Grain Boundary Wetting Transition in the Mg-Based ZEK 100 Alloy
Abstract
:1. Introduction
2. Materials and Methods
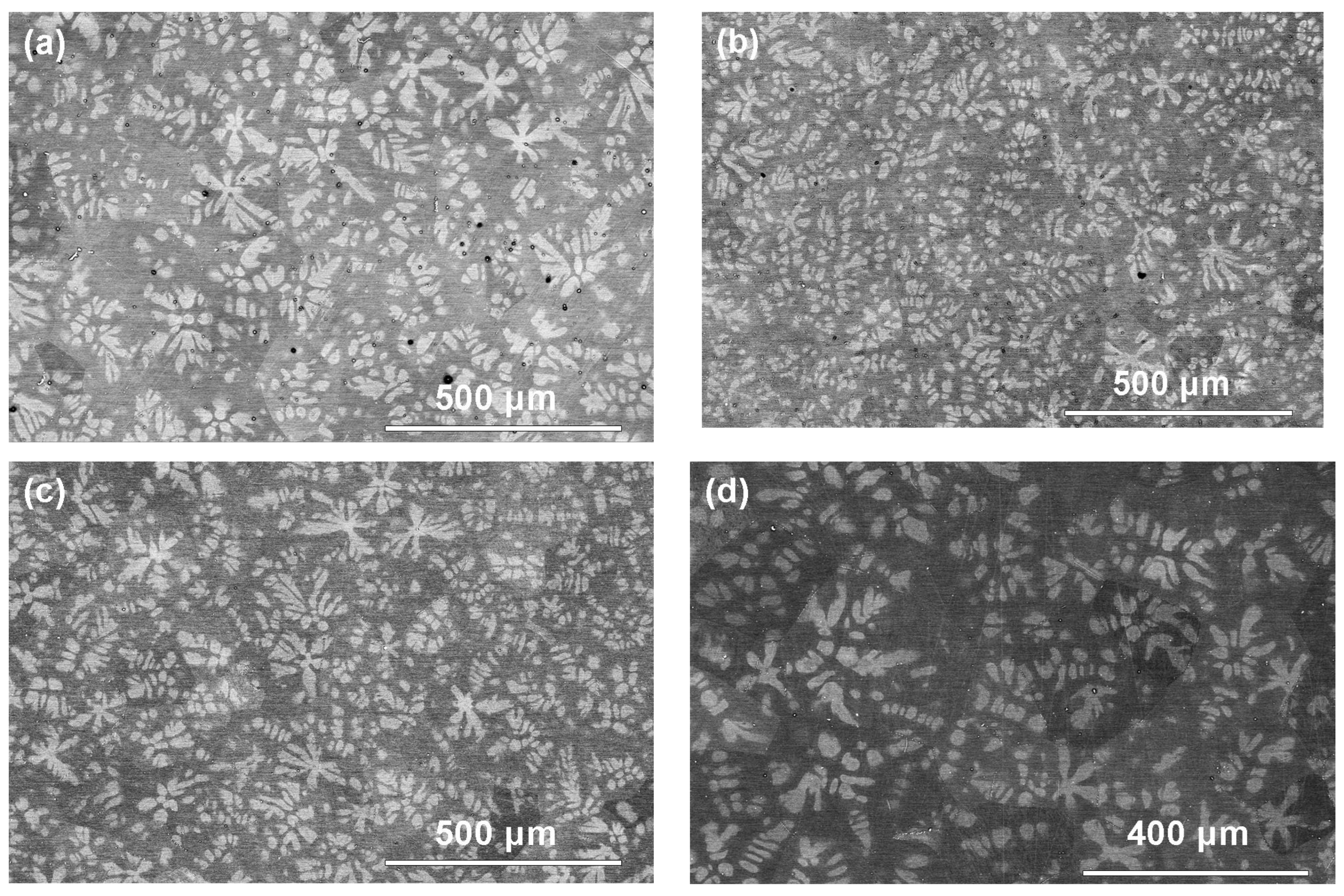
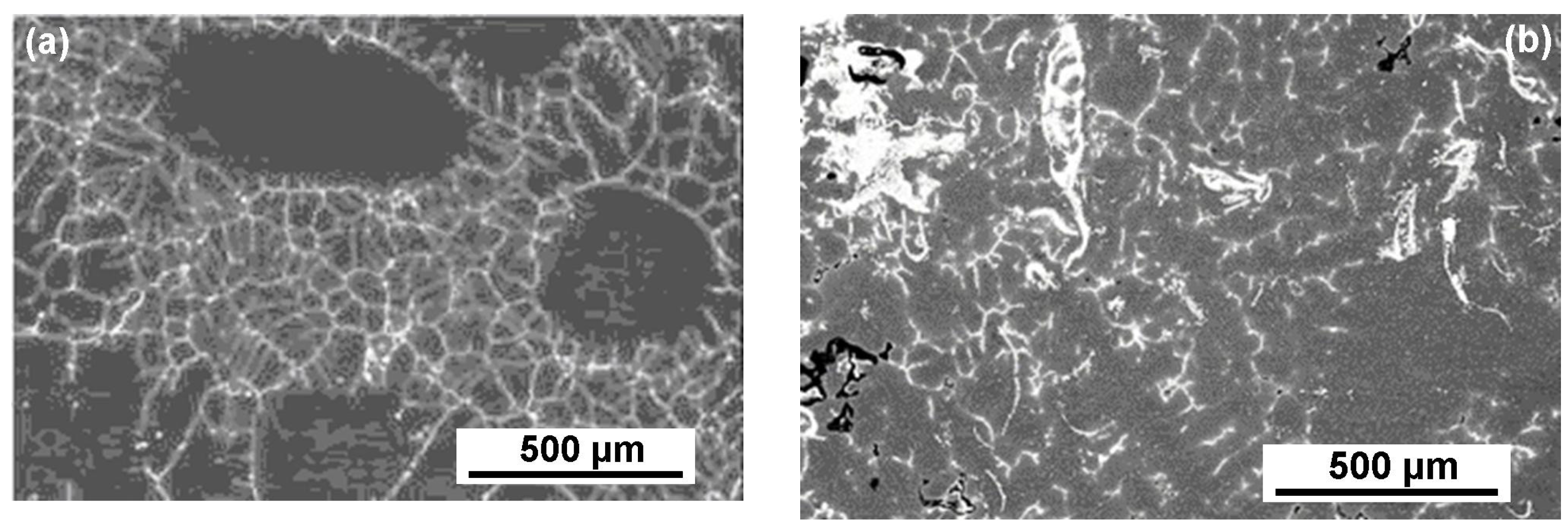
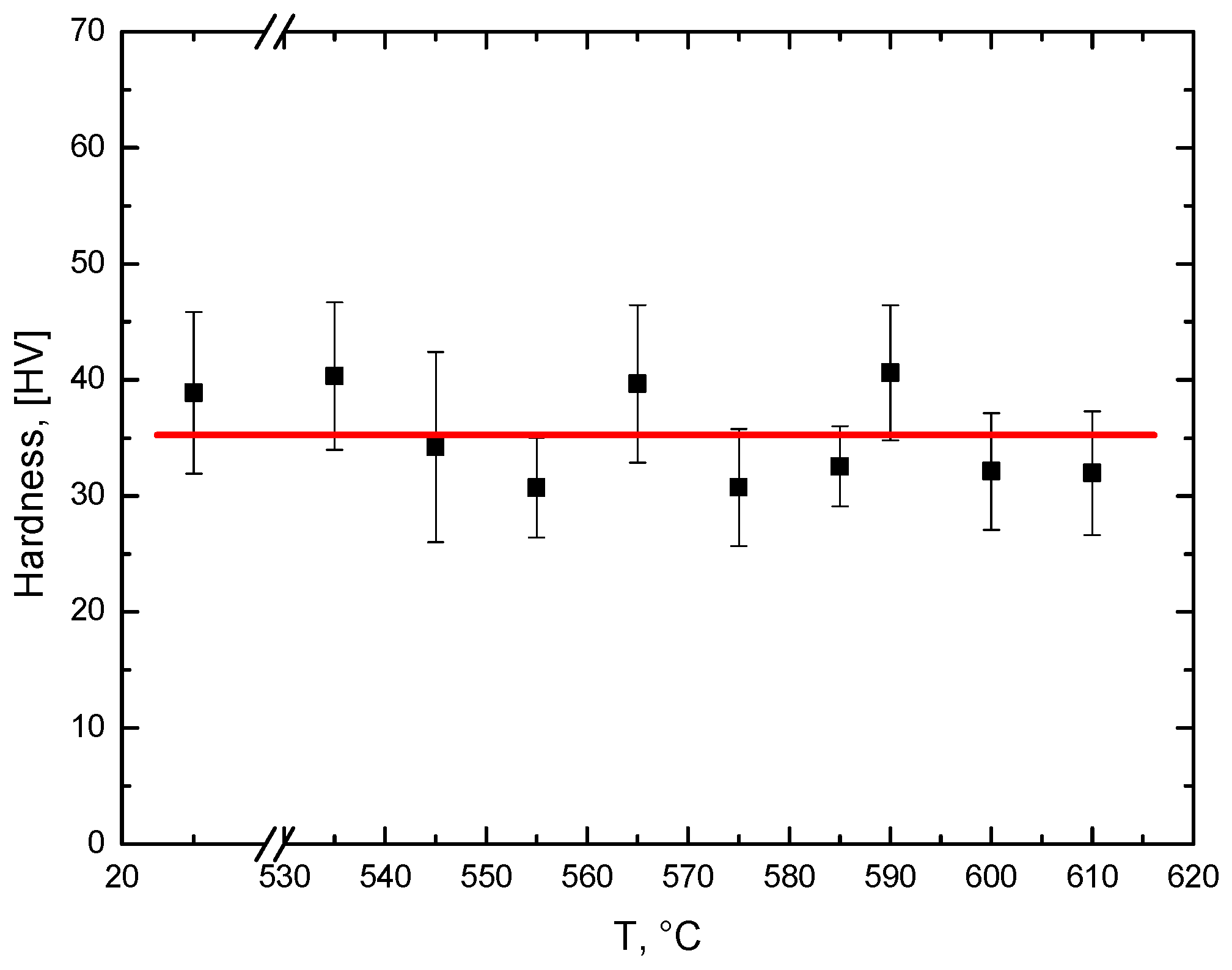
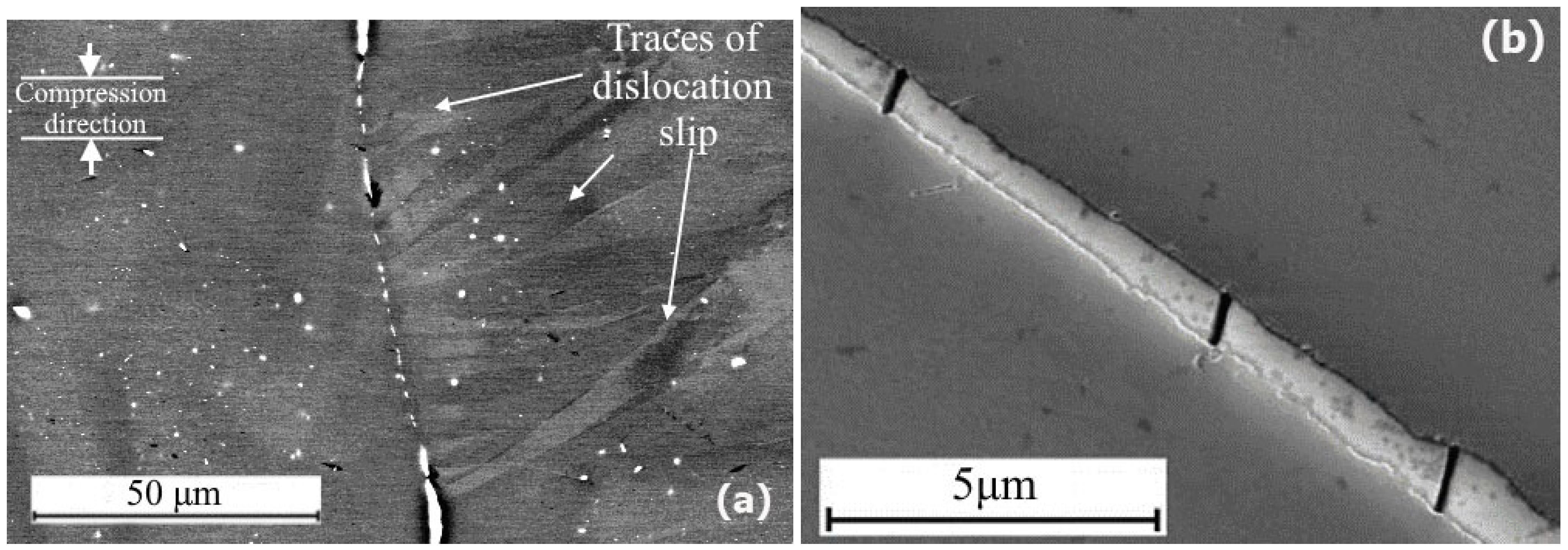
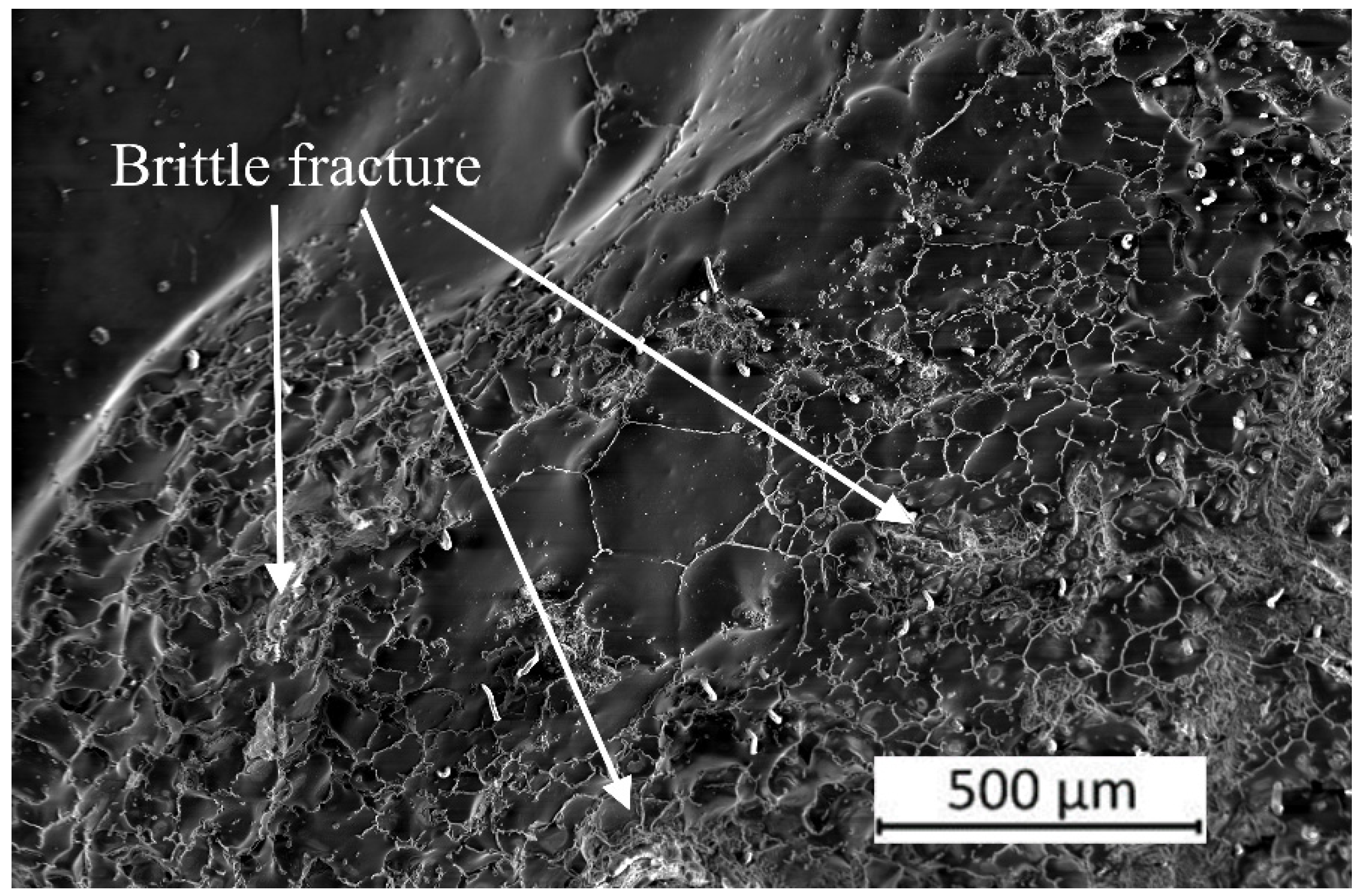
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryła, K.; Krystian, M.; Horky, J.; Mingler, B.; Mroczka, K.; Kurtyka, P.; Lityńska-Dobrzyńska, L. Improvement of Strength and Ductility of an EZ Magnesium Alloy by Applying Two Different ECAP Concepts to Processable Initial States. Mater. Sci. Eng. A 2018, 737, 318–327. [Google Scholar] [CrossRef]
- Bryła, K.; Morgiel, J.; Faryna, M.; Edalati, K.; Horita, Z. Effect of High-Pressure Torsion on Grain Refinement, Strength Enhancement and Uniform Ductility of EZ Magnesium Alloy. Mater. Lett. 2018, 212, 323–326. [Google Scholar] [CrossRef]
- Straumal, A.B.; Tsoy, K.V.; Mazilkin, I.A.; Nekrasov, A.N.; Bryła, K. Grain Boundary Wetting and Material Performance in an Industrial EZ33a Mg Cast Alloy. Arch. Metall. Mater. 2019, 64, 869–873. [Google Scholar] [CrossRef]
- Straumal, A.; Mazilkin, I.; Tzoy, K.; Straumal, B.; Bryła, K.; Baranchikov, A.; Eggeler, G. Bulk and Surface Low Temperature Phase Transitions in the Mg-Alloy EZ33A. Metals 2020, 10, 1127. [Google Scholar] [CrossRef]
- Trang, T.T.T.; Zhang, J.H.; Kim, J.H.; Zargaran, A.; Hwang, J.H.; Suh, B.C.; Kim, N.J. Designing a Magnesium Alloy with High Strength and High Formability. Nat. Commun. 2018, 9, 2522. [Google Scholar] [CrossRef]
- Oshida, Y. Magnesium Materials. From Mountain Bikes to Degradable Bone Grafts; De Gruyter Publishing: Berlin, Germany, 2021; p. 808. [Google Scholar] [CrossRef]
- Atrens, A.; Dietzel, W.; Srinivasan, P.B.; Winzer, N.; Kannan, M.B. Chapter 9: Stress Corrosion Cracking (SCC) of Magnesium Alloys. In Stress Corrosion Cracking: Theory and Practice; Woodhead Publishing: Cambridge, UK, 2011; pp. 341–380. [Google Scholar] [CrossRef]
- Martinez, D.C.; Dobkowska, A.; Marek, R.; Cwieka, H.; Jaroszewicz, J.; Plocinski, T.P.; Donik, C.; Helmholz, H.; Luthringer-Feyerabend, B.; Zeller-Plumhoff, B.; et al. In Vitro and In Vivo Degradation Behavior of Mg-0.45Zn-0.45Ca (ZX00) Screws for Orthopedic Applications. Bioact. Mat. 2023, 28, 132–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Kurukuri, S.; Worswick, M.J.; Li, D.Y.; Peng, Y.H.; Wu, P.D. The Strain Rate Sensitive and Anisotropic Behavior of Rare-Earth Magnesium Alloy ZEK100 Sheet. J. Magn. Alloys 2023, 11, 882–891. [Google Scholar] [CrossRef]
- Al-Samman, T.; Li, X. Sheet Texture Modification in Magnesium-Based Alloys by Selective Rare Earth Alloying. Mater. Sci. Eng. A 2011, 528, 3809–3822. [Google Scholar] [CrossRef]
- Patel, M.; Paudel, Y.; Mujahid, S.; Rhee, H.; El Kadiri, H. Self-Consistent Crystal Plasticity Modeling of Slip-Twin Interactions in Mg Alloys. Crystals 2023, 13, 653. [Google Scholar] [CrossRef]
- Ishiguro, Y.; Huang, X.; Tsukada, Y.; Koyama, T.; Chino, Y. Effect of Bending and Tension Deformation on the Texture Evolution and Stretch Formability of Mg–Zn–RE–Zr Alloy. Int. J. Miner. Metall. Mater. 2022, 29, 1334. [Google Scholar] [CrossRef]
- Griffits, D.; Davis, B.; Robson, J.D. The Influence of Strain Path on Rare Earth Recrystallization Textures in a Magnesium-Zinc-Rare Earth Alloy. Metal. Mater. Trans. A 2018, 49, 321–332. [Google Scholar] [CrossRef]
