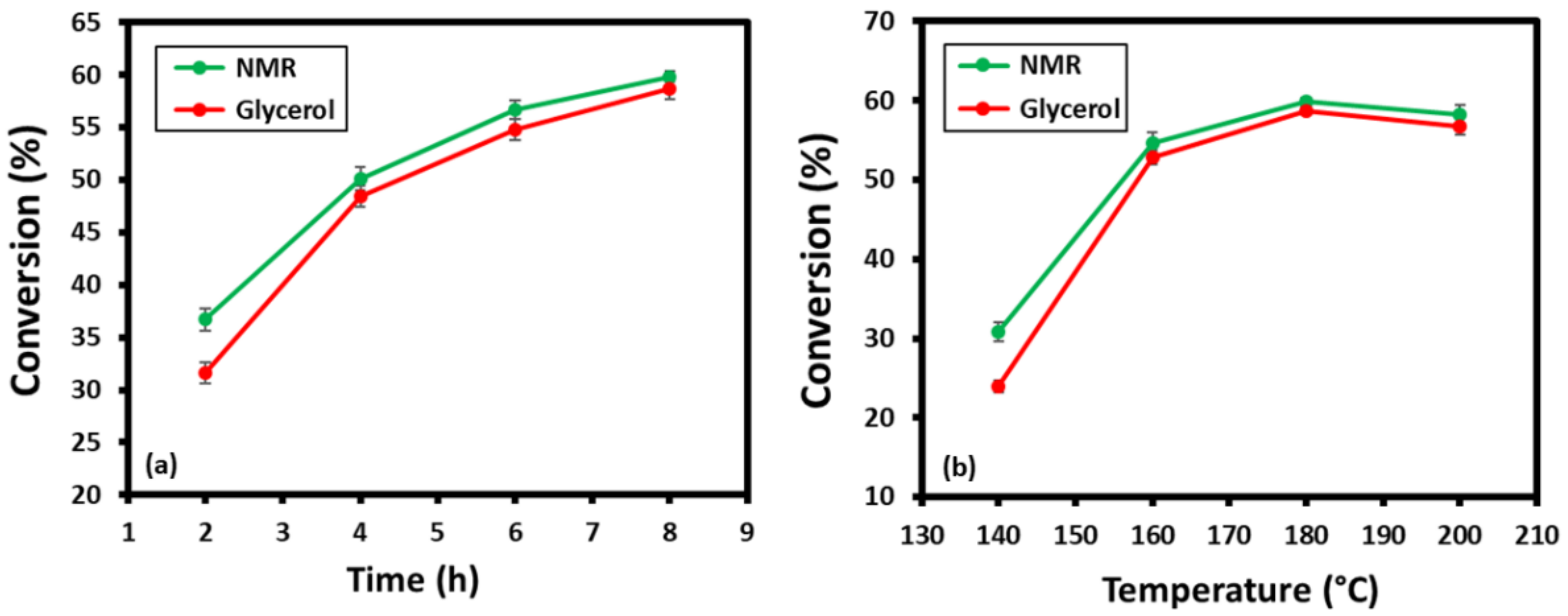
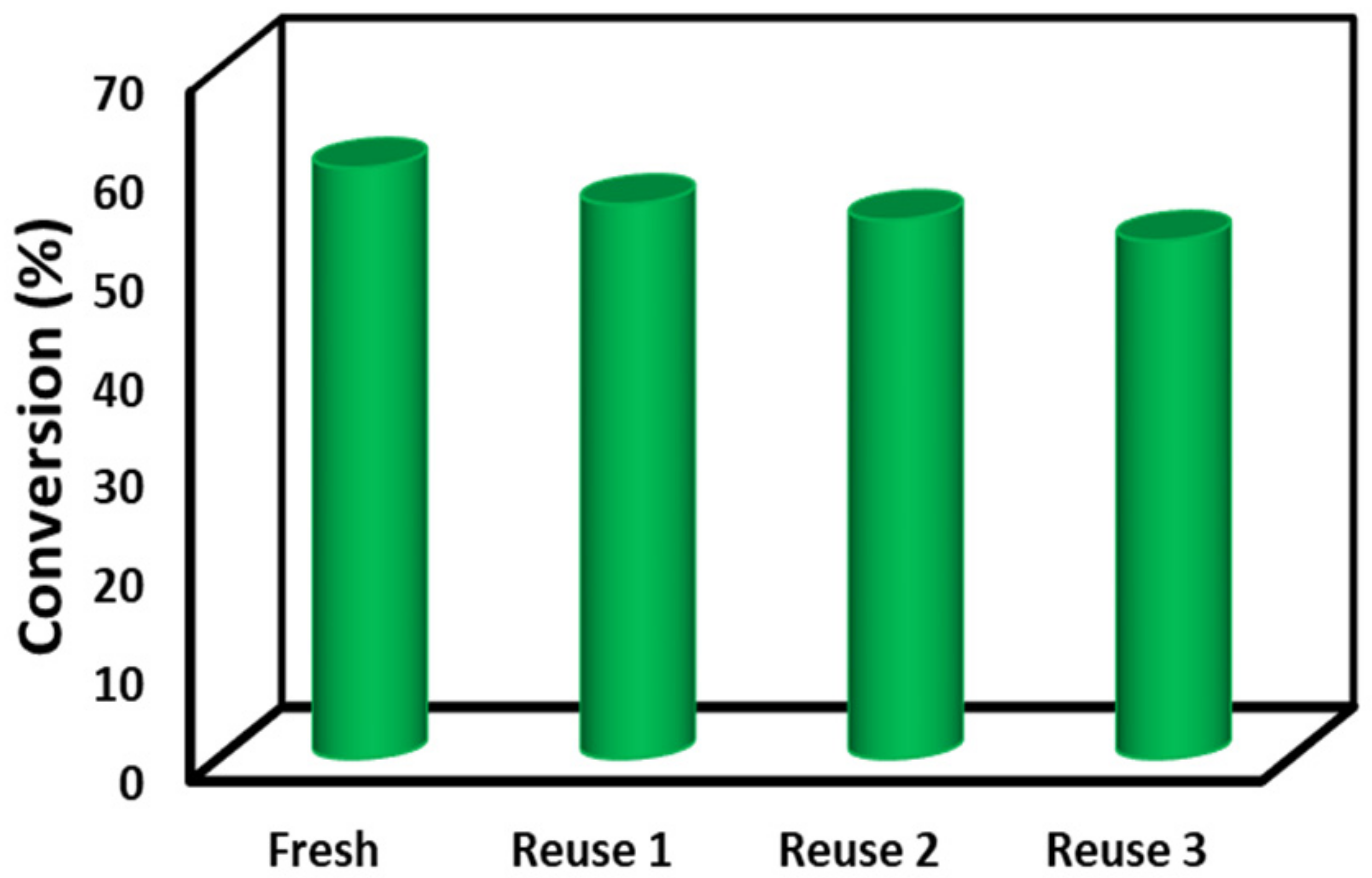
3.6. Catalytic Evaluation of MOF-801
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Dacosta, C.; Shen, L.; Schakel, W.; Ramirez, A.; Kramer, G.J. Potential and challenges of low-carbon energy options: Comparative assessment of alternative fuels for the transport sector. Appl. Energy 2019, 236, 590–606. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.; Taufiq-Yap, Y. Biofuels: Past, Present, Future. In Innovations in Sustainable Energy and Cleaner Environment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 489–504. [Google Scholar]
- Nguyen, D.C. Development of biofuels for a green future. Eur. J. Eng. Technol. Res. 2019, 4, 115–120. [Google Scholar]
- Liu, H.; Huang, Y.; Yuan, H.; Yin, X.; Wu, C. Life cycle assessment of biofuels in China: Status and challenges. Renew. Sustain. Energy Rev. 2018, 97, 301–322. [Google Scholar] [CrossRef]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. A review of cleaner intensification technologies in biodiesel production. J. Clean. Prod. 2017, 146, 181–193. [Google Scholar] [CrossRef]
- Okoye, P.; Hameed, B. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R. Trends in catalytic production of biodiesel from various feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Janaun, J.; Ellis, N. Perspectives on biodiesel as a sustainable fuel. Renew. Sustain. Energy Rev. 2010, 14, 1312–1320. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Shi, Y.; Yuan, H.; Shi, J. Catalysts from renewable resources for biodiesel production. Energy Convers. Manag. 2018, 178, 277–289. [Google Scholar] [CrossRef]
- Vicente, G.; Martınez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Benessere, V.; Cucciolito, M.E.; Esposito, R.; Lega, M.; Turco, R.; Ruffo, F.; Di Serio, M. A novel and robust homogeneous supported catalyst for biodiesel production. Fuel 2016, 171, 1–4. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Kumar, A.; Raheman, H. Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass Bioenergy 2007, 31, 569–575. [Google Scholar] [CrossRef]
