Magnetic β-Cyclodextrin Polymer Nanoparticles for Efficient Adsorption of U(VI) from Wastewater
Abstract
:1. Introduction
2. Reagents and Methods
2.1. Reagents
2.2. Preparation of PCDP and CA-PCDP
2.3. Preparation of CA-PCDP@MNP
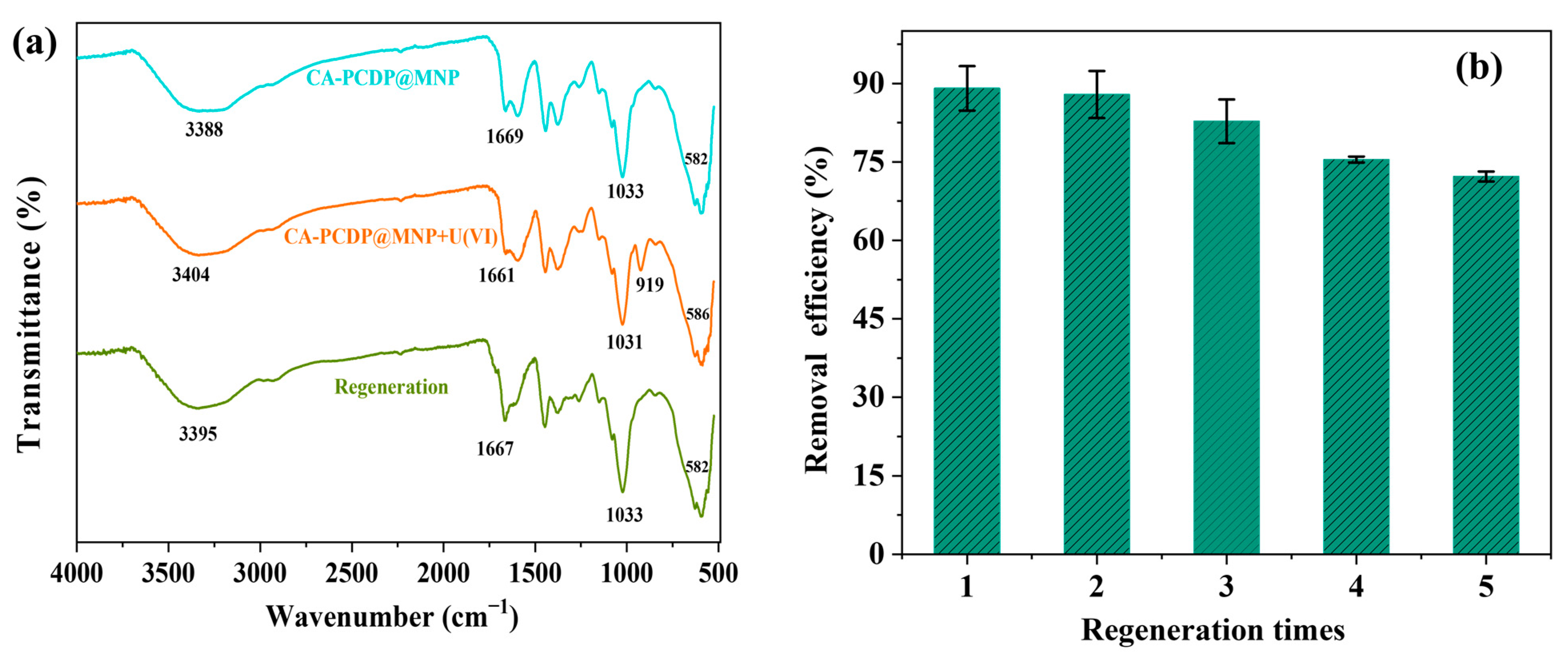
2.4. Characterization
2.5. Different Factors’ Effect on U(VI) Adsorption
2.6. Kinetic Adsorption
2.7. Adsorption Isotherm and Thermodynamic
2.8. Recycling and Regeneration
3. Results and Discussion
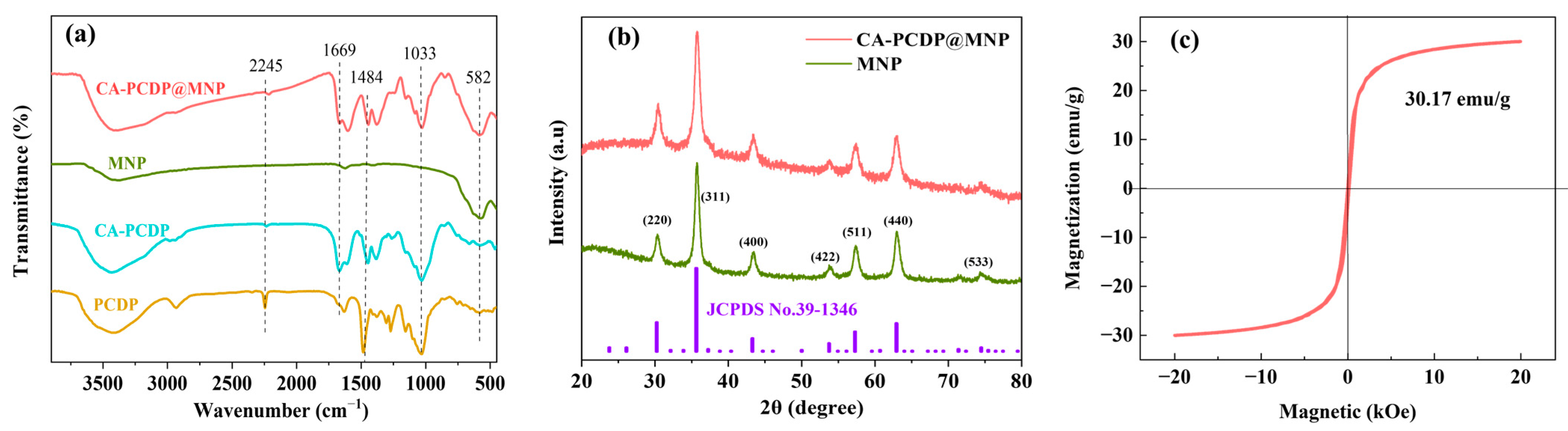
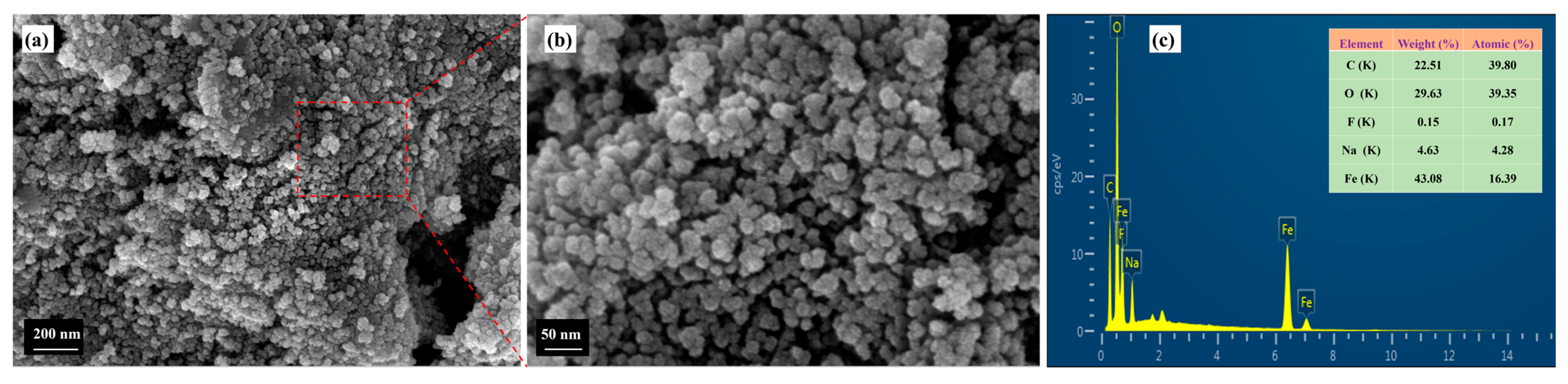
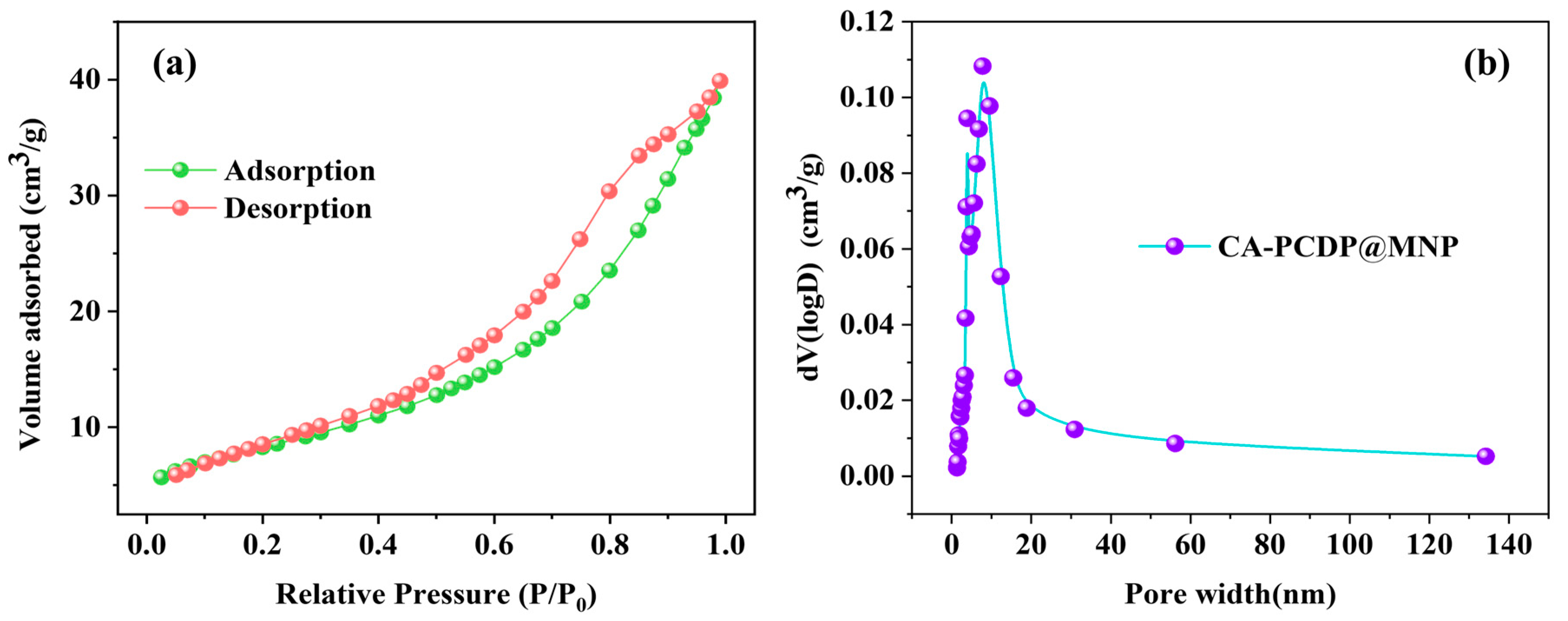
3.1. Characterization
3.2. Effect of Sorbent Dosage and pH
3.3. Adsorption Kinetics
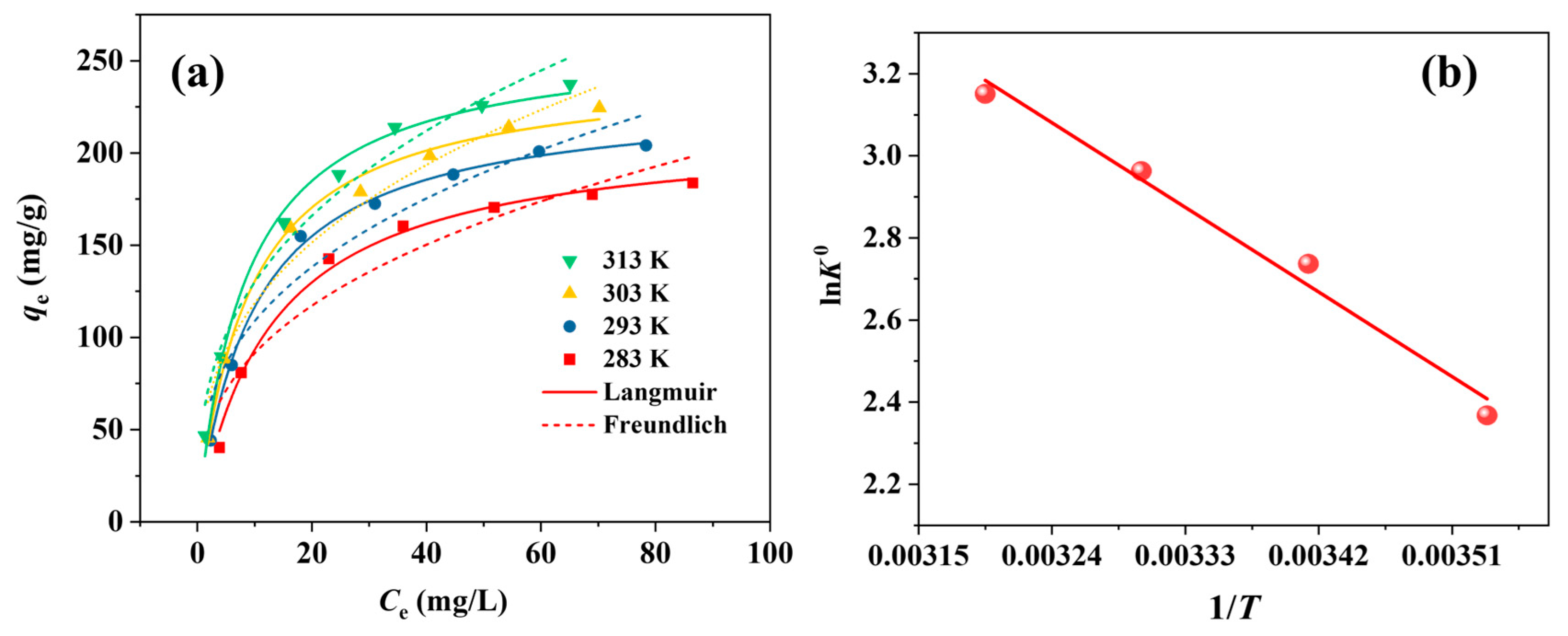
3.4. Adsorption Isotherms and Thermodynamics
3.5. Explanation of Adsorption Mechanism
3.6. Recycling and Regeneration of CA-PCDP@MNP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Liu, J.; Zhang, W.; Zhou, J.; Luo, D.; Li, Z. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Qiu, M.; Hu, B.; Wang, G.; Lu, Y. Recent advances in the removal of U(VI) by magnetic metal oxides. J. Mol. Liq. 2023, 385, 122295. [Google Scholar] [CrossRef]
- Prusty, S.; Somu, P.; Sahoo, J.K.; Panda, D.; Sahoo, S.K.; Sahoo, S.K.; Lee, Y.R.; Jarin, T.; Sundar, L.S.; Rao, K.S. Adsorptive sequestration of noxious uranium (VI) from water resources: A comprehensive review. Chemosphere 2022, 308, 136278. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yuan, Q.; Wang, Q.; Hu, C.; Guo, K.; Ouyang, J.; Chen, M. Maleic anhydride-β-cyclodextrin functionalized magnetic nanoparticles for the removal of uranium (VI) from wastewater. Crystals 2022, 12, 1731. