Enhanced Microwave Dielectric Properties and Sintering Behaviors of Mg2SiO4-Li2TiO3-LiF Ceramics by Adding CaTiO3 for LTCC and GPS Antenna Applications
Abstract
:1. Introduction
2. Materials and Methods
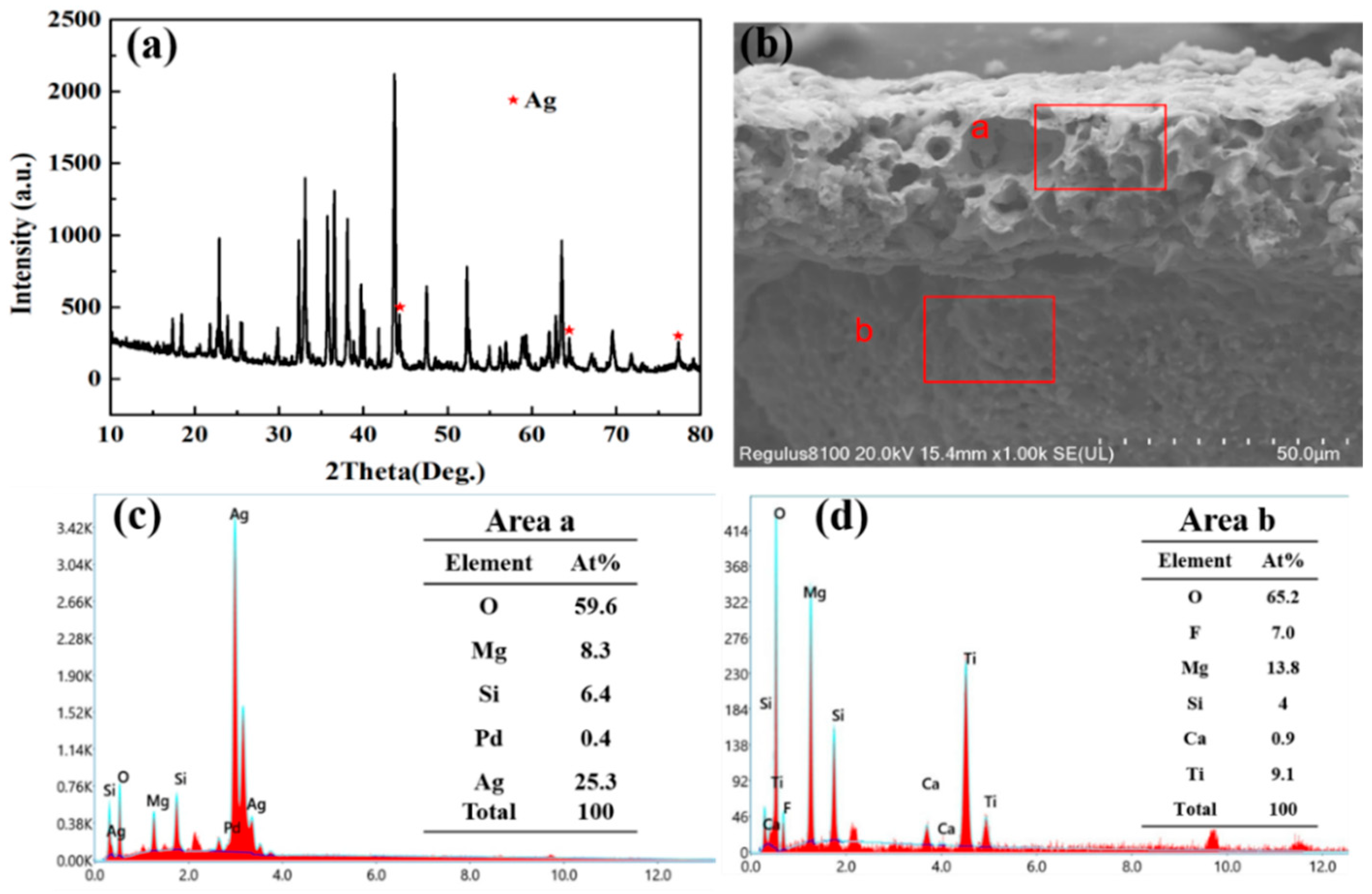
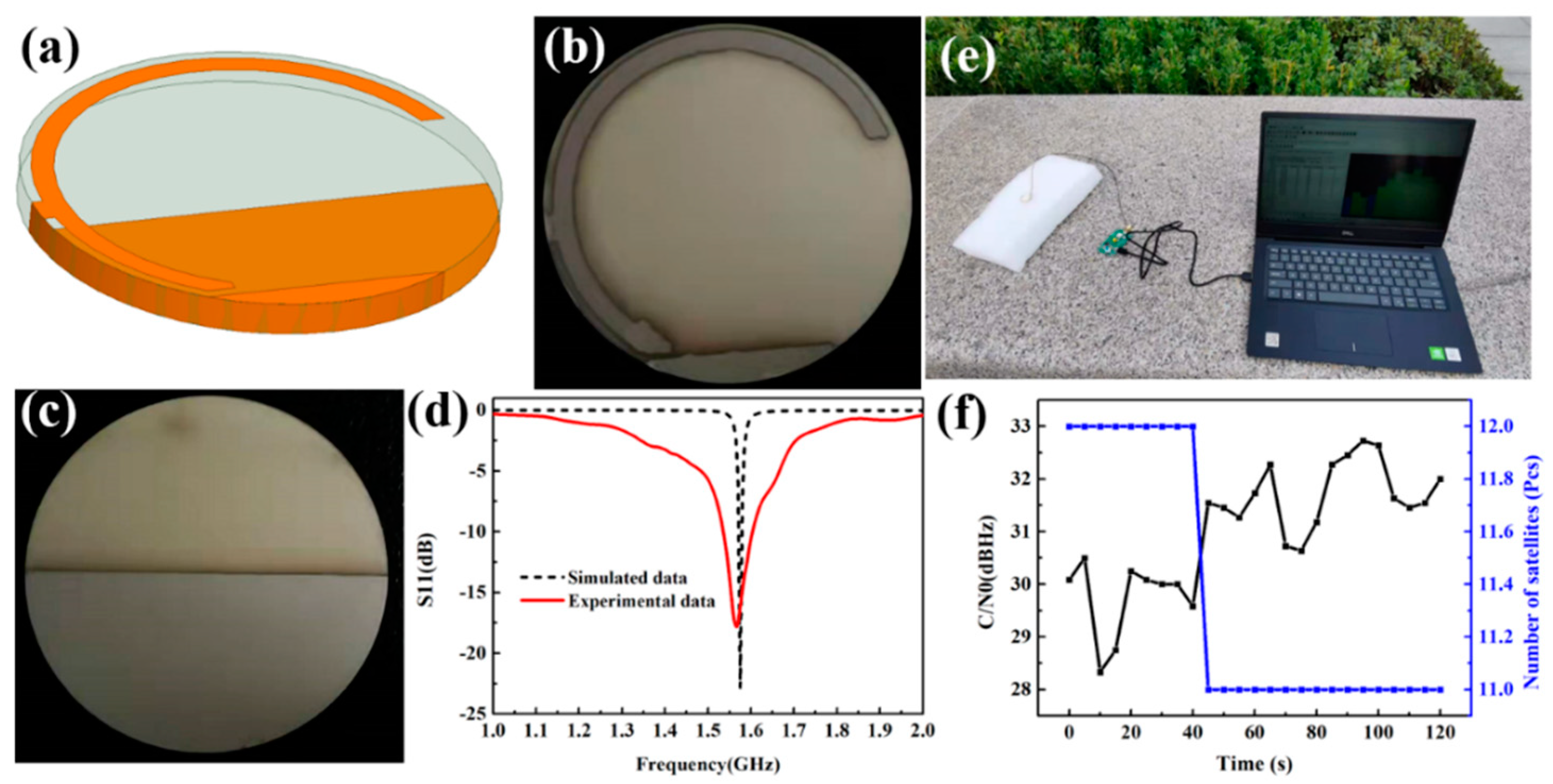
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, X.; Du, K.; Li, J.; Muhammad, R.; Lu, W.; Wang, X.; Lei, W. Crystal structures and microwave dielectric properties of novel low-permittivity Ba1−xSrxZnSi3O8 ceramics. Mater. Res. Bull. 2019, 112, 178–181. [Google Scholar] [CrossRef]
- Lai, Y.; Su, H.; Wang, G.; Tang, X.; Liang, X.; Huang, X.; Li, Y.; Zhang, H.; Ye, C.; Wang, X.R. Improved microwave dielectric properties of CaMgSi2O6 ceramics through CuO doping. J. Alloys Compd. 2019, 772, 40–48. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, X.; Zhang, H.; Liang, X.; Huang, X.; Li, Y.; Su, H. Correlation between structure and microwave dielectric properties of low-temperature-fired Mg2SiO4 ceramics. Mater. Res. Bull. 2018, 99, 496–502. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Yang, W.; Feng, S.; Ma, H.; Jia, G.; Xu, S. Effects of Al2O3 addition on the sintering behavior and microwave dielectric properties of CaSiO3 ceramics. J. Eur. Ceram. Soc. 2012, 32, 541–545. [Google Scholar] [CrossRef]
