Construction of Energetic Complexes Based on LLM-105 and Transition Metal Cations (Ni, Co, Mn, and Cu)
Abstract
:1. Introduction
2. Results and Discussion
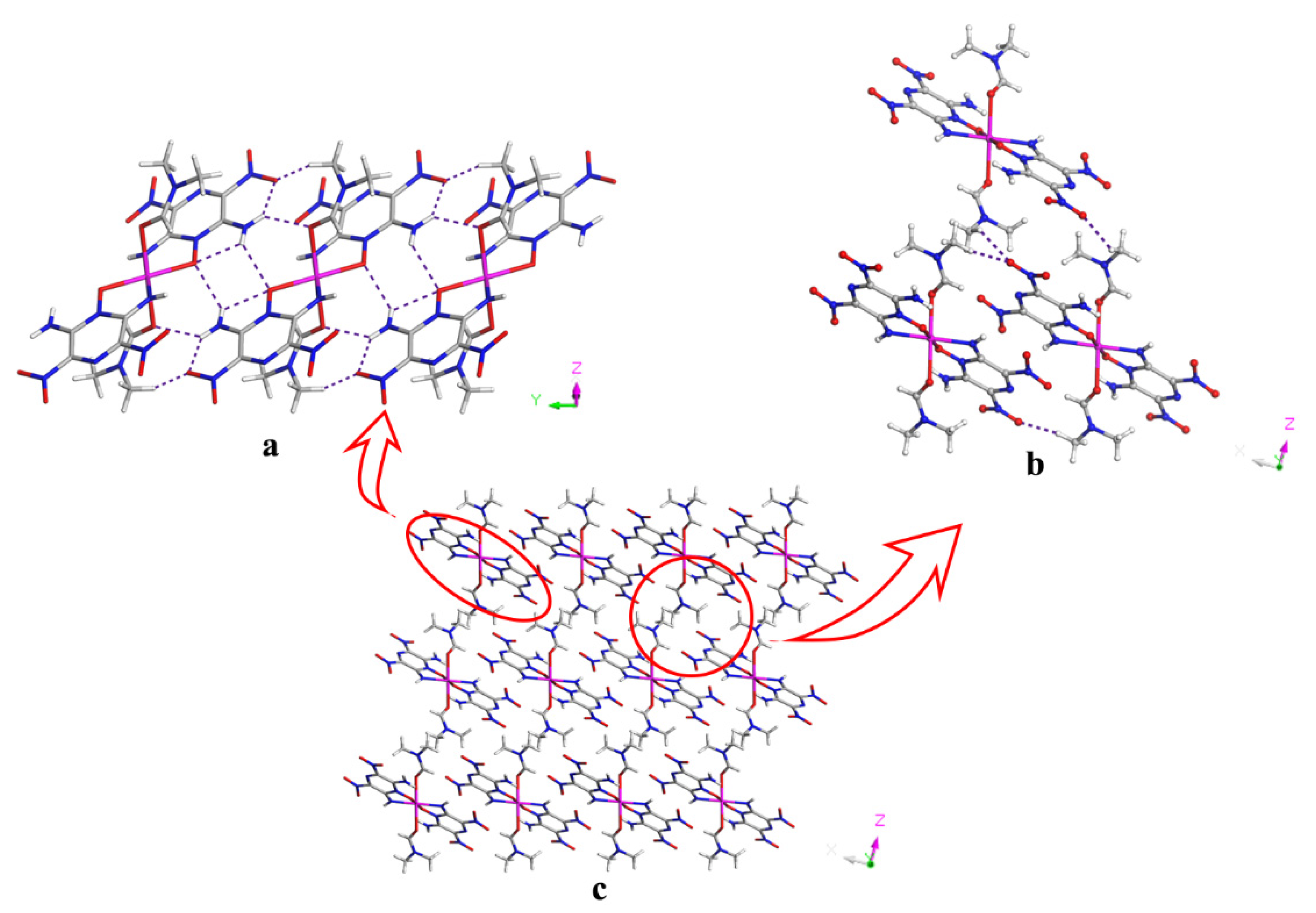
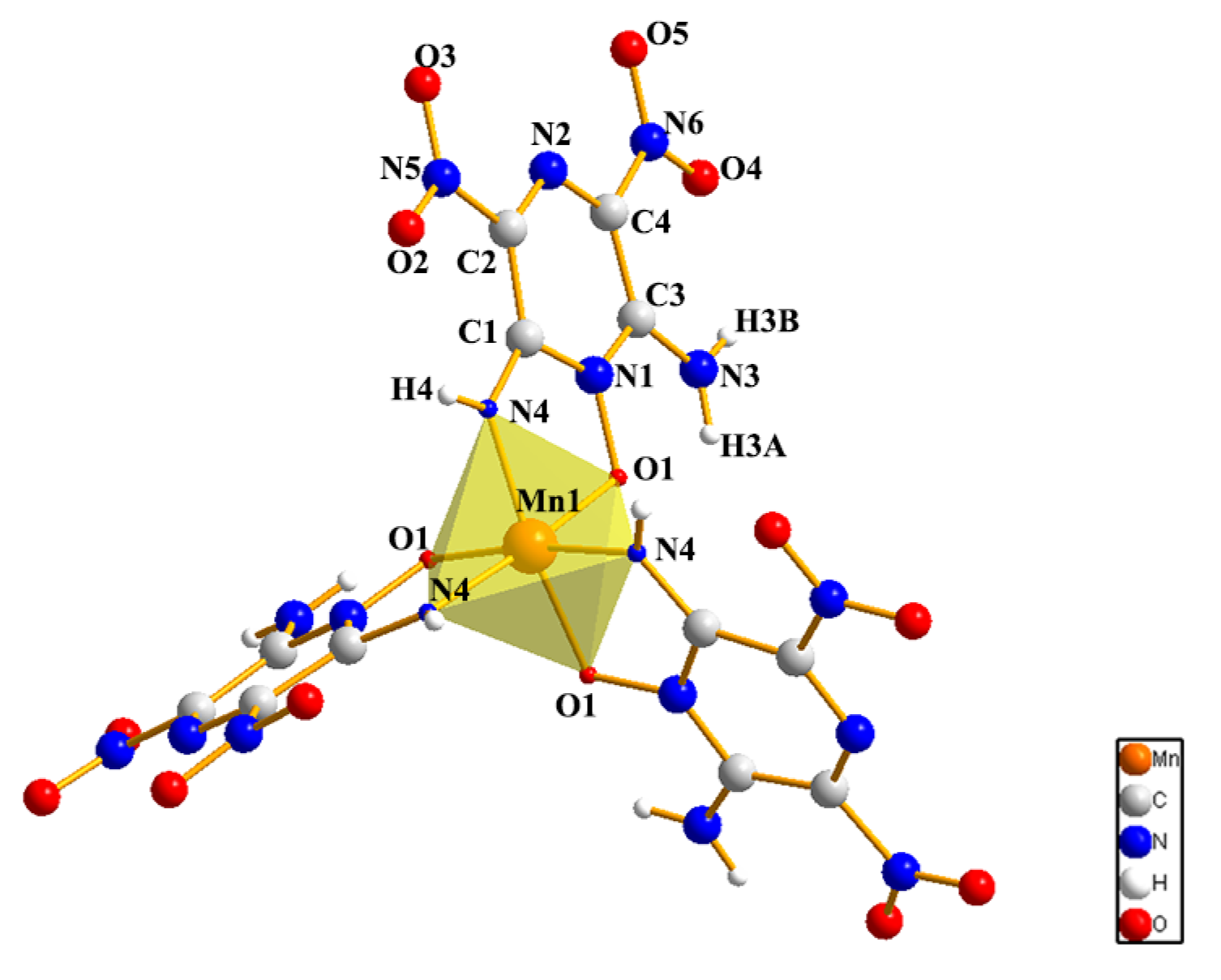
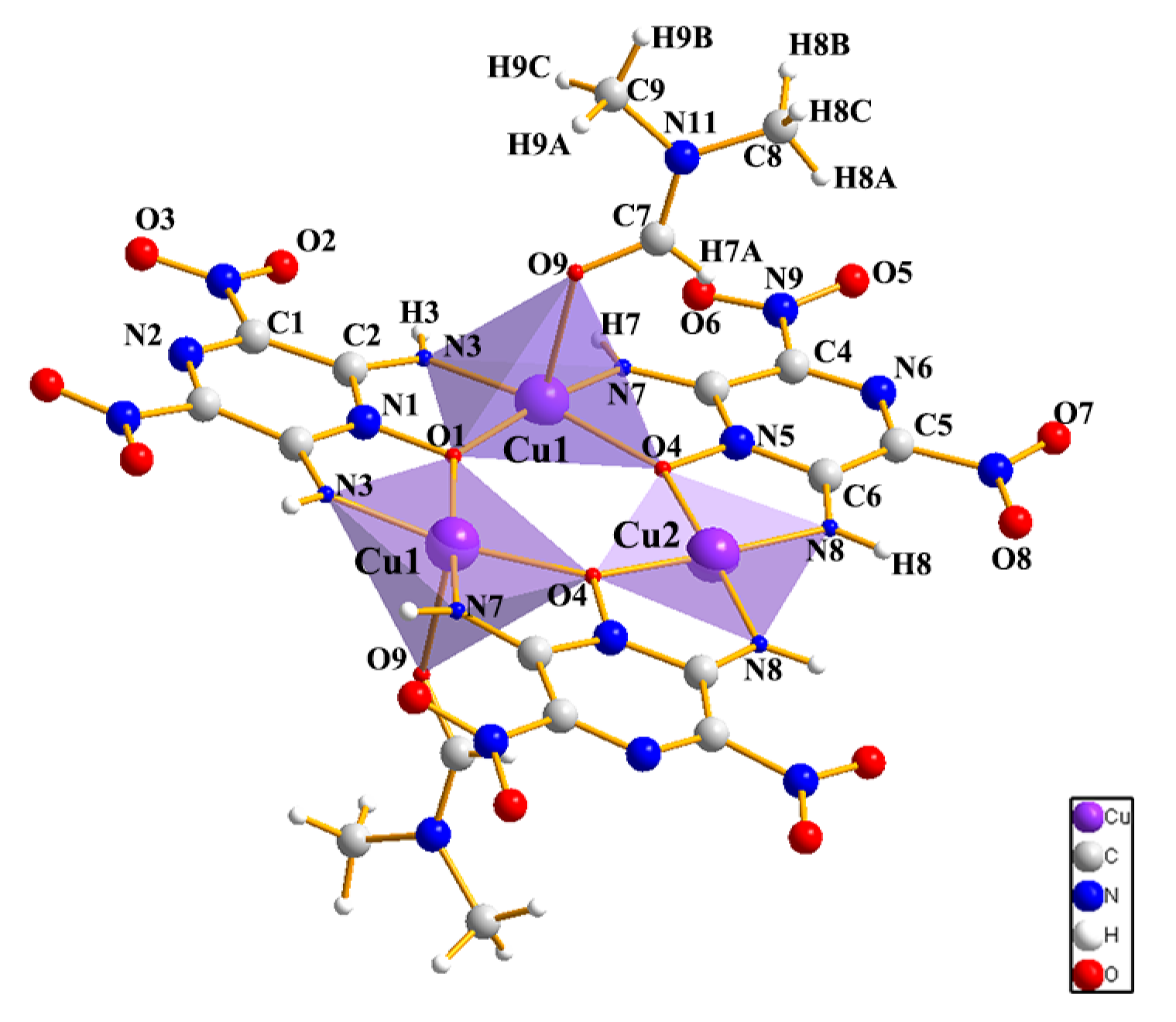
2.1. Structures
2.2. Energetic Properties
3. Conclusions
4. Experimental Section
4.1. Caution
4.2. Materials
4.3. Synthesis of Ni(LLM-105)2·DMF (1)
4.4. Synthesis of Co(LLM-105)2·2DMF (2)
4.5. Synthesis of Mn(LLM-105)3·3/2DMF (3)
4.6. Synthesis of Cu3(LLM-105)3·3DMF (4)
4.7. Structure Determination
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pagoria, P.F.; Lee, G.S.; Mitchell, A.R.; Schmidt, R.D. A review of energetic materials synthesis. Thermochim. Acta 2002, 384, 187–204. [Google Scholar] [CrossRef]
- Zeman, S.; Jungová, M. Sensitivity and performance of energetic materials. Propell. Explos. Pyrot. 2016, 41, 426–451. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C. Review of the molecular and crystal correlations on sensitivities of energetic materials. J. Hazard. Mater. 2020, 398, 122910. [Google Scholar] [CrossRef]