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, J.; Chen, F.; Dionysiou, D.D.; Feng, Y.; Liang, B.; Cheng, H.-Y.; Qin, Z.; Tang, X.; Li, H. Removal and recovery of aqueous U(VI) by heterogeneous photocatalysis: Progress and challenges. Chem. Eng. J. 2022, 450, 138317. [Google Scholar] [CrossRef]
- Tang, N.; Liang, J.; Niu, C.; Wang, H.; Luo, Y.; Xing, W.; Ye, S.; Liang, C.; Guo, H.; Guo, J.; et al. Amidoxime-based materials for uranium recovery and removal. J. Mater. Chem. A 2020, 8, 7588–7625. [Google Scholar] [CrossRef]
- Abney, C.W.; Mayes, R.T.; Saito, T.; Dai, S. Materials for the recovery of uranium from seawater. Chem. Rev. 2017, 117, 13935–14013. [Google Scholar] [CrossRef]
- Djellabi, R.; Yang, B.; Sharif, H.M.A.; Zhang, J.; Ali, J.; Zhao, X. Sustainable and easy recoverable magnetic TiO2-lignocellulosic biomass@Fe3O4 for solar photocatalytic water remediation. J. Clean. Prod. 2019, 233, 841–847. [Google Scholar] [CrossRef]
- Rasheed, T. Magnetic nanomaterials: Greener and sustainable alternatives for the adsorption of hazardous environmental contaminants. J. Clean. Prod. 2022, 362, 132338. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Zhao, L.; Zhang, S.; Huang, Y.; Wu, X.; Wang, X. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core–shell magnetic microspheres for highly efficient sorption of U(VI). Chem. Eng. J. 2014, 235, 275–283. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, Y.; Liu, L.; Fang, Y.; Ma, F.; Zhang, C.; Dong, H. Efficient and magnetically recoverable U (VI) adsorbent: Fe3O4 loaded hypercrosslink copoly (styrene/maleic anhydride). Colloids Surf. A 2022, 632, 127644. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Ji, X.; Ren, J.; Gao, B.; Deng, W.; Wang, Z. Bioinspired succinyl-β-cyclodextrin membranes for enhanced uranium extraction and reclamation. Environ. Sci. Nano. 2020, 7, 3124–3135. [Google Scholar] [CrossRef]