- Habib, S.A.; Khan, A.S.; Gnäupel-Herold, T.; Lloyd, J.T.; Schoenfeld, S.E. Anisotropy, Tension-Compression Asymmetry and Texture Evolution of a Rare-Earth-Containing Magnesium Alloy Sheet, ZEK100, at Different Strain Rates and Temperatures: Experiments and Modeling. Int. J. Plast. 2017, 95, 163–190. [Google Scholar] [CrossRef]
- Becker, R.; Lloyd, J.T. A Reduced-Order Crystal Model for HCP Metals: Application to Mg. Mech. Mater. 2016, 98, 98–110. [Google Scholar] [CrossRef]
- Omer, K.; Butcher, C.; Worswick, M. Characterization of Heat Transfer Coefficient for Non-isothermal Elevated Temperature Forming of Metal Alloys. Int. J. Mater. Form. 2020, 13, 177–201. [Google Scholar] [CrossRef]
- Hadadzadeh, A.; Wells, M.A.; Javaid, A. Warm and Hot Deformation Behavior of As-delivered ZEK100 Magnesium Alloy. Exp. Mech. 2016, 56, 259–271. [Google Scholar] [CrossRef]
- Abedini, A.; Butcher, C.; Worswick, M.J. Experimental Fracture Characterisation of an Anisotropic Magnesium Alloy Sheet in Proportional and Non-proportional Loading Conditions. Int. J. Solids Struct. 2018, 144–145, 1–19. [Google Scholar] [CrossRef]
- Abedini, A.; Butcher, C.; Worswick, M.J. Fracture Characterization of Rolled Sheet Alloys in Shear Loading: Studies of Specimen Geometry, Anisotropy, and Rate Sensitivity. Exp. Mech. 2017, 57, 75–88. [Google Scholar] [CrossRef]
- Ray, A.K.; Wilkinson, D.S. The Effect of Microstructure on Damage and Fracture in AZ31B and ZEK100 Magnesium Alloys. Mater. Sci. Eng. A 2016, 658, 33–41. [Google Scholar] [CrossRef]
- Li, Q.; Yea, W.; Gaoa, H.; Gao, L. Improving the Corrosion Resistance of ZEK100 Magnesium Alloy by Combining High-pressure Torsion Technology with Hydroxyapatite Coating. Mater. Des. 2019, 181, 107933. [Google Scholar] [CrossRef]
- Min, J.; Hector, L.G., Jr.; Lin, J.; Carter, J.T.; Sachdev, A.K. Spatio-temporal Characteristics of Propagative Plastic Instabilities in a Rare Earth Containing Magnesium Alloy. Int. J. Plast. 2014, 57, 52–76. [Google Scholar] [CrossRef]
- Kamrani, S.; Fleck, C. Effects of Calcium and Rare-earth Elements on the Microstructure and Tension–Compression Yield Asymmetry of ZEK100 Alloy. Mater. Sci. Eng. A 2014, 618, 238–243. [Google Scholar] [CrossRef]
- Ertürk, S.; Brocks, W.; Bohlen, J.; Letzig, D.; Steglich, D. A Constitutive Law for the Thermo-mechanical Modelling of Magnesium Alloy Extrusion. Int. J. Mater. Form. 2012, 5, 325–339. [Google Scholar] [CrossRef]
- Dobroñ, P.; Chmelík, F.; Parfenenko, K.; Letzig, D.; Bohlen, J. On the Effect of the Extrusion Speed on Microstructure and Plastic Deformation of ZE10 and ZEK100 Magnesium Alloys—An Acoustic Emission Study. Acta Phys. Pol. 2012, 122, 593–596. [Google Scholar] [CrossRef]
- Javaid, A.; Czerwinski, F. Effect of Hot Rolling on Microstructure and Properties of the ZEK100 Alloy. J. Magn. Alloys 2019, 7, 27–37. [Google Scholar] [CrossRef]
- Boba, M.; Butcher, C.; Panahi, N.; Worswick, M.J.; Mishra, R.K.; Carter, J.T. Warm Forming Limits of Rare Earth-magnesium Alloy ZEK100 Sheet. Int. J. Mater. Form. 2017, 10, 181–191. [Google Scholar] [CrossRef]
- Roostaei, A.A.; Ling, Y.; Jahed, H.; Glinka, G. Applications of Neuber’s and Glinka’s Notch Plasticity Correction Rules to Asymmetric Magnesium Alloys Under Cyclic Load. Theor. Appl. Fract. Mech. 2020, 105, 102431. [Google Scholar] [CrossRef]