- Li, J.; Fang, L.; Luo, H.; Khaliq, J.; Tang, Y.; Li, C. Li4WO5: A temperature stable low-firing microwave dielectric ceramic with rock salt structure. J. Eur. Ceram. Soc. 2016, 36, 243–246. [Google Scholar] [CrossRef]
- Zhou, D.; Randall, C.A.; Pang, L.; Wang, H.; Guo, J.; Zhang, G.; Wu, X.; Shui, L.; Yao, X. Microwave Dielectric Properties of Li2WO4 Ceramic with Ultra-Low Sintering Temperature. J. Am. Ceram. Soc. 2011, 94, 348–350. [Google Scholar] [CrossRef]
- Dong, H.L.; Hu, C.X.; Wang, W.J.; Bao, H.P.; Liu, W.J.; Yang, B. Novel Low-Permittivity, Low-Sintering-Temperature Na2WO4 Microwave Dielectric Ceramics for LTCC Applications. J. Ceram. Sci. Technol. 2018, 9, 471–475. [Google Scholar]
- Cheng, K.; Li, C.; Xiang, H.; Sun, Y.; Fang, L. LiYGeO4: Novel low-permittivity microwave dielectric ceramics with intrinsic low sintering temperature. Mater. Lett. 2018, 228, 96–99. [Google Scholar] [CrossRef]
- Yin, C.; Xiang, H.; Li, C.; Porwal, H.; Fang, L. Low-temperature sintering and thermal stability of Li2GeO3-based microwave dielectric ceramics with low permittivity. J. Am. Ceram. Soc. 2018, 101, 4608–4614. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Kweon, S.; Im, M.; Nahm, S. Low-temperature sintering and microwave dielectric properties of B2O3-added ZnO-deficient Zn2GeO4 ceramics for advanced substrate application. J. Eur. Ceram. Soc. 2018, 38, 4682–4688. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, M.; Duan, L.; Chen, J.; Li, C.; Xiang, H.; Fang, L. Structure, microwave dielectric properties, and infrared reflectivity spectrum of olivine type Ca2GeO4 ceramic. J. Eur. Ceram. Soc. 2019, 39, 2354–2359. [Google Scholar] [CrossRef]
- Luo, H.; Fang, L.; Xiang, H.; Tang, Y.; Li, C. Two novel low-firing germanates Li2MGe3O8 (M = Ni, Co) microwave dielectric ceramics with spinel structure. Ceram. Int. 2017, 43, 1622–1627. [Google Scholar] [CrossRef]
- Chang, S.; Pai, H.; Tseng, C.; Tsai, C. Microwave dielectric properties of ultra-low temperature fired Li3BO3 ceramics. J. Alloys Compd. 2017, 698, 814–818. [Google Scholar] [CrossRef]
- Ren, J.; Bi, K.; Fu, X.; Peng, Z. Novel Al2Mo3O12-based temperature-stable microwave dielectric ceramics for LTCC applications. J. Mater. Chem. C 2018, 6, 11465–11470. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, S.; Xiao, M. The microwave dielectric properties and crystal structure of low temperature sintering LiNiPO4 ceramics. J. Eur. Ceram. Soc. 2018, 38, 4433–4439. [Google Scholar] [CrossRef]
- Peng, R.; Li, Y.; Yu, G.; Lu, Y.; Li, S. Effect of Co2+ Substitution on the Microwave Dielectric Properties of LiZnPO4 Ceramics. J. Electron. Mater. 2018, 47, 7281–7287. [Google Scholar] [CrossRef]