- Wu, B.D.; Bi, Y.G.; Li, F.G.; Yang, L.; Zhou, Z.N.; Zhang, J.G.; Zhang, T.L. A Novel Stable High-Nitrogen Energetic Compound: Copper (II) 1, 2–Diaminopropane Azide. Z. Anorg. Allg. Chem. 2014, 640, 224–228. [Google Scholar] [CrossRef]
- Wu, B.D.; Zhou, Z.N.; Li, F.G.; Yang, L.; Zhang, T.L.; Zhang, J.G. Preparation, crystal structures, thermal decompositions and explosive properties of two new high-nitrogen azide ethylenediamine energetic compounds. New. J. Chem. 2013, 37, 646–653. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, J.G.; Liu, Z.H.; Zhang, T.L.; Yang, L.; Qiao, X.J. Synthesis, structural characterization and thermal analysis of a high nitrogen-contented cadmium (II) coordination polymer based on 1,5-diaminotetrazole. J. Mol. Struct. 2011, 1004, 8–12. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Brown, P.; Maiti, A.; Gee, R.H.; Peterson, G.R.; Weeks, B.L.; Hope-Weeks, L.J. Ionic polymers as a new structural motif for high-energy-density materials. J. Am. Chem. Soc. 2012, 134, 1422–1425. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Peterson, G.R.; Brown, P.; Maiti, A.; Gee, R.H.; Weeks, B.L.; Hope-Weeks, L.J. Metal–organic frameworks (MOFs) as safer, structurally reinforced energetics. Chem.-Euro. J. 2013, 19, 1706–1711. [Google Scholar] [CrossRef]