- Ling, Y.; Roostaei, A.A.; Glinka, G.; Jahed, H. Fatigue of ZEK100-F Magnesium Alloy: Characterisation and Modelling. Int. J. Fatigue 2019, 125, 179–186. [Google Scholar] [CrossRef]
- Chen, G.; Ren, J.; Gao, H.; Cui, Y.; Chen, X. Pseudoelastic and Corrosion Behaviors of Mg ZEK100 Alloy under Cyclic Loading. Int. J. Fatigue 2017, 103, 466–477. [Google Scholar] [CrossRef]
- Mokdad, F.; Chen, D.L. Cyclic Deformation and Anelastic Behavior of ZEK100 Magnesium Alloy: Effect of Strain Ratio. Mater. Sci. Eng. A 2015, 640, 243–258. [Google Scholar] [CrossRef]
- Mokdad, F.; Chen, D.L. Strain-controlled Low Cycle Fatigue Properties of a Rare-earth Containing ZEK100 Magnesium Alloy. Mater. Des. 2015, 67, 436–447. [Google Scholar] [CrossRef]
- Min, J.; Lin, J. Anelastic Behavior and Phenomenological Modeling of Mg ZEK100-O Alloy Sheet under Cyclic Tensile Loading–Unloading. Mater. Sci. Eng. A 2013, 561, 174–182. [Google Scholar] [CrossRef]
- Aslam, I.; Li, B.; McClelland, Z.; Horstemeyer, S.J.; Maa, Q.; Wang, P.T.; Horstemeyer, M.F. Three-point Bending Behavior of a ZEK100 Mg Alloy at Room Temperature. Mater. Sci. Eng. A 2014, 590, 168–173. [Google Scholar] [CrossRef]
- Noder, J.; Abedini, A.; Butcher, C. Evaluation of the VDA 238–100 Tight Radius Bend Test for Plane Strain Fracture Characterization of Automotive Sheet Metals. Exp. Mech. 2020, 60, 787–800. [Google Scholar] [CrossRef]
- Paudel, Y.R.; Indeck, J.; Hazeli, K.; Priddy, M.W.; Inal, K.; Rhee, H.; Barrett, C.D.; Whittington, W.R.; Limmer, K.R.; El Kadiri, H. Characterization and Modeling of {1012} Twin Banding in Magnesium. Acta Mater. 2020, 183, 438–451. [Google Scholar] [CrossRef]
- Min, J.; Lin, J.; Li, J. Forming Limits of Mg alloy ZEK100 Sheet in Preform Annealing Process. Mater. Des. 2014, 53, 947–953. [Google Scholar] [CrossRef]
- Min, J.; Hector, L.G., Jr.; Lin, J.; Carter, J.T. Analytical Method for Forming Limit Diagram Prediction with Application to a Magnesium ZEK100-O Alloy. J. Mater. Eng. Perform. 2013, 22, 3324–3336. [Google Scholar] [CrossRef]
- Antoniswamy, A.R.; Carpenter, A.J.; Carter, J.T.; Hector, L.G., Jr.; Taleff, E.M. Forming-Limit Diagrams for Magnesium AZ31B and ZEK100 Alloy Sheets at Elevated Temperatures. J. Mater. Eng. Perform. 2013, 22, 3389–3397. [Google Scholar] [CrossRef]
- Bong, H.J.; Hu, X.; Sun, X.; Ren, Y. Temperature-Dependent Constitutive Modeling of a Magnesium Alloy ZEK100 Sheet Using Crystal Plasticity Models Combined with In Situ High-Energy X-ray Diffraction Experiment. J. Magnes. Alloys 2022, 10, 2801–2816. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, B.; Jiang, Y.; Wu, P.; Wang, H. Multi-Island Genetic-Algorithm-Based Approach to Uniquely Calibrate Polycrystal Plasticity Models for Magnesium Alloys. JOM 2021, 73, 1395–1403. [Google Scholar] [CrossRef]
- Bong, H.J.; Hu, X.; Sun, X.; Ren, Y. Mechanism-Based Constitutive Modeling of ZEK100 Magnesium Alloy with Crystal Plasticity and In-Situ HEXRD Experiment. Int. J. Plast. 2019, 113, 35–51. [Google Scholar] [CrossRef]
- Abedini, A.; Butcher, C.; Worswick, M.J. Application of an Evolving Non-Associative Anisotropic-Asymmetric Plasticity Model for a Rare-Earth Magnesium Alloy. Metals 2018, 8, 1013. [Google Scholar] [CrossRef]