- Yang, S.; Zong, P.; Hu, J.; Sheng, G.; Wang, Q.; Wang, X. Fabrication of β-cyclodextrin conjugated magnetic HNT/iron oxide composite for high-efficient decontamination of U(VI). Chem. Eng. J. 2013, 214, 376–385. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, R.; Xu, G.; Guo, K.; Hu, C. Adsorptive removal of uranium (VI) from wastewater using a crosslinked amidoxime-functionalized β-cyclodextrin polymer. New J. Chem. 2022, 46, 20592–20601. [Google Scholar] [CrossRef]
- Zong, P.; Xu, X.; Shao, M.; Cao, D.; Wang, S.; Zhang, H. Efficient elimination of uranium by carboxymethyl-β-cyclodextrin nanoparticles decorated iron oxides: Water chemistry influences and mechanism researches. Int. J. Hydrog. Energ. 2021, 46, 1370–1384. [Google Scholar] [CrossRef]
- Xu, G.; Xie, X.; Qin, L.; Hu, X.; Zhang, D.; Xu, J.; Li, D.; Ji, X.; Huang, Y.; Tu, Y.; et al. Simple synthesis of a swellable porous β-cyclodextrin-based polymer in the aqueous phase for the rapid removal of organic micro-pollutants from water. Green Chem. 2019, 21, 6062–6072. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, J.; Hai, W.; Tan, H.; Luo, Y.; Xie, X.; Cao, Y.; He, F. A novel crosslinked β-cyclodextrin-based polymer for removing methylene blue from water with high efficiency. Colloids Surf. A 2019, 560, 59–68. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, L.; Ma, F.; Zhu, X.; Dong, H.; Zhang, C.; Zhao, F. Synthesis of phosphorylated hyper-cross-linked polymers and their efficient uranium adsorption in water. J. Hazard. Mater. 2021, 419, 126538. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, Y.; Jiang, Y.; Pan, N.; Liu, C.; Wang, J.; Ma, C.; Xia, X.; Liu, M.; Zhang, H.; et al. Efficient extraction of U(VI) from uranium enrichment process wastewater by amine-aminophosphonate-modified polyacrylonitrile fibers. Sci. Total Environ. 2022, 831, 154743. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Lu, Y.; Zhang, X.; Chen, W.; Chen, X.; Li, L. Amidoxime-modified ultrathin polyethylene fibrous membrane for uranium extraction from seawater. Desalination 2022, 539, 115965. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Zhang, S.; Ding, C.; Sheng, G.; Alsaedi, A.; Hayat, T.; Li, J.; Song, Y. Amidoxime-Functionalized Hollow Carbon Spheres for Efficient Removal of Uranium from Wastewater. ACS Sustain. Chem. Eng. 2019, 7, 10800–10807. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Jiang, H.-L.; Xie, Z.-W.; Li, Z.-T.; Xu, D.; He, F.-A. Highly efficient selective adsorption of anionic dyes by modified β-cyclodextrin polymers. J. Taiwan Inst. Chem. Eng. 2020, 108, 114–128. [Google Scholar] [CrossRef]
- Dong, S.; Wang, S.; Wang, X.; Zhai, L. Superparamagnetic nanocomposite Fe3O4@SiO2-NH2/CQDs as fluorescent probe for copper (II) detection. Mater. Lett. 2020, 278, 128404. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, G.; Xian, G.; Ren, Z.; Wei, T.; Li, Q.; Zhang, Y.; Zou, Z. Tetracycline degradation by persulfate activated with magnetic γ-Fe2O3/CeO2 catalyst: Performance, activation mechanism and degradation pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Chen, B.; Sun, J.; Fan, F.; Zhang, X.; Qin, Z.; Wang, P.; Li, Y.; Zhang, X.; Liu, F.; Liu, Y. Ferumoxytol of ultrahigh magnetization produced by hydrocooling and magnetically internal heating co-precipitation. Nanoscale 2018, 10, 7369–7376. [Google Scholar] [CrossRef]