- Penn, S.J.; Alford, N.M.; Templeton, A.; Wang, X.R.; Xu, M.S.; Reece, M.; Schrapel, K. Effect of porosity and grain size on the microwave dielectric properties of sintered alumina. J. Am. Ceram. Soc. 1997, 80, 1885–1888. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.H.; Zhu, J.Y.; Chen, X.M. B2O3-modified fused silica microwave dielectric materials with ultra-low dielectric constant. J. Eur. Ceram. Soc. 2015, 35, 1799–1805. [Google Scholar] [CrossRef]
- Tsunooka, T.; Androu, M.; Higashida, Y.; Sugiura, H.; Ohsato, H. Effects of TiO2 on sinterability and dielectric properties of high-Q forsterite ceramics. J. Eur. Ceram. Soc. 2003, 23, 2573–2578. [Google Scholar] [CrossRef]
- Hameed, I.; Li, L.; Liu, X.Q.; Chen, X.M. Ultra low loss (Mg1−xCax)2SiO4 dielectric ceramics (x = 0 to 0.15) for millimeter wave applications. J. Am. Ceram. Soc. 2022, 105, 2010–2019. [Google Scholar] [CrossRef]
- Song, K.X.; Chen, X.M. Phase evolution and microwave dielectric characteristics of Ti-substituted Mg2SiO4 forsterite ceramics. Mater. Lett. 2008, 62, 520–522. [Google Scholar] [CrossRef]
- Cheng, Z.; Hu, X.; Li, Y.; Ling, Z. Fabrication and Microwave Dielectric Properties of Mg2SiO4-LiMgPO4-TiO2 Composite Ceramics. J. Am. Ceram. Soc. 2016, 99, 2688–2692. [Google Scholar] [CrossRef]