- Zhang, K.; Badreddine, H.; Saanouni, K. Thermodynamically-Consistent Constitutive Modeling of Hardening Asymmetry Including Isotropic Ductile Damage for Mg Alloys. Eur. J. Mech. A Solids 2019, 73, 169–180. [Google Scholar] [CrossRef]
- Abedini, A.; Butcher, C.; Nemcko, M.J.; Kurukuri, S.; Worswick, M.J. Constitutive Characterization of a Rare-Earth Magnesium Alloy Sheet (ZEK100-O) in Shear Loading: Studies of Anisotropy and Rate Sensitivity. Int. J. Mech. Sci. 2017, 128–129, 54–69. [Google Scholar] [CrossRef]
- Muhammad, W.; Mohammadi, M.; Kang, J.; Mishra, R.K.; Inal, K. An Elasto-Plastic Constitutive Model for Evolving Asymmetric/anisotropic Hardening Behavior of AZ31B and ZEK100 Magnesium Alloy Sheets Considering Monotonic and Reverse Loading Paths. Int. J. Plast. 2015, 70, 30–59. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saxena, K.K.; Malik, V.; Mohammed, K.A.; Prakash, C.; Buddhi, D.; Dixit, S. Significance of Alloying Elements on the Mechanical Characteristics of Mg-Based Materials for Biomedical Applications. Crystals 2022, 12, 1138. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, M.; Zhao, J.; Gao, L.; Li, M. In Vitro and in Vivo Degradation and Mechanical Properties of ZEK100 Magnesium Alloy Coated with Alginate, Chitosan and Mechano-growth Factor. Mater. Sci. Eng. C 2016, 63, 450–461. [Google Scholar] [CrossRef]
- Grillo, C.A.; Alvarez, F.; Fernández Lorenzo de Mele, M.A. Degradation of Bioabsorbable Mg-Based Alloys: Assessment of the Effects of Insoluble Corrosion Products and Joint Effects of Alloying Components on Mammalian Cells. Mater. Sci. Eng. C 2016, 58, 372–380. [Google Scholar] [CrossRef]
- Reifenrath, J.; Marten, A.-K.; Angrisani, N.; Eifler, R.; Weizbauer, A. In Vitro and In Vivo Corrosion of the Novel Magnesium Alloy Mg–La–Nd–Zr: Influence of the Measurement Technique and in vivo Implant Location. Biomed. Mater. 2015, 10, 045021. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, L.-L.; Gao, H.; Yuan, X.; Chen, X. Biodegradable Behaviour and Fatigue Life of ZEK100 Magnesium Alloy in Simulated Physiological Environment. Fatigue Fract. Eng. Mater. Struct. 2015, 38, 904–913. [Google Scholar] [CrossRef]
- Bondarenko, A.; Angrisani, N.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Reifenrath, J. Magnesium-based Bone Implants: Immunohistochemical Inalysis of Peri-implant Osteogenesis by Evaluation of Osteopontin and Osteocalcin Expression. J. Biomed. Mater. Res. Part A 2014, 102, 1449–1457. [Google Scholar] [CrossRef]
- Weizbauer, A.; Modrejewski, C.; Behrens, S.; Klein, H.; Helmecke, P.; Seitz, J.-M.; Windhagen, H.; Möhwald, K.; Reifenrath, J.; Waizy, H. Comparative in vitro Study and Biomechanical Testing of Two Different Magnesium Alloys. J. Biomater. Appl. 2014, 28, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-term in vivo Degradation Behaviour and Biocompatibility of the Magnesium Alloy ZEK100 for use as a Biodegradable Bone Implant. Acta Biomater. 2013, 9, 8548–8560. [Google Scholar] [CrossRef]
- Reifenrath, J.; Angrisani, N.; Erdmann, N.; Lucas, A.; Waizy, H.; Seitz, J.M.; Bondarenko, A.; Meyer-Lindenberg, A. Degrading Magnesium Screws ZEK100: Biomechanical Testing, Degradation Analysis and Soft-tissue Biocompatibility in a Rabbit Model. Biomed. Mater. 2013, 8, 045012. [Google Scholar] [CrossRef]