- Le Jeune, M.; Secret, E.; Trichet, M.; Michel, A.; Ravault, D.; Illien, F.; Siaugue, J.-M.; Sagan, S.; Burlina, F.; Ménager, C. Conjugation of oligo-his peptides to magnetic γ-Fe2O3@SiO2 core–shell nanoparticles promotes their access to the cytosol. ACS Appl. Mater. Interfaces 2022, 14, 15021–15034. [Google Scholar] [CrossRef]
- Sobhani, S.; Khakzad, F. A novel hydrophobic copper complex supported on γ-Fe2O3 as a magnetically heterogeneous catalyst for one-pot three-component synthesis of α-aminophosphonates. Appl. Organomet. Chem. 2017, 31, 3877. [Google Scholar] [CrossRef]
- Ma, H.; Pu, S.; Ma, J.; Yan, C.; Zinchenko, A.; Pei, X.; Chu, W. Formation of multi-layered chitosan honeycomb spheres via breath-figure-like approach in combination with co-precipitation processing. Mater. Lett. 2018, 211, 91–95. [Google Scholar] [CrossRef]
- Khan, G.A.; Esentürk, E.N.; Bek, A.; Bhatti, A.S.; Ahmed, W. Fabrication of highly catalytically active gold nanostructures on filter-paper and their applications towards degradation of environmental pollutants. ChemistrySelect 2021, 6, 10655–10660. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, C.; Ji, H.; Zhang, L.; Li, F. Facile synthesis of nitrogen-deficient mesoporous graphitic carbon nitride for highly efficient photocatalytic performance. Appl. Surf. Sci. 2019, 478, 304–312. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Mei, B.; Tian, X.; Jia, L.; Zhu, W. Mussel inspired synthesis of polydopamine/polyethyleneimine-grafted fly ash composite adsorbent for the effective separation of U (VI). Sci. Total Environ. 2023, 876, 162841. [Google Scholar] [CrossRef] [PubMed]