- Liu, X.; Gao, W.; Sun, P.; Su, Z.; Chen, S.; Wei, Q.; Xie, G.; Gao, S. Environmentally friendly high-energy MOFs: Crystal structures, thermostability, insensitivity and remarkable detonation performances. Green Chem. 2015, 17, 831–836. [Google Scholar] [CrossRef]
- Song, H.; Li, B.; Gao, X.; Shan, F.; Ma, X.; Tian, X.; Chen, X. Thermodynamics and catalytic properties of two novel energetic complexes based on 3-amino-1, 2, 4-triazole-5-carboxylic acid. ACS Omega 2022, 7, 3024–3029. [Google Scholar] [CrossRef]
- Gong, L.; Chen, G.; Liu, Y.; Wang, T.; Zhang, J.; Yi, X.; He, P. Energetic metal–organic frameworks achieved from furazan and triazole ligands: Synthesis, crystal structure, thermal stability and energetic performance. New. J. Chem. 2021, 45, 22299–22305. [Google Scholar] [CrossRef]
- Friedrich, M.; Gálvez-Ruiz, J.C.; Klapötke, T.M.; Mayer, P.; Weber, B.; Weigand, J.J. BTA copper complexes. Inorg. Chem. 2005, 44, 8044–8052. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, B.; Zhang, Q.; Shang, Y.; Guo, Z.; Tan, B.; Peng, R. Nitrogen-Rich Energetic Metal-Organic Framework: Synthesis, Structure, Properties, and Thermal Behaviors of Pb (II) Complex Based on N, N-Bis (1 H-tetrazole-5-yl)-Amine. Materials 2016, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Yang, L.; Feng, J.L.; Wu, B.D.; Zhang, J.G.; Zhang, T.L.; Zhou, Z.N. Synthesis, Crystal Structure, Thermal Decomposition, and Sensitive Properties of Two Novel Energetic Cadmium (II) Complexes Based on 4-Amino-1,2,4-triazole. Z. Anorg. Allg. Chem. 2011, 637, 2215–2222. [Google Scholar] [CrossRef]
- McDonald, K.A.; Seth, S.; Matzger, A.J. Coordination polymers with high energy density: An emerging class of explosives. Cryst. Growth Des. 2015, 15, 5963–5972. [Google Scholar] [CrossRef]
- Xu, Z.; Su, H.; Zhou, X.; Wang, X.; Wang, J.; Gao, C.; Sun, X.; Dai, R.; Wang, Z.; Li, H. Pressure-and temperature-dependent structural stability of LLM-105 crystal. J. Phys. Chem. C 2018, 123, 1110–1119. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, C.; Li, J. Thermal behaviors of LLM-105: A brief review. J. Therm. Anal. Calorim. 2022, 147, 12965–12974. [