Assessment of Physicochemical, Anticancer, Antimicrobial, and Biofilm Activities of N-Doped Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
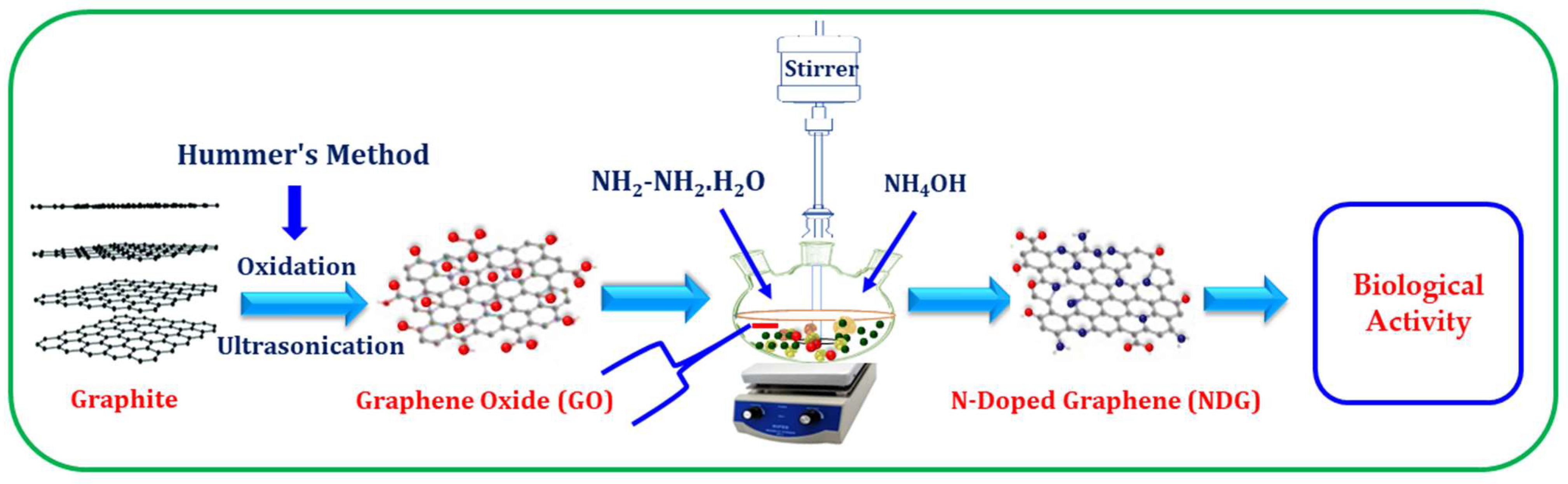
2.2. Synthesis of Graphene Oxide (GO)
2.3. Synthesis of N-Doped Graphene (NDG)
2.4. Screening of Synthesized NDG for Antimicrobial Analysis
2.5. Screening of Synthesized NDG for Biofilm Activity
2.6. Determination of Minimum Biofilm Inhibitory Concentration (MBIC)
2.7. Determination of Antibiofilm Activity by Inverted Microscope
2.8. Determination of Cell Cytotoxicity by 2,5-Diphenyl-2H-Tetrazolium Bromide (MTT) Assay
2.9. Statistical Analysis
3. Results and Discussions
3.1. UV-Vis Analysis
3.2. FT-IR Analysis
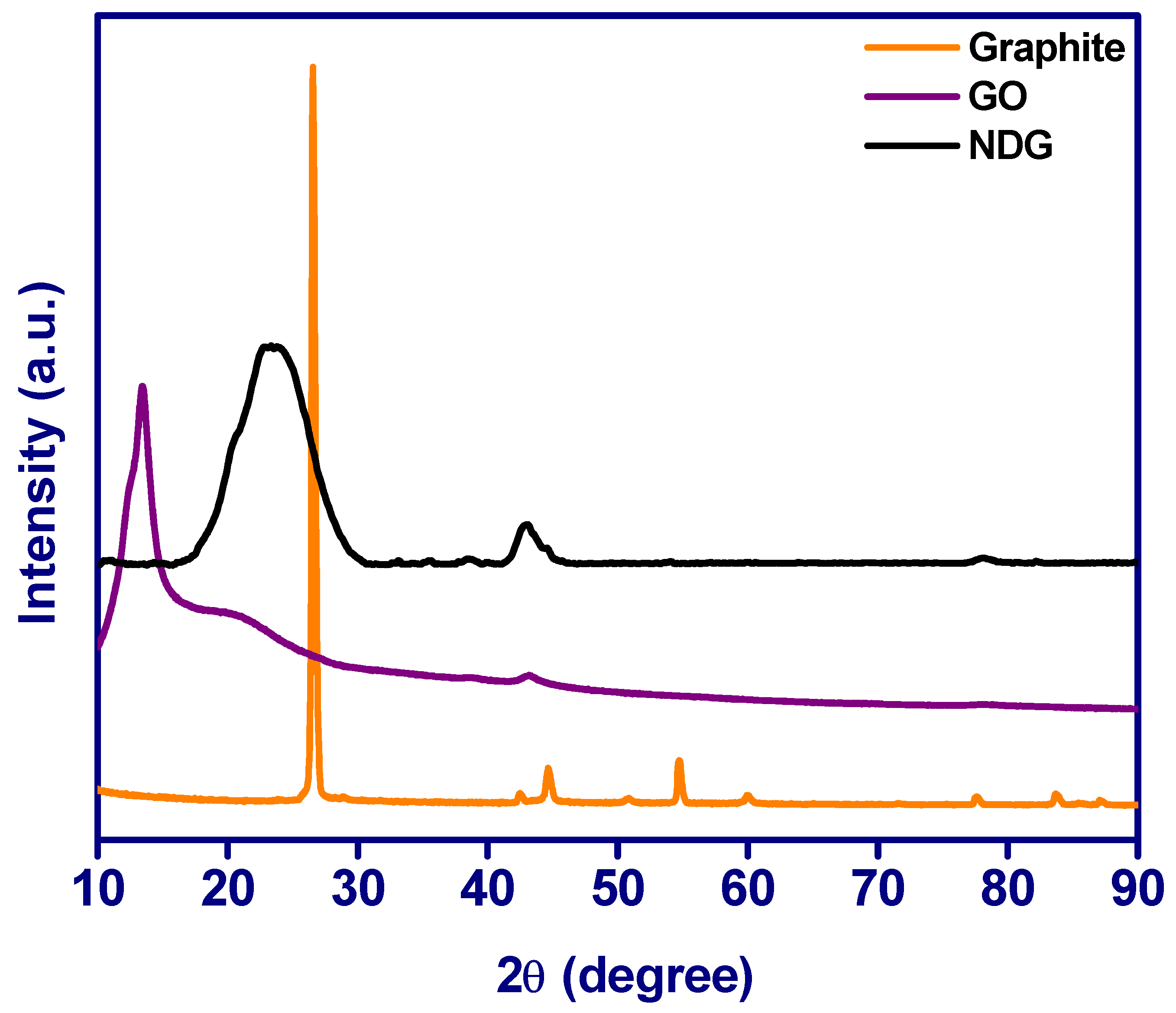
3.3. XRD Analysis
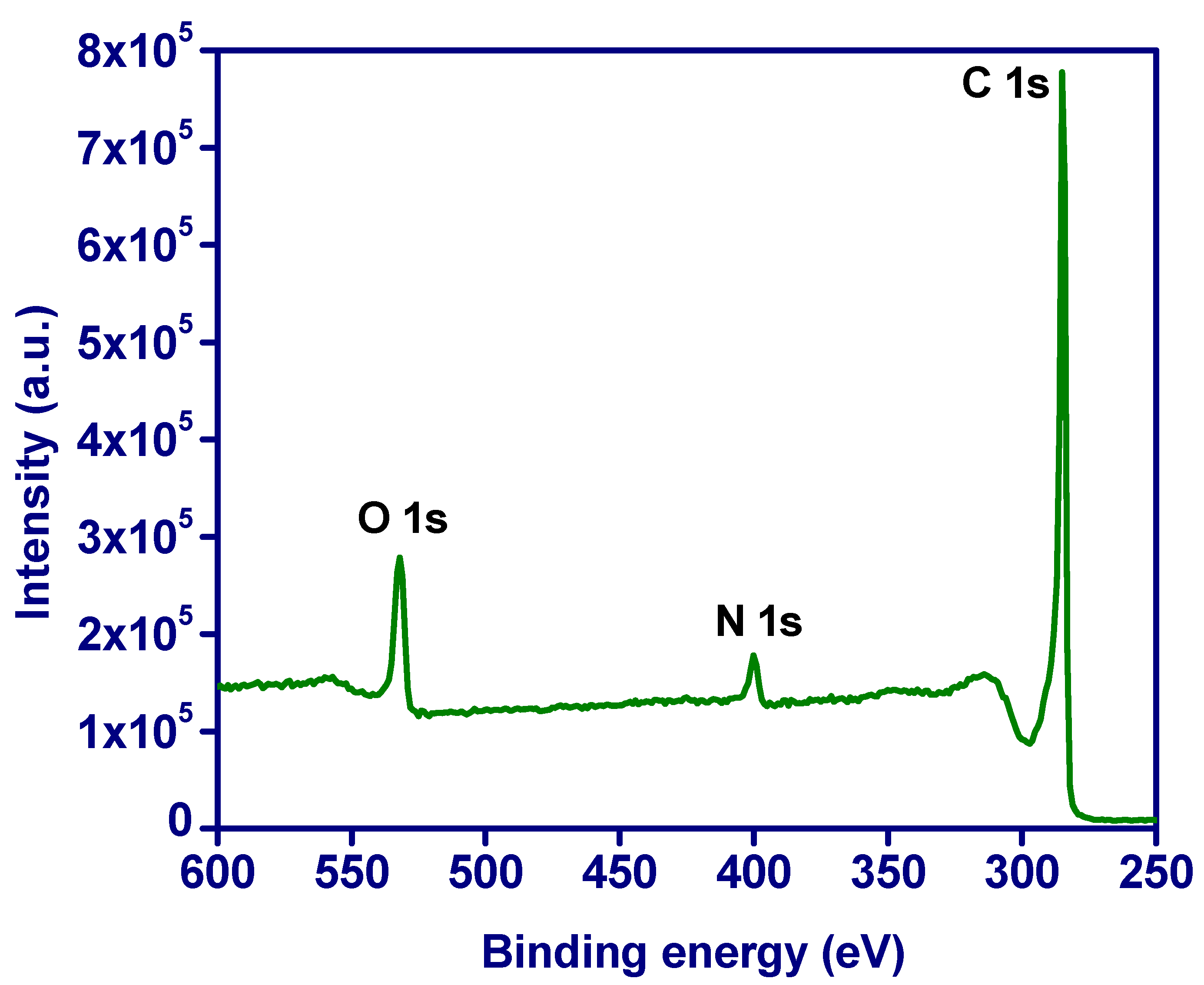
3.4. XPS Analysis
3.5. N2 Sorption Studies
3.6. TEM Analysis
3.7. Antimicrobial Analysis
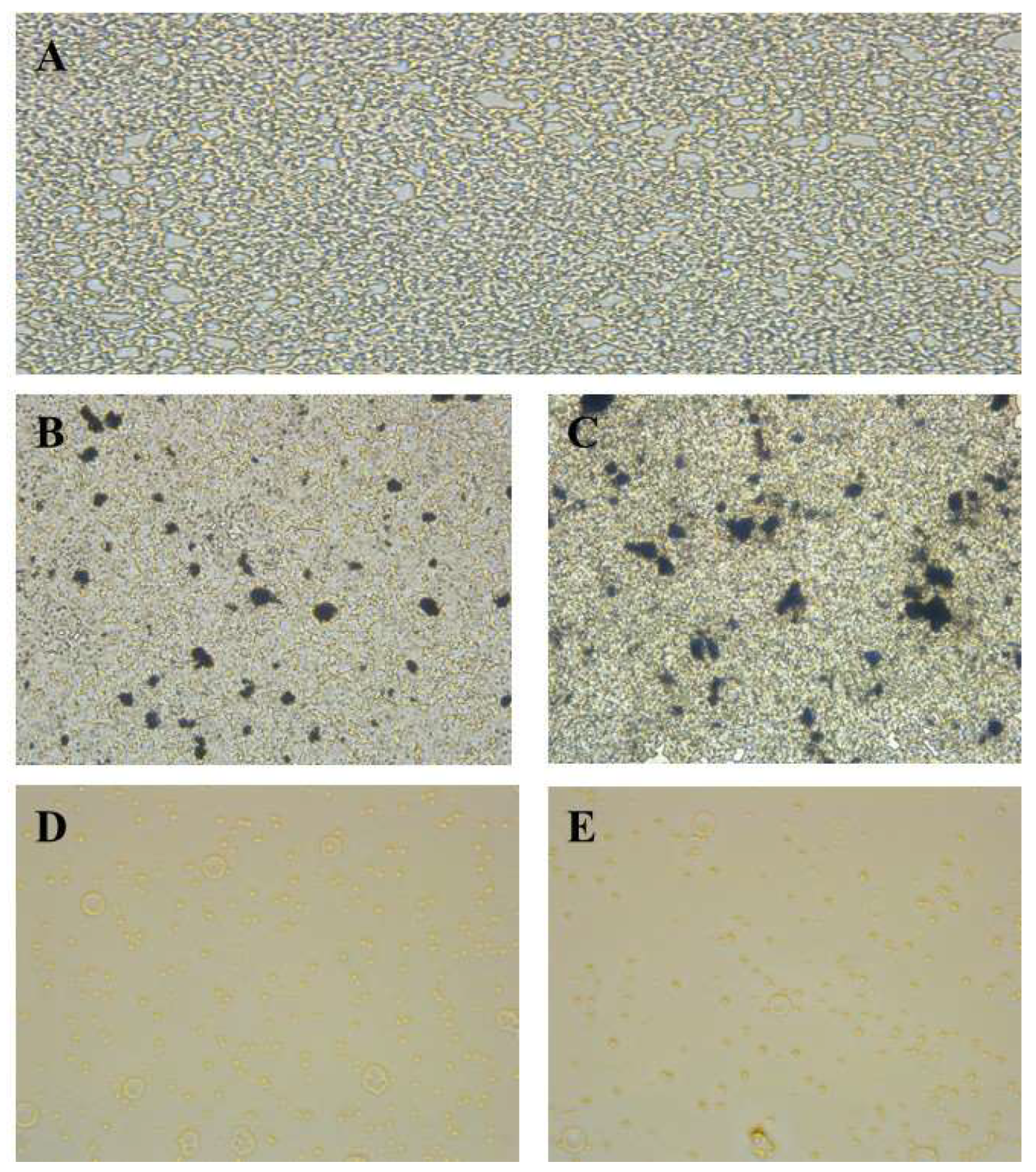
3.8. Biofilm Inhibition and MBIC Assay Results
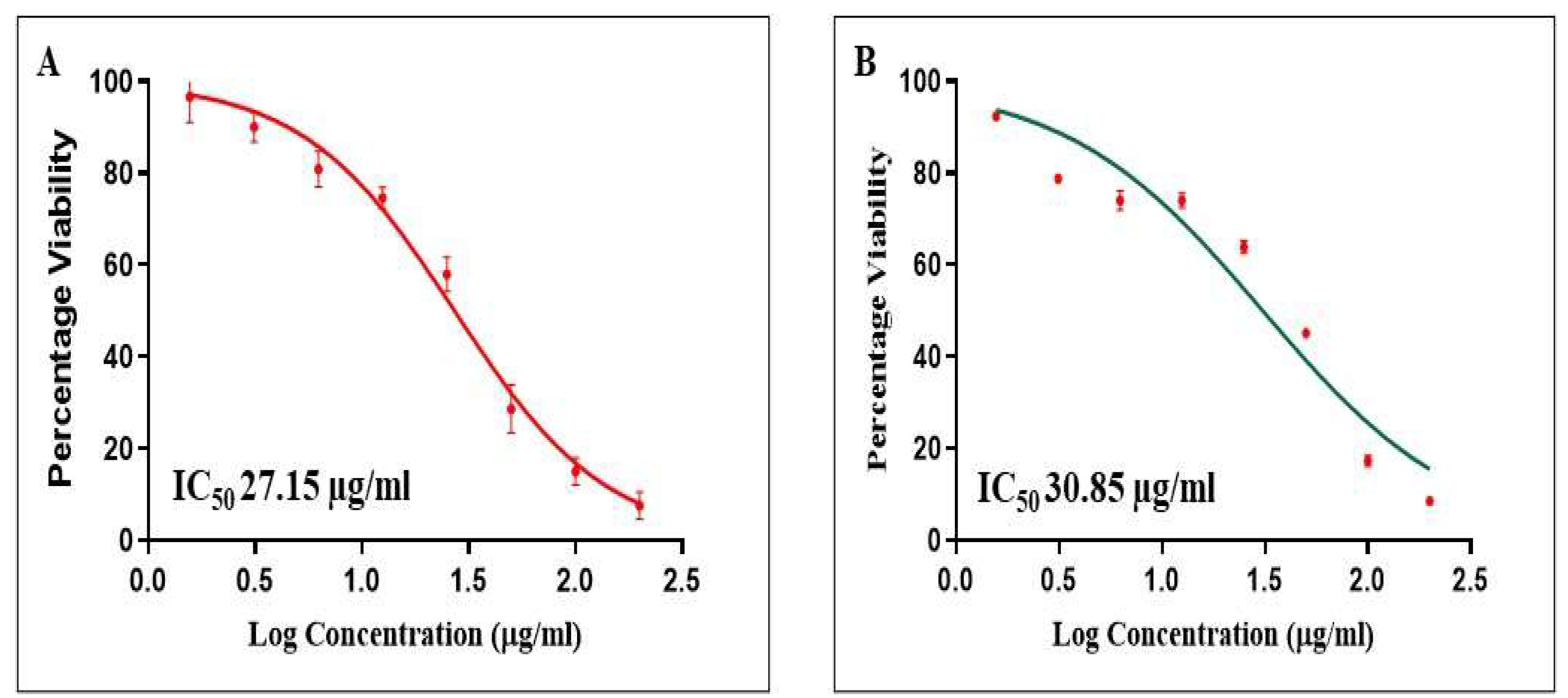
3.9. Cell Proliferation Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawyers, C. Targeted Cancer Therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Khang, D. Multiple Cues on the Physiochemical, Mesenchymal, and Intracellular Trafficking Interactions with Nanocarriers to Maximize Tumor Target Efficiency. Int. J. Nanomed. 2015, 10, 3989–4008. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Yoo, K.H.; Sym, S.J.; Khang, D. Mesenchymal Stem Cell Therapy Assisted by Nanotechnology: A Possible Combinational Treatment for Brain Tumor and Central Nerve Regeneration. Int. J. Nanomed. 2019, 14, 5925–5942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, E. Cancer Immunotherapy. Nature 2017, 552, S61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, C.; Kadri, S.S.; Dekker, J.P.; Danner, R.L.; Chen, H.-C.; Fram, D.; Zhang, F.; Wang, R.; Klompas, M. Prevalence of Antibiotic-Resistant Pathogens in Culture-Proven Sepsis and Outcomes Associated with Inadequate and Broad-Spectrum Empiric Antibiotic Use. JAMA Netw. Open 2020, 3, e202899. [Google Scholar] [CrossRef] [PubMed]
- Alqasim, A. Colistin-resistant gram-negative bacteria in Saudi Arabia: A literature review. J. King Saud Univ. Sci. 2021, 33, 101610. [Google Scholar] [CrossRef]
- Almamad, M.F.; Aldujaili, N.H. Antibacterial and Antibiofilm Activity of Bacterially Reduced Graphene Oxide against Some MDR Bacterial Pathogens Isolated from Urinary Tract Infections. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2022; p. 020003. [Google Scholar]
- Tré-Hardy, M.; Macé, C.; El Manssouri, N.; Vanderbist, F.; Traore, H.; Devleeschouwer, M.J. Effect of Antibiotic Co-Administration on Young and Mature Biofilms of Cystic Fibrosis Clinical Isolates: The Importance of the Biofilm Model. Int. J. Antimicrob. Agents 2009, 33, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Fortner, J.D.; Zhu, D.; Qi, Z.; Chen, W. Transport of Sulfide-Reduced Graphene Oxide in Saturated Quartz Sand: Cation-Dependent Retention Mechanisms. Environ. Sci. Technol. 