- Dastbaz, A.; Keshtkar, A.R. Adsorption of Th4+, U6+, Cd2+, and Ni2+ from aqueous solution by a novel modified polyacrylonitrile composite nanofiber adsorbent prepared by electrospinning. Appl. Surf. Sci. 2014, 293, 336–344. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, L.; Wang, Y.; Ma, F.; Tian, Y.; Dong, H.; Zhang, C. Synthesis of hypercrosslinked calix [4] arenes anchored with Fe (III) via phosphate groups for excellent uranyl extraction from wastewater and seawater. J. Water Process. Eng. 2023, 53, 103615. [Google Scholar] [CrossRef]
- Guo, X.; Shang, Y.; Liang, X.; Diao, Z.; Song, G.; Chen, D.; Wang, S.; Kong, L. A comparison of Ni-Co layered double oxides with memory effect on recovering U(VI) from wastewater to hydroxides. Chem. Eng. J. 2022, 446, 137220. [Google Scholar] [CrossRef]
- Li, W.; Han, X.; Wang, X.; Wang, Y.; Wang, W.; Xu, H.; Tan, T.; Wu, W.; Zhang, H. Recovery of uranyl from aqueous solutions using amidoximated polyacrylonitrile/exfoliated Na-montmorillonite composite. Chem. Eng. J. 2015, 279, 735–746. [Google Scholar] [CrossRef]
- Song, S.; Huang, Q.; Cheng, G.; Wang, W.; Lu, Z.; Zhang, R.; Wen, T.; Zhang, Y.; Wang, J.; Wang, X. Immobilization of U (VI) on hierarchical NiSiO@ MgAl and NiSiO@ NiAl nanocomposites from wastewater. ACS Sustain. Chem. Eng. 2019, 7, 3475–3486. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.-X.; Wang, Z.-Y.; Zhang, F.; Wu, Q.; Sha, L.-T.; Wang, Y.; Yan, Z.-Y. Design and synthesis of a novel bifunctional polymer with malonamide and carboxyl group for highly selective separation of uranium (VI). Sep. Purif. Technol. 2022, 302, 122115. [Google Scholar] [CrossRef]