- Kagomiya, I.; Sugihara, J.; Kakimoto, K.; Ohsato, H. Mg2SiO4-TiO2 composite ceramics prepared using a liquid phase deposition process. J. Electroceram. 2009, 22, 327–333. [Google Scholar] [CrossRef]
- Dou, G.; Zhou, D.; Guo, M.; Gong, S.; Hu, Y. Low-temperature sintered Mg2SiO4-CaTiO3 ceramics with near-zero temperature coefficient of resonant frequency. J. Mater. Sci.-Mater. Electron. 2013, 24, 1431–1438. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, P.; Fu, Z.; Zhao, L.; Ren, Y. Microwave dielectric properties of low-fired (1−x)Mg2SiO4−xCa0.9Sr0.1TiO3 ceramics by using nanopowders from high energy ball milling. J. Mater. Sci. Mater. Electron. 2017, 28, 15398–15404. [Google Scholar] [CrossRef]
- Song, K.X.; Chen, X.M.; Fan, X.C. Effects of Mg/Si Ratio on Microwave Dielectric Characteristics of Forsterite Ceramics. J. Am. Ceram. Soc. 2007, 90, 1808–1811. [Google Scholar] [CrossRef]
- He, G.; Li, Z.; Dong, Y.; Wang, G. Effects of excessive magnesium oxide and sintering regime on microstructure and dielectric properties of forsterite ceramics for millimeterwave dielectrics. J. Mater. Sci.-Mater. Electron. 2022, 33, 3129–3138. [Google Scholar] [CrossRef]
- Roshni, S.B.; Arun, S.; Sebastian, M.T.; Mohanan, P.; Surendran, K.P. Low κ Mg2SiO4 ceramic tapes and their role as screen printed microstrip patch antenna substrates. Mater. Sci. Eng. B 2021, 264, 114947. [Google Scholar] [CrossRef]