2015, 49, 11468–11475. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Di Lodovico, S.; Fontana, A.; Traini, T.; Di Campli, E.; Pilato, S.; D’Ercole, S.; Cellini, L. Graphene Oxide Affects Staphylococcus Aureus and Pseudomonas Aeruginosa Dual Species Biofilm in Lubbock Chronic Wound Biofilm Model. Sci. Rep. 2020, 10, 18525. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Bostan, H.B.; Rezaee, R.; Valokala, M.G.; Tsarouhas, K.; Golokhvast, K.; Tsatsakis, A.M.; Karimi, G. Cardiotoxicity of Nano-Particles. Life Sci. 2016, 165, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 2002, 80, 1339. [Google Scholar] [CrossRef]
- Alangari, A.; Alqahtani, M.S.; Mateen, A.; Kalam, M.A.; Alshememry, A.; Ali, R.; Kazi, M.; AlGhamdi, K.M.; Syed, R. Iron Oxide Nanoparticles: Preparation, Characterization, and Assessment of Antimicrobial and Anticancer Activity. Adsorpt. Sci. Technol. 2022, 2022, 1562051. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Nasr, M.; Elkhatib, W.F.; Eltayeb, W.N. In Vitro Evaluation of Antimicrobial Activity and Cytotoxicity of Different Nanobiotics Targeting Multidrug Resistant and Biofilm Forming Staphylococci. Biomed Res. Int. 2018, 2018, 7658238. [Google Scholar] [CrossRef] [Green Version]
- Fais, R.; Di Luca, M.; Rizzato, C.; Morici, P.; Bottai, D.; Tavanti, A.; Lupetti, A. The N-Terminus of Human Lactoferrin Displays Anti-Biofilm Activity on Candida Parapsilosis in Lumen Catheters. Front. Microbiol. 2017, 8, 2218. [Google Scholar] [CrossRef]