- Husnain, S.M.; Um, W.; Woojin-Lee, W.-L.; Chang, Y.-S. Magnetite-based adsorbents for sequestration of radionuclides: A review. RSC Adv. 2018, 8, 2521–2540. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Xu, X.; Alsaedi, A.; Hayat, T.; Li, J. Adsorption and desorption of U (VI) on different-size graphene oxide. Chem. Eng. J. 2019, 360, 941–950. [Google Scholar] [CrossRef]
- Liao, J.; Xiong, T.; Zhao, Z.; Ding, L.; Zhu, W.; Zhang, Y. Synthesis of a novel environmental-friendly biocarbon composite and its highly efficient removal of uranium(VI) and thorium(IV) from aqueous solution. J. Clean. Prod. 2022, 374, 134059. [Google Scholar] [CrossRef]
- Fan, F.-L.; Qin, Z.; Bai, J.; Rong, W.-D.; Fan, F.-Y.; Tian, W.; Wu, X.-L.; Wang, Y.; Zhao, L. Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles. J. Environ. Radioactiv. 2012, 106, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, P.; Dong, F.; Nie, X.; Ding, C.; Wang, S.; Zhang, Y.; Liu, H.; Zhou, S. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem. Eng. J. 2019, 367, 198–207. [Google Scholar] [CrossRef]
- He, Y.; Lin, X.; Yan, T.; Zhang, X.; Zhou, J.; Chen, Y.; Luo, X. Selective adsorption of uranium from salt lake-simulated solution by phenolic-functionalized hollow sponge-like adsorbent. J. Chem. Technol. Biot. 2019, 94, 455–467. [Google Scholar] [CrossRef]
- Atrei, A.; Lesiak-Orlowska, B.; Tóth, J. Magnetite nanoparticles functionalized with citrate: A surface science study by XPS and ToF-SIMS. Appl. Surf. Sci. 2022, 602, 154366. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]




| Pseudo-First-Order Model | Pseudo-Second-Order Model | Internal Diffusion Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe,cal | qe,exp | R2 | k2 | qe,cal | qe,exp | R2 | kid | C | R2 |