- Ma, J.; Yang, T.; Fu, Z.; Liu, P.; Feng, Q.; Zhao, L. Low-fired Mg2SiO4-based dielectric ceramics with temperature stable for LTCC applications. J. Alloys Compd. 2017, 695, 3198–3201. [Google Scholar] [CrossRef]
- Sasikala, T.S.; Pavithran, C.; Sebastian, M.T. Effect of lithium magnesium zinc borosilicate glass addition on densification temperature and dielectric properties of Mg2SiO4 ceramics. J. Mater. Sci.-Mater. Electron. 2010, 21, 141–144. [Google Scholar] [CrossRef]
- Yang, H.; Li, E.; Sun, C.; Duan, S.; Yuan, Y.; Tang, B. The Influence of Sintering Temperature on the Microwave Dielectric Properties of Mg2SiO4 Ceramics with CaO-B2O3-SiO2 Addition. J. Electron. Mater. 2017, 46, 1048–1054. [Google Scholar] [CrossRef]
- Meng, S.; Yue, Z.; Zhuang, H.; Zhao, F.; Li, L. Microwave Dielectric Properties of Ba3(VO4)2-Mg2SiO4 Composite Ceramics. J. Am. Ceram. Soc. 2010, 93, 359–361. [Google Scholar] [CrossRef]
- Bian, J.J.; Dong, Y.F. Sintering behavior, microstructure and microwave dielectric properties of Li2+xTiO3 (0 ≤ x ≤ 0.2). Mater. Sci. Eng. B 2011, 176, 147–151. [Google Scholar] [CrossRef]
- Song, X.; Du, K.; Li, J.; Lan, X.; Lu, W.; Wang, X.; Lei, W. Low-fired fluoride microwave dielectric ceramics with low dielectric loss. Ceram. Int. 2019, 45, 279–286. [Google Scholar] [CrossRef]