- Kalam, M.A.; Iqbal, M.; Alshememry, A.; Alkholief, M.; Alshamsan, A. Development and Evaluation of Chitosan Nanoparticles for Ocular Delivery of Tedizolid Phosphate. Molecules 2022, 27, 2326. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascon, J.M. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Vinoth, R.; Babu, S.G.; Bahnemann, D.; Neppolian, B. Nitrogen Doped Reduced Graphene Oxide Hybrid Metal Free Catalyst for Effective Reduction of 4-Nitrophenol. Sci. Adv. Mater. 2015, 7, 1443–1449. [Google Scholar] [CrossRef]
- Kumar, M.P.; Kesavan, T.; Kalita, G.; Ragupathy, P.; Narayanan, T.N.; Pattanayak, D.K. On the Large Capacitance of Nitrogen Doped Graphene Derived by a Facile Route. RSC Adv. 2014, 4, 38689–38697. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.M.; Wallace, R.; Chhowalla, M.; Cho, K.; Chabal, Y. The Role of Oxygen during Thermal Reduction of Graphene Oxide Studied by Infrared Absorption Spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Dahryn Trivedi, A.B.; Khemraj Bairwa, H.S. Fourier Transform Infrared and Ultraviolet-Visible Spectroscopic Characterization of Biofield Treated Salicylic Acid and Sparfloxacin. Nat. Prod. Chem. Res. 2015, 5, 186. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, T.; Zhou, Y.; Li, Y.; Lu, J.; Tian, X.; Wang, D.; Wang, J. Facile Synthesis of Boron and Nitrogen-Doped Graphene as Efficient Electrocatalyst for the Oxygen Reduction Reaction in Alkaline Media. Int. J. Hydrogen Energy 2014, 39, 16043–16052. [Google Scholar] [CrossRef]