| 0.0126 | 53.96 | 178.85 | 0.718 | 0.0012 | 183.15 | 178.85 | 0.999 | kid,1 = 26.759 | 30.349 | 0.991 |
| kid,2 = 9.820 | 99.665 | 0.993 | ||||||||
| kid,3 = 0.246 | 175.184 | 0.971 | ||||||||
| Sample | T/K | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| qm/(mg/g) | KL/(L/mg) | RL2 | KF/(mg/g) | n | RF2 | ||
| CA-PCDP@MNP | 283 | 213.58 | 0.07761 | 0.992 | 206.90 | 3.449 | 0.856 |
| 293 | 231.25 | 0.10145 | 0.998 | 235.57 | 3.636 | 0.844 | |
| 303 | 245.66 | 0.11337 | 0.994 | 280.75 | 4.155 | 0.845 | |
| 313 | 262.52 | 0.11906 | 0.992 | 313.32 | 4.371 | 0.875 | |
| Sample | T/K | ∆G0/(kJ∙mol−1) | ∆H0/(kJ∙mol−1) | ∆S0/(J∙mol−1∙K−1) |
|---|---|---|---|---|
| CA-PCDP@MNP | 283 | −5.571 | 19.048 | 87.330 |
| 293 | −6.667 | |||
| 303 | −7.463 | |||
| 313 | −8.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Lv, N.; Zhang, M.; Tan, Y.; Yuan, Q.; Hu, C.; Ma, M.; Wu, Y.; Ouyang, J. Magnetic β-Cyclodextrin Polymer Nanoparticles for Efficient Adsorption of U(VI) from Wastewater. Crystals 2023, 13, 1496. https://doi.org/10.3390/cryst13101496
Zhong X, Lv N, Zhang M, Tan Y, Yuan Q, Hu C, Ma M, Wu Y, Ouyang J. Magnetic β-Cyclodextrin Polymer Nanoparticles for Efficient Adsorption of U(VI) from Wastewater. Crystals. 2023; 13(10):1496. https://doi.org/10.3390/cryst13101496
Chicago/Turabian StyleZhong, Xing, Nan Lv, Meicheng Zhang, Yubin Tan, Qiaozhulin Yuan, Caixia Hu, Mingyang Ma, Yongchuan Wu, and Jinbo Ouyang. 2023. "Magnetic β-Cyclodextrin Polymer Nanoparticles for Efficient Adsorption of U(VI) from Wastewater" Crystals 13, no. 10: 1496. https://doi.org/10.3390/cryst13101496