- Bauer, M.; Biskup, C.; Schilling, T.; Haverich, A.; Bach, F.W.; Maier, H.J.; Hassel, T. Influence of Shot Peening on Surface Rougness and in vitro Load Cycles of Magnesium Alloys. Biomed. Tech. 2013, 58, 4060. [Google Scholar] [CrossRef]
- Weidling, M.; Besdo, S.; Schilling, T.; Bauer, M.; Hassel, T.; Haverich, A.; Wriggers, P. Finite Element Simulation of Myocardial Stabilising Structures and Development of New Designs. Biomed. Tech. 2013, 58, 4061. [Google Scholar] [CrossRef]
- Schulman, J.; Meyer-Lindenberg, A.; Goblet, F.; Bormann, D.; Stiller, W.; Seifert, H. Phantomuntersuchungen an einem Hochauflösenden CT zur Ex-vivo-Darstellung von Degradierbaren Magnesiumimplantaten und Simulierten Periimplantären Knochenschichten in Kaninchentibiae. Fortschr. Röntgenstr. 2012, 184, 455–460. [Google Scholar] [CrossRef]
- Huehnerschulte, T.A.; Angrisani, N.; Rittershaus, D.; Bormann, D.; Windhagen, H.; Meyer-Lindenberg, A. In Vivo Corrosion of Two Novel Magnesium Alloys ZEK100 and AX30 and Their Mechanical Suitability as Biodegradable Implants. Materials 2011, 4, 1144–1167. [Google Scholar] [CrossRef] [PubMed]
- Huehnerschulte, T.A.; Reifenrath, J.; von Rechenberg, B.; Dziuba, D.; Seitz, J.M.; Bormann, D.; Windhagen, H.; Meyer-Lindenberg, A. In Vivo Assessment of the Host Reactions to the Biodegradation of the Two Novel Magnesium Alloys ZEK100 and AX30 in an Animal Model. BioMed. Eng. OnLine 2012, 11, 14. [Google Scholar] [CrossRef]
- Seitz, J.-M.; Utermöhlen, D.; Wulf, E.; Klose, C.; Bach, F.-W. The Manufacture of Resorbable Suture Material from Magnesium—Drawing and Stranding of Thin Wires. Adv. Eng. Mater. 2011, 13, 1087–1095. [Google Scholar] [CrossRef]
- Seitz, J.-M.; Wulf, E.; Freytag, P.; Bormann, D.; Bach, F.-W. The Manufacture of Resorbable Suture Material from Magnesium. Adv. Eng. Mater. 2010, 12, 1099–1105. [Google Scholar] [CrossRef]
- Zaghloul, B.; Kish, J.R. Corrosion Inhibition of Mg Alloy ZEK100 Sheet Metal by Dissolved Lithium Carbonate. J. Electrochem. Soc. 2021, 168, 081507. [Google Scholar] [CrossRef]
- Brady, M.P.; Rother, G.; Frith, M.G.; Ievlev, A.E.; Leonard, D.N.; Littrell, K.C.; Cakmak, E.; MeyerIII, H.M.; Anovitz, L.M.; Davis, B. Temporal Evolution of Corrosion Film Nano-Porosity and Magnesium Alloy Hydrogen Penetration in NaCl Solution. J. Electrochem. Soc. 2020, 167, 131513. [Google Scholar] [CrossRef]
- Brady, M.P.; Leonard, D.N.; McNally, E.A.; Kish, J.R.; Meyer, H.M.; Cakmak, E.; Davis, B. Magnesium Alloy Effects on Plasma Electrolytic Oxidation Electro-Ceramic and Electro-Coat Formation and Corrosion Resistance. J. Electrochem. Soc. 2019, 166, C492–C508. [Google Scholar] [CrossRef]
- Binns, W.J.; Zargarzadah, F.; Dehnavi, V.; Chen, J.; Noël, J.J.; Shoesmith, D.W. Physical and Electrochemical Evidence for the Role of a Mg Hydride Species in Mg Alloy Corrosion. Corrosion 2019, 75, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.P.; Leonard, D.N.; Meyer III, H.M.; Thomson, J.K.; Unocic, K.A.; Elsentriecy, H.H.; Song, G.-L.; Kitchen, K.; Davis, B. Advanced Characterization Study of Commercial Conversion and Electrocoating Structures on Magnesium Alloys AZ31B and ZE10A. Surf. Coat. Technol. 2016, 294, 164–176. [Google Scholar] [CrossRef]