- Divya, K.S.; Chandran, A.; Reethu, V.N.; Mathew, S. Enhanced Photocatalytic Performance of RGO/Ag Nanocomposites Produced via a Facile Microwave Irradiation for the Degradation of Rhodamine B in Aqueous Solution. Appl. Surf. Sci. 2018, 444, 811–818. [Google Scholar] [CrossRef]
- Hassan, F.M.; Chabot, V.; Li, J.; Kim, B.K.; Ricardez-Sandoval, L.; Yu, A. Pyrrolic-Structure Enriched Nitrogen Doped Graphene for Highly Efficient next Generation Supercapacitors. J. Mater. Chem. A 2013, 1, 2904. [Google Scholar] [CrossRef]
- Matsuoka, K.; Yamagishi, Y.; Yamazaki, T.; Setoyama, N.; Tomita, A.; Kyotani, T. Extremely High Microporosity and Sharp Pore Size Distribution of a Large Surface Area Carbon Prepared in the Nanochannels of Zeolite Y. Carbon N. Y. 2005, 43, 876–879. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Moritz, M.; Geszke-Moritz, M. The Newest Achievements in Synthesis, Immobilization and Practical Applications of Antibacterial Nanoparticles. Chem. Eng. J. 2013, 228, 596–613. [Google Scholar] [CrossRef]
- Huang, C.; Li, C.; Shi, G. Graphene Based Catalysts. Energy Environ. Sci. 2012, 5, 8848. [Google Scholar] [CrossRef]
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and Characterization of Antibacterial Electrospun Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide Composite Nanofibrous Membrane. Appl. Surf. Sci. 2018, 435, 832–840. [Google Scholar] [CrossRef]