- Macario, A.; Giordano, G.; Onida, B.; Cocina, D.; Tagarelli, A.; Giuffrè, A.M. Biodiesel production process by homogeneous/heterogeneous catalytic system using an acid–base catalyst. Appl. Catal. A Gen. 2010, 378, 160–168. [Google Scholar] [CrossRef]
- De Lima, A.L.; Ronconi, C.M.; Mota, C.J. Heterogeneous basic catalysts for biodiesel production. Catal. Sci. Technol. 2016, 6, 2877–2891. [Google Scholar] [CrossRef]
- Alsalme, A.; Alsharif, A.A.; Al-Enizi, H.; Khan, M.; Alshammari, S.G.; Alotaibi, M.A.; Khan, R.A.; Siddiqui, M.R.H. Probing the catalytic efficiency of supported heteropoly acids for esterification: Effect of weak catalyst support interactions. J. Chem. 2018, 2018, 7037461. [Google Scholar] [CrossRef] [Green Version]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Meira, M.; Quintella, C.; Ribeiro, E.; Silva, H.; Guimarães, A. Overview of the challenges in the production of biodiesel. Biomass Convers. Biorefinery 2015, 5, 321–329. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Hayyan, A.; Alam, M.Z.; Mirghani, M.E.; Kabbashi, N.A.; Hakimi, N.I.N.M.; Siran, Y.M.; Tahiruddin, S. Reduction of high content of free fatty acid in sludge palm oil via acid catalyst for biodiesel production. Fuel Process. Technol. 2011, 92, 920–924. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Gebremariam, S.; Marchetti, J. Economics of biodiesel production. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Plechkova, N.V.; Earle, M.J.; Fabregat, A.; Stüber, F.; Fortuny, A.; Font, J.; Bengoa, C. Biodiesel production from sewage sludge lipids catalysed by Brønsted acidic ionic liquids. Appl. Catal. B Environ. 2016, 181, 738–746. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Oduwale, A.I.; Owootomo, Y.; Obisesan, O.R.; Elugoke, S.E.; Durodola, S.S.; Akintunde, S.B.; Oluwafemi, O.S. Biodiesel potential of used vegetable oils transesterified with biological catalysts. Energy Rep. 2020, 6, 2861–2871. [Google Scholar] [CrossRef]
- Kuniyil, M.; Kumar, J.S.; Adil, S.F.; Assal, M.E.; Shaik, M.R.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H. Production of biodiesel from waste cooking oil using ZnCuO/N-doped graphene nanocomposite as an efficient heterogeneous catalyst. Arab. J. Chem. 2021, 14, 102982. [Google Scholar] [CrossRef]