- Dauphin-Ducharme, P.; Binns, W.J.; Snowden, M.E.; Shoesmith, D.W.; Mauzeroll, J. Determination of the Local Corrosion Rate of Magnesium Alloys Using a Shear Force Mounted Scanning Microcapillary Method. Faraday Discuss. 2015, 180, 331–345. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Binns, W.J.; Jakupi, P.; Shoesmith, D. The Influence of Microstructure on the Corrosion of Magnesium Alloy ZEK100. Corrosion 2015, 71, 242–254. [Google Scholar] [CrossRef]
- Waizy, H.; Weizbauer, A.; Modrejewski, C.; Witte, F.; Windhagen, H.; Lucas, A.; Kieke, M.; Denken, B.; Behrens, P.; Meyer-Lindenberg, A. In Vitro Corrosion of ZEK100 Plates in Hank’s Balanced Salt Solution. BioMed. Eng. OnLine 2012, 11, 12. [Google Scholar] [CrossRef]
- Bauer, M.; Hassel, T.; Biskup, C.; Hartung, D.; Schilling, T.; Weidling, M.; Wriggers, P.; Wacker, F.; Bach, F.W.; Haverich, A. Geometric Adaption of Resorbable Myocardial Stabilizing Structures Based on the Magnesium Alloys LA63 and ZEK100 for the Support of Myocardial Grafts on the Left Ventricle. Biomed. Eng. 2012, 57, 22–25. [Google Scholar] [CrossRef]
- Gao, H.; Ye, W.; Zhang, Z.; Gao, L. Ratcheting Behavior of ZEK100 Magnesium Alloy with Various Loading Conditions and Different Immersing Time. J. Mater. Res. 2017, 32, 2143–2152. [Google Scholar] [CrossRef]
- Krüger, R.; Seitz, J.-M.; Ewald, A.; Bach, F.-W.; Groll, J. Strong and Tough Magnesium Wire Reinforced Phosphate Cement Composites for Load-bearing Bone Replacement. J. Mech. Behav. Biomed. Mater. 2013, 20, 36–44. [Google Scholar] [CrossRef]
- Peng, H.; Chen, D.L.; Bai, X.F.; Wang, P.Q.; Li, D.Y.; Jiang, X.Q. Microstructure and Mechanical Properties of Mg-to-Al Dissimilar Welded Joints with an Ag Interlayer using Ultrasonic Spot Welding. J. Magn. Alloys 2020, 8, 552–563. [Google Scholar] [CrossRef]
- Macwan, A.; Chen, D.L. Ultrasonic Spot Welding of a Rare-Earth Containing ZEK100 Magnesium Alloy: Effect of Welding Energy. Metal. Mater Trans. A 2016, 47, 1686–1697. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Amirkhiz, B.S.; Worswick, M.J.; Gerlich, A.P. Microstructures and Properties of Mg alloy/DP600 Steel Dissimilar Refill Friction Stir Spot Welds. Sci. Technol. Weld. Join. 2015, 20, 494–501. [Google Scholar] [CrossRef]
- Rodriguez, R.I.; Jordon, J.B.; Rao, H.M.; Badarinarayan, H.; Yuan, W.; ElKadiri, H.; Allison, P.G. Microstructure, Texture, and Mechanical Properties of Friction Stir Spot Welded Rare-Earth Containing ZEK100 Magnesium Alloy Sheets. Mater. Sci. Eng. A 2014, 618, 637–644. [Google Scholar] [CrossRef]
- Peng, H.; Chen, D.L.; Bai, X.F.; She, X.W.; Li, D.Y.; Jiang, X.Q. Ultrasonic Spot Welding of Magnesium-to-Aluminum Alloys with a Copper Interlayer: Microstructural Evolution and Tensile Properties. J. Manuf. Proc. 2019, 37, 91–100. [Google Scholar] [CrossRef]
- Peng, H.; Jiang, X.; Bai, X.; Li, D.; Chen, D. Microstructure and Mechanical Properties of Ultrasonic Spot Welded Mg/Al Alloy Dissimilar Joints. Metals 2018, 8, 229. [Google Scholar] [CrossRef]
- Macwan, A.; Chen, D.L. Ultrasonic Spot Welding of Rare-Earth Containing ZEK100 Magnesium Alloy to 5754 Aluminum Alloy. Mater. Sci. Eng. A 2016, 666, 139–148. [Google Scholar] [CrossRef]
- Kuang, J.; Li, X.; Ye, X.; Tang, J.; Liu, H.; Wang, J.; Tang, G. Microstructure and Texture Evolution of Magnesium Alloys During Electropulse Treatment. Metall. Mater. Trans. A 2015, 46, 1789–1804. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Z.; Li, Y.; Zhang, H.; Zhou, N. Laser Welding/Brazing of 5182 Aluminium Alloy to ZEK100 Magnesium Alloy Using a Nickel Interlayer. Sci. Technol. Weld. Join. 2018, 23, 543–550. [Google Scholar] [CrossRef]