- Huang, S.-Z.; Ma, Q.-Y.; Fang, W.-W.; Xu, F.-Q.; Peng, H.; Dai, H.-F.; Zhou, J.; Zhao, Y.-X. Three New Isopimarane Diterpenoids from Excoecaria Acerifolia. J. Asian Nat. Prod. Res. 2013, 15, 750–755. [Google Scholar] [CrossRef]
- Hou, H.-M.; Zhu, Y.-L.; Wang, J.-Y.; Jiang, F.; Qu, W.-Y.; Zhang, G.-L.; Hao, H.-S. Characteristics of N-Acylhomoserine Lactones Produced by Hafnia Alvei H4 Isolated from Spoiled Instant Sea Cucumber. Sensors 2017, 17, 772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, S.; Jia, H.; Deng, Y.; Zhou, J.; Huang, B.; Yu, Y.; Lan, J.; Wang, W.; Lou, Y.; et al. A Novel Neutralizing Monoclonal Antibody Targeting the N-Terminal Domain of the MERS-CoV Spike Protein. Emerg. Microbes Infect. 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Lin, S.; Soteyome, T.; Peters, B.M.; Li, Y.; Chen, H.; Su, J.; Li, L.; Li, B.; Xu, Z.; et al. Biofilm Formation of Staphylococcus Aureus under Food Heat Processing Conditions: First Report on CML Production within Biofilm. Sci. Rep. 2019, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Neihaya, H.Z.; Zaman, H.H. Investigating the Effect of Biosynthesized Silver Nanoparticles as Antibiofilm on Bacterial Clinical Isolates. Microb. Pathog. 2018, 116, 200–208. [Google Scholar] [CrossRef]