- Ding, Y.; Bian, J. Structural evolution, sintering behavior and microwave dielectric properties of (1−x)Li2TiO3 + xLiF ceramics. Mater. Res. Bull. 2013, 48, 2776–2781. [Google Scholar] [CrossRef]
- Bian, J.J.; Dong, Y.F. New high Q microwave dielectric ceramics with rock salt structures: (1−x)Li2TiO3 + xMgO system (0 ≤ x≤ 0.5). J. Eur. Ceram. Soc. 2010, 30, 325–330. [Google Scholar] [CrossRef]
- Lai, Y.; Hong, C.; Jin, L.; Tang, X.; Zhang, H.; Huang, X.; Li, J.; Su, H. Temperature stability and high-Qf of low temperature firing Mg2SiO4-Li2TiO3 microwave dielectric ceramics. Ceram. Int. 2017, 43, 16167–16173. [Google Scholar] [CrossRef]
- Kang, S.-J.L. Sintering: Densification, Grain Growth, and Microstructure, 1st ed.; Elsevier Butterworth-Heinemann: Oxford, UK; Burlington, MA, USA, 2005; pp. 67–68. [Google Scholar]
- Wise, P.L.; Reaney, I.M.; Lee, W.E.; Price, T.J.; Iddles, D.M.; Cannell, D.S. Structure-microwave property relations of Ca and Sr titanates. J. Eur. Ceram. Soc. 2001, 21, 2629–2632. [Google Scholar] [CrossRef]
- Tian, F.; Huang, Y.B.; Xu, B.; Ou, G. An Optimized Low-Power-Consumption Tracking Algorithm Based on Navigation Message Structure for Weak Signals. Appl. Mech. Mater. 2015, 733, 854–859. [Google Scholar] [CrossRef]

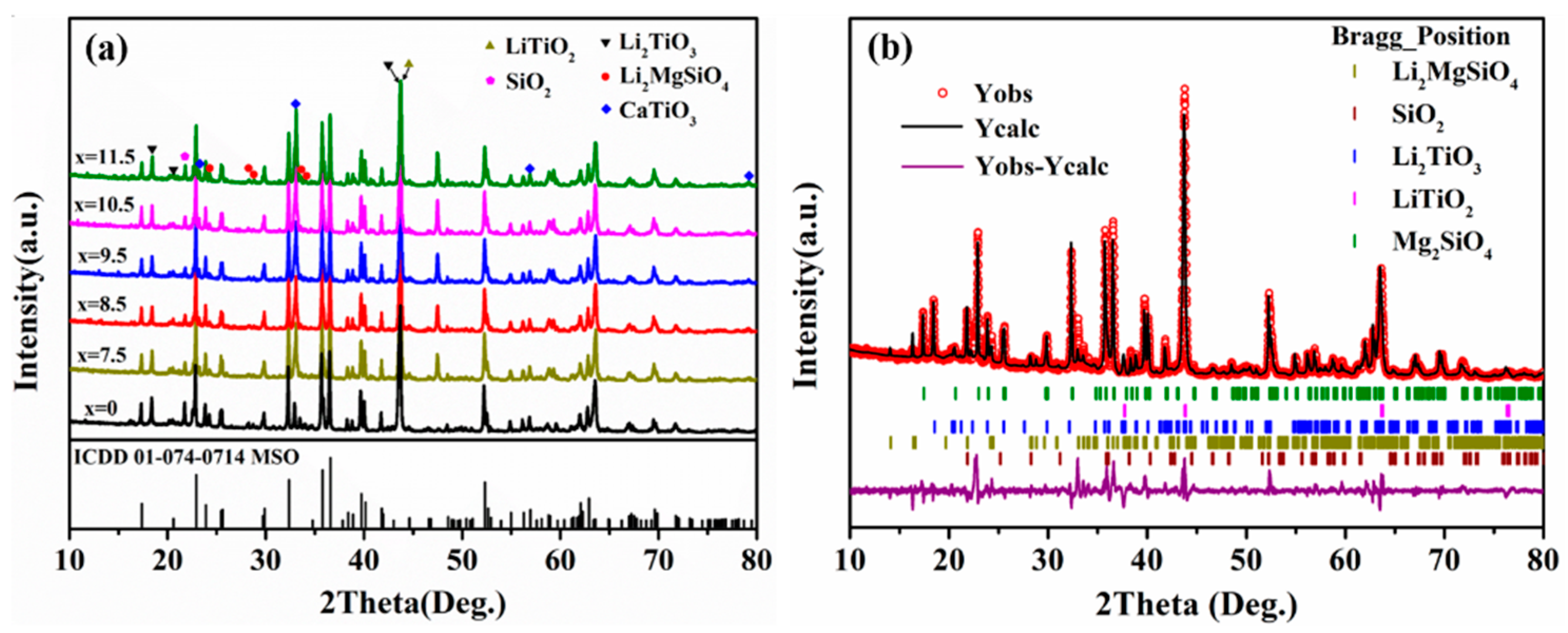
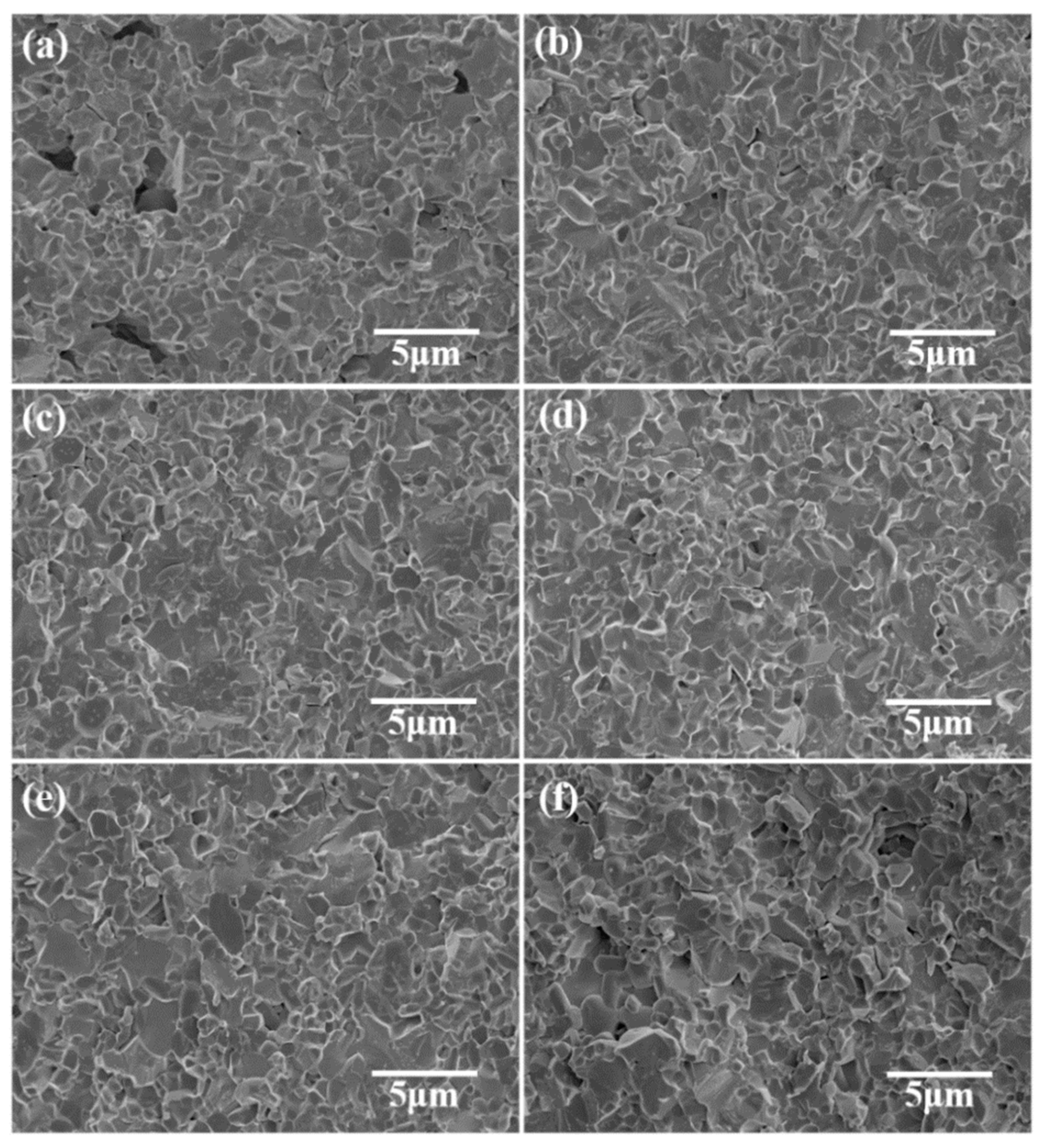
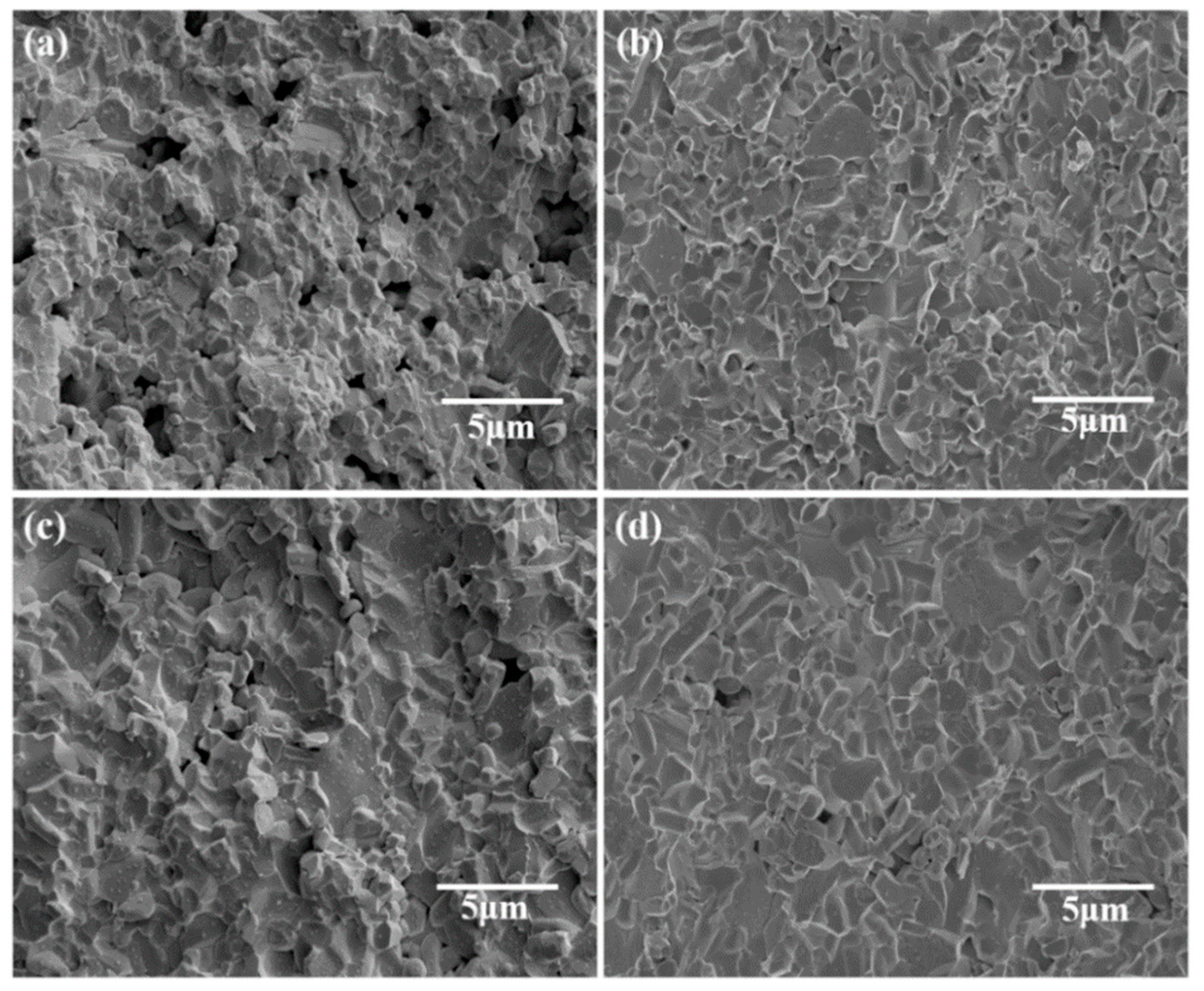