- Yang, J.; Su, J.H.; Yu, Z.S.; Zhang, G.Z.; Lin, S.B.; Li, Y.L.; Zhou, N.Y. Influence of Ni Interlayer Width on Interfacial Reactions and Mechanical Properties in Laser Welding/Brazing of Al/Mg Lap Joint. Sci. Technol. Weld. Join. 2019, 25, 37–44. [Google Scholar] [CrossRef]
- Cahn, J.W. Critical Point Wetting. J. Chem. Phys. 1977, 66, 3667. [Google Scholar] [CrossRef]
- Ebner, C.; Saam, W.F. New Phase-Transition Phenomena in Thin Argon Films. Phys. Rev. Lett. 1977, 38, 1486. [Google Scholar] [CrossRef]
- Straumal, B.B.; Polyakov, S.A.; Bischoff, E.; Gust, W.; Mittemeijer, E.J. Faceting of Σ3 and Σ9 grain boundaries in copper. Interface Sci. 2001, 9, 287–292. [Google Scholar] [CrossRef]
- Maksimova, E.L.; Shvindlerman, L.S.; Straumal, B.B. Transformation of Σ17 special tilt boundaries to general boundaries in tin. Acta Metall. 1988, 36, 1573–1583. [Google Scholar] [CrossRef]
- Laporte, D.; Watson, E.B. Experimental and Theoretical Constraints on Melt Distribution in Crustal Sources: The Effect of Crystalline Anisotropy on Melt Interconnectivity. Chem. Geol. 1995, 124, 161–184. [Google Scholar] [CrossRef]
- Noskovich, O.I.; Rabkin, E.I.; Semenov, V.N.; Straumal, B.B.; Shvindlerman, L.S. Wetting and premelting phase transitions in 38° [100] tilt grain boundaries in (Fe–12at.%Si) Zn alloy in the vicinity of the A2–B2 bulk ordering in Fe–12at.%Si alloy. Acta Metall. Mater. 1991, 39, 3091–3098. [Google Scholar] [CrossRef]
- Chang, L.-S.; Rabkin, E.; Straumal, B.B.; Hoffmann, S.; Baretzky, B.; Gust, W. Grain boundary segregation in the Cu–Bi system. Defect Diff. Forum 1998, 156, 135–146. [Google Scholar] [CrossRef]
- Ross, D.; Bonn, D.; Meunier, J. Observation of Short-Range Critical Wetting. Nature 1999, 400, 737–739. [Google Scholar] [CrossRef]
- Straumal, B.B.; Gust, W.; Watanabe, T. Tie lines of the grain boundary wetting phase transition in the Zn-rich part of the Zn–Sn phase diagram. Mater. Sci. Forum 1999, 294–296, 411–414. [Google Scholar] [CrossRef]
- Rabkin, E.I.; Shvindlerman, L.S.; Straumal, B.B. Grain Boundaries: Phase Transitions and Critical Phenomena. Int. J. Mod. Phys. B 1991, 5, 2989–3028. [Google Scholar] [CrossRef]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid Phase Sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Straumal, B.; Rabkin, E.; Lojkowski, W.; Gust, W.; Shvindlerman, L.S. Pressure influence on the grain boundary wetting phase transition in Fe–Si alloys. Acta Mater. 1997, 45, 1931–1940. [Google Scholar] [CrossRef]
- Straumal, A.B.; Mazilkin, I.A.; Tsoi, K.V.; Baretzky, B.; Straumal, B.B. “Wetting” Phase Transitions by the Second Solid Phase for Linear Defects (Grain Boundary Triple Junctions). JETP Lett. 2020, 112, 257–261. [Google Scholar] [CrossRef]
- Straumal, A.B.; Bokstein, B.S.; Petelin, A.L.; Straumal, B.B.; Baretzky, B.; Rodin, A.O.; Nekrasov, A.N. Apparently Complete Grain Boundary Wetting in Cu–In Alloys. J. Mater. Sci. 2012, 47, 8336–8343. [Google Scholar] [CrossRef]
- Jandaghi, M.R.; Pouraliakbar, H.; Hong, S.I.; Pavese, M. Grain Boundary Transition Associated Intergranular Failure Analysis at TMAZ/SZ Interface of Dissimilar AA7475-AA2198 Joints by Friction Stir Welding. Mater. Lett. 2020, 280, 128557. [Google Scholar] [CrossRef]