- Seth, A.K.; Geringer, M.R.; Hong, S.J.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. In Vivo Modeling of Biofilm-Infected Wounds: A Review. J. Surg. Res. 2012, 178, 330–338. [Google Scholar] [CrossRef]
- Neethu, S.; Midhun, S.J.; Radhakrishnan, E.K.; Jyothis, M. Surface Functionalization of Central Venous Catheter with Mycofabricated Silver Nanoparticles and Its Antibiofilm Activity on Multidrug Resistant Acinetobacter Baumannii. Microb. Pathog. 2020, 138, 103832. [Google Scholar] [CrossRef]
- De Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; de Fátima, S.M.; dos Santos Medeiros, R.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and Antibiofilm Potential of Silver Nanoparticles against Antibiotic-Sensitive and Multidrug-Resistant Pseudomonas Aeruginosa Strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as Promising Alternative in the Infection Treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef] [Green Version]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated Nano-Graphene Oxide as a Nanocarrier for Delivering Mixed Anticancer Drugs to Improve Anticancer Activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef]





| Microorganisms | Zone of Inhibitions (mm), Mean ± SD, n = 3 | |
|---|---|---|
| Ampicillin | NDG | |
| Klebsiella pneumoniae | 19.21 ± 1.39 | 14.2 ± 1.86 |
| Pseudomonas aeruginosa | 19.5 ± 2.75 | 17.8 ± 1.98 |
| MRSA | 20.3 ± 1.12 | 24.7 ± 1.89 * |
| Streptococcus mutans | 18.21 ± 1.33 | 22.31 ± 1.67 * |
| Streptococcus pneumoniae | 19.16 ± 1.72 | 25.45 ± 2.89 * |
| Bacillus subtillis | 19.22 ± 1.87 | 25.12 ± 2.35 * |