- Bilal, M.; Qamar, S.A.; Yadav, V.; Cheng, H.; Khan, M.; Adil, S.F.; Taherzadeh, M.J.; Iqbal, H.M. Exploring the potential of ligninolytic armory for lignin valorization–A way forward for sustainable and cleaner production. J. Clean. Prod. 2021, 326, 129420. [Google Scholar] [CrossRef]
- Clohessy, J.; Kwapinski, W. Carbon-based catalysts for biodiesel production—A review. Appl. Sci. 2020, 10, 918. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal–organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Cong, W.-J.; Nanda, S.; Li, H.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Metal–organic framework-based functional catalytic materials for biodiesel production: A review. Green Chem. 2021, 23, 2595–2618. [Google Scholar] [CrossRef]
- Ma, X.; Liu, F.; Helian, Y.; Li, C.; Wu, Z.; Li, H.; Chu, H.; Wang, Y.; Wang, Y.; Lu, W. Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review. Energy Convers. Manag. 2021, 229, 113760. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Cheng, J.; Li, H.; Ma, P. An Overview of Metal-organic Frameworks-based Acid/Base Catalysts for Biofuel Synthesis. Curr. Org. Chem. 2020, 24, 1876–1891. [Google Scholar] [CrossRef]
- Trickett, C.A.; Popp, T.M.O.; Su, J.; Yan, C.; Weisberg, J.; Huq, A.; Urban, P.; Jiang, J.; Kalmutzki, M.J.; Liu, Q. Identification of the strong Brønsted acid site in a metal–organic framework solid acid catalyst. Nat. Chem. 2019, 11, 170–176. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, J.; Zhang, X.; Liao, Y.; Wang, R.; Zhang, K.; Lyu, J.; Farha, O.K.; Hupp, J.T. Node-Accessible Zirconium MOFs. J. Am. Chem. Soc. 2020, 142, 21110–21121. [Google Scholar] [CrossRef]
- Deria, P.; Gómez-Gualdrón, D.A.; Hod, I.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. Framework-topology-dependent catalytic activity of zirconium-based (porphinato) zinc (II) MOFs. J. Am. Chem. Soc. 2016, 138, 14449–14457. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Nauert, S.L.; Buru, C.T.; Rimoldi, M.; Choi, H.; Schweitzer, N.M.; Hupp, J.T.; Farha, O.K.; Notestein, J.M. Pushing the limits on metal–organic frameworks as a catalyst support: NU-1000 supported tungsten catalysts for o-xylene isomerization and disproportionation. J. Am. Chem. Soc. 2018, 140, 8535–8543. [Google Scholar] [CrossRef] [PubMed]