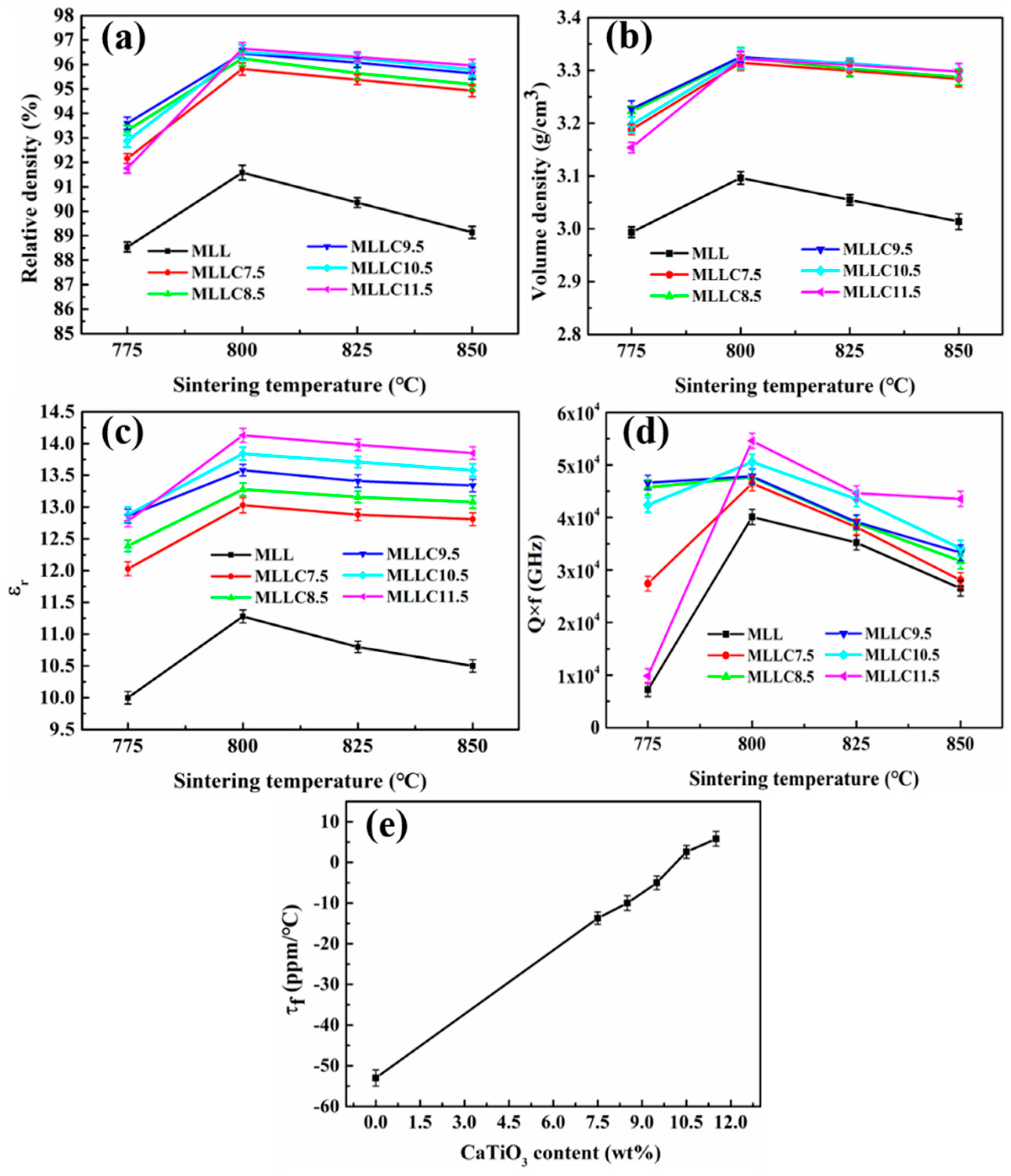
| Phase | Dt (g/cm3) | Vt (×106 pm3) | ρt (wt%) | Rwp (%) | Rp (%) | χ2 | D (g/cm3) |
|---|---|---|---|---|---|---|---|
| Mg2SiO4 | 3.311 | 290.337 | 59.07 | 13.9 | 9.82 | 5.42 | 3.381 |
| LiTiO2 | 4.068 | 70.890 | 24.18 | ||||
| Li2TiO3 | 3.404 | 428.252 | 8.60 | ||||
| Li2MgSiO4 | 2.575 | 336.059 | 6.27 | ||||
| SiO2 | 2.270 | 175.838 | 1.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Pan, F.; Liu, L.; Du, Q.; Tang, R.; Ai, J.; Zhang, H.; Chen, Y. Enhanced Microwave Dielectric Properties and Sintering Behaviors of Mg2SiO4-Li2TiO3-LiF Ceramics by Adding CaTiO3 for LTCC and GPS Antenna Applications. Crystals 2022, 12, 512. https://doi.org/10.3390/cryst12040512
Wang Z, Pan F, Liu L, Du Q, Tang R, Ai J, Zhang H, Chen Y. Enhanced Microwave Dielectric Properties and Sintering Behaviors of Mg2SiO4-Li2TiO3-LiF Ceramics by Adding CaTiO3 for LTCC and GPS Antenna Applications. Crystals. 2022; 12(4):512. https://doi.org/10.3390/cryst12040512
Chicago/Turabian StyleWang, Zhijian, Feng Pan, Lanlan Liu, Qifeng Du, Ruitao Tang, Jun Ai, Hong Zhang, and Ying Chen. 2022. "Enhanced Microwave Dielectric Properties and Sintering Behaviors of Mg2SiO4-Li2TiO3-LiF Ceramics by Adding CaTiO3 for LTCC and GPS Antenna Applications" Crystals 12, no. 4: 512. https://doi.org/10.3390/cryst12040512