| S. No | Tested Microorganism | Drug | MIC (µg/mL) | Parentage of Inhibition | IC50 (µg) |
|---|---|---|---|---|---|
| 1 | P. aeruginosa | NDG | 25 | 94.26 | 12.98 |
| NDG | 50 | 94.23 | 26.53 | ||
| Ampicillin | 1000 | 90.45 | 110.55 | ||
| Ampicillin | 2000 | 91.31 | 547.58 | ||
| 2 | MRSA | NDG | 50 | 98.12 | 23.11 |
| NDG | 100 | 93.76 | 53.32 | ||
| Ampicillin | 200 | 89.79 | 111.37 | ||
| Ampicillin | 1000 | 91.07 | 137.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alangari, A.; Aldakheel, F.M.; Mateen, A.; Alqhatani, M.S.; Alaofi, A.L.; Shahid, M.; Ali, R.; Syed, R.; Adil, S.F.; Khan, M.; et al. Assessment of Physicochemical, Anticancer, Antimicrobial, and Biofilm Activities of N-Doped Graphene. Crystals 2022, 12, 1035. https://doi.org/10.3390/cryst12081035
Alangari A, Aldakheel FM, Mateen A, Alqhatani MS, Alaofi AL, Shahid M, Ali R, Syed R, Adil SF, Khan M, et al. Assessment of Physicochemical, Anticancer, Antimicrobial, and Biofilm Activities of N-Doped Graphene. Crystals. 2022; 12(8):1035. https://doi.org/10.3390/cryst12081035
Chicago/Turabian StyleAlangari, Abdulaziz, Fahad M. Aldakheel, Ayesha Mateen, Mohammed S. Alqhatani, Ahmed L. Alaofi, Mudassar Shahid, Raisuddin Ali, Rabbani Syed, Syed Farooq Adil, Mujeeb Khan, and et al. 2022. "Assessment of Physicochemical, Anticancer, Antimicrobial, and Biofilm Activities of N-Doped Graphene" Crystals 12, no. 8: 1035. https://doi.org/10.3390/cryst12081035