- Mautschke, H.-H.; Drache, F.; Senkovska, I.; Kaskel, S.; i Xamena, F.L. Catalytic properties of pristine and defect-engineered Zr-MOF-808 metal organic frameworks. Catal. Sci. Technol. 2018, 8, 3610–3616. [Google Scholar] [CrossRef]
- Cirujano, F.; Llabrés i Xamena, F.X. Tuning the Catalytic Properties of UiO-66 Metal–Organic Frameworks: From Lewis to Defect-Induced Brønsted Acidity. J. Phys. Chem. Lett. 2020, 11, 4879–4890. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Jiang, M.; Yao, J. Tailoring the properties of UiO-66 through defect engineering: A review. Ind. Eng. Chem. Res. 2019, 58, 17646–17659. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; García Gómez, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Ke, F.; Peng, C.; Zhang, T.; Zhang, M.; Zhou, C.; Cai, H.; Zhu, J.; Wan, X. Fumarate-based metal-organic frameworks as a new platform for highly selective removal of fluoride from brick tea. Sci. Rep. 2018, 8, 939. [Google Scholar] [CrossRef] [Green Version]
- Butova, V.V.; Pankin, I.A.; Burachevskaya, O.A.; Vetlitsyna-Novikova, K.S.; Soldatov, A.V. New fast synthesis of MOF-801 for water and hydrogen storage: Modulator effect and recycling options. Inorg. Chim. Acta 2021, 514, 120025. [Google Scholar] [CrossRef]
- Diab, K.E.; Salama, E.; Hassan, H.S.; Abd El-moneim, A.; Elkady, M.F. Biocompatible MIP-202 Zr-MOF tunable sorbent for cost-effective decontamination of anionic and cationic pollutants from waste solutions. Sci. Rep. 2021, 11, 6619. [Google Scholar] [CrossRef]
- Butova, V.V.; Burachevskaya, O.A.; Ozhogin, I.V.; Borodkin, G.S.; Starikov, A.G.; Bordiga, S.; Damin, A.; Lillerud, K.P.; Soldatov, A.V. UiO-66 type MOFs with mixed-linkers—1,4-Benzenedicarboxylate and 1,4-naphthalenedicarboxylate: Effect of the modulator and post-synthetic exchange. Microporous Mesoporous Mater. 2020, 305, 110324. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.V.R.K.; Dudhe, P.; Chelvam, V. Role of oxygen defects in basicity of Se doped ZnO nanocatalyst for enhanced triglyceride transesterification in biodiesel production. Catal. Commun. 2021, 149, 106258. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Nik Abdul Khalil, N.N.A.; Islam, A.; Rashid, U.; Ibrahim, S.F.; Sinar Mashuri, S.I.; Taufiq-Yap, Y.H. Preparation of Na2O supported CNTs nanocatalyst for efficient biodiesel production from waste-oil. Energy Convers. Manag. 2020, 205, 112445. [Google Scholar] [CrossRef]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal–air batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Jahan, I.; Rupam, T.H.; Palash, M.L.; Rocky, K.A.; Saha, B.B. Energy efficient green synthesized MOF-801 for adsorption cooling applications. J. Mol. Liq. 2022, 345, 117760. [Google Scholar] [CrossRef]
- Kim, Z.H.; Park, H.; Ryu, Y.-J.; Shin, D.-W.; Hong, S.-J.; Tran, H.-L.; Lim, S.-M.; Lee, C.-G. Algal biomass and biodiesel production by utilizing the nutrients dissolved in seawater using semi-permeable membrane photobioreactors. J. Appl. Phycol. 2015, 27, 1763–1773. [Google Scholar] [CrossRef]



| Catalyst | Oil (g) | Amount of Catalyst | Product Yield (g) | Conversion (%) | |||
|---|---|---|---|---|---|---|---|
| wt.% to Oil | Amount (g) | Biodiesel | Glycerol | 1H-NMR | Yield of Glycerol | ||
| MOF-801 | 1.5 | 5 | 0.075 | 1.311 | 0.055 | 40.3 | 36.1 |
| MOF-801 | 10 | 0.150 | 1.406 | 0.091 | 59.8 | 58.7 | |
| MOF-801 | 15 | 0.225 | 1.399 | 0.090 | 59.6 | 58.1 | |
| MOF-801 | 20 | 0.300 | 1.394 | 0.087 | 57.4 | 56.1 | |
| MOF-801/HCl | 10 | 0.150 | 1.482 | 0.107 | 70.0 | 69 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik, M.R.; Adil, S.F.; ALOthman, Z.A.; Alduhaish, O.M. Fumarate Based Metal–Organic Framework: An Effective Catalyst for the Transesterification of Used Vegetable Oil. Crystals 2022, 12, 151. https://doi.org/10.3390/cryst12020151
Shaik MR, Adil SF, ALOthman ZA, Alduhaish OM. Fumarate Based Metal–Organic Framework: An Effective Catalyst for the Transesterification of Used Vegetable Oil. Crystals. 2022; 12(2):151. https://doi.org/10.3390/cryst12020151
Chicago/Turabian StyleShaik, Mohammed Rafi, Syed Farooq Adil, Zeid A. ALOthman, and Osamah M. Alduhaish. 2022. "Fumarate Based Metal–Organic Framework: An Effective Catalyst for the Transesterification of Used Vegetable Oil" Crystals 12, no. 2: 151. https://doi.org/10.3390/cryst12020151