- Jandaghi, M.R.; Pouraliakbar, H.; Saboori, A.; Hong, S.I.; Pavese, M. Comparative Insight into the Interfacial Phase Evolutions during Solution Treatment of Dissimilar Friction Stir Welded AA2198-AA7475 and AA2198-AA6013 Aluminum Sheets. Materials 2021, 14, 1290. [Google Scholar] [CrossRef] [PubMed]
- Molodov, D.A.; Straumal, B.B.; Shvindlerman, L.S. The Effect of Pressure on Migration of the [001] Tilt Grain Boundaries in the Tin Bicrystals. Scripta metall. 1984, 18, 207–211. [Google Scholar] [CrossRef]
- Straumal, B.B.; Gornakova, A.S.; Kucheev, Y.O.; Baretzky, B.; Nekrasov, A.N. Grain Boundary Wetting by a Second Solid Phase in the Zr–Nb Alloys. J. Mater. Eng. Perform. 2012, 21, 721–724. [Google Scholar] [CrossRef]
- Mazilkin, A.A.; Abrosimova, G.E.; Protasova, S.G.; Straumal, B.B.; Schütz, G.; Dobatkin, S.V.; Bakai, A.S. Transmission Electron Microscopy Investigation of Boundaries Between Amorphous “Grains” in Ni50Nb20Y30 Alloy. J. Mater. Sci. 2011, 46, 4336–4342. [Google Scholar] [CrossRef]
- Chang, L.-S.; Straumal, B.B.; Rabkin, E.; Gust, W.; Sommer, F. The Solidus Line of the Cu–Bi Phase Diagram. J. Phase Equil. 1997, 18, 128–135. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Protasova, S.G.; Dobatkin, S.V.; Rodin, A.O.; Baretzky, B.; Goll, D.; Schütz, G. Fe–C Nanograined Alloys Obtained by High Pressure Torsion: Structure and Magnetic Properties. Mater. Sci. Eng. A 2009, 503, 185–189. [Google Scholar] [CrossRef]
- Khisamov, R.K.; Kistanov, A.A.; Nazarov, K.S.; Shayakhmetov, R.U.; Korznikova, G.F.; Yumaguzin, Y.M.; Dmitriev, S.V.; Mulyukov, R.R. Work Function of Chemical Compounds of Aluminum-Magnesium System. IOP Conf. Ser. Mater. Sci. Eng. 2020, 1008, 012032. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kilmametov, A.R.; Ivanisenko, Y.; Mazilkin, A.A.; Kogtenkova, O.A.; Kurmanaeva, L.; Korneva, A.; Zięba, P.; Baretzky, B. Phase Transitions Induced by Severe Plastic Deformation: Steady-State and Equifinality. Int. J. Mater. Res. 2015, 106, 657–664. [Google Scholar] [CrossRef]
- Khisamov, R.K.; Shayakhmetov, R.U.; Yumaguzin, Y.M.; Kistanov, A.A.; Korznikova, G.F.; Korznikova, E.A.; Nazarov, K.S.; Khalikova, G.R.; Timiryaev, R.R.; Mulyukov, R.R. Work Function, Sputtering Yield and Microhardness of an Al-Mg Metal-Matrix Nanostructured Composite Obtained with High-Pressure Torsion. Appl. Sci. 2023, 13, 5007. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Baretzky, B. Grain Boundary Complexions and Pseudopartial Wetting. Curr. Opin. Solid State Mater. Sci. 2016, 20, 247–256. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straumal, B.; Khrapova, N.; Druzhinin, A.; Tsoy, K.; Davdian, G.; Orlov, V.; Gerstein, G.; Straumal, A. Grain Boundary Wetting Transition in the Mg-Based ZEK 100 Alloy. Crystals 2023, 13, 1538. https://doi.org/10.3390/cryst13111538
Straumal B, Khrapova N, Druzhinin A, Tsoy K, Davdian G, Orlov V, Gerstein G, Straumal A. Grain Boundary Wetting Transition in the Mg-Based ZEK 100 Alloy. Crystals. 2023; 13(11):1538. https://doi.org/10.3390/cryst13111538
Chicago/Turabian StyleStraumal, Boris, Natalya Khrapova, Aleksandr Druzhinin, Kristina Tsoy, Gregory Davdian, Valery Orlov, Gregory Gerstein, and Alexander Straumal. 2023. "Grain Boundary Wetting Transition in the Mg-Based ZEK 100 Alloy" Crystals 13, no. 11: 1538. https://doi.org/10.3390/cryst13111538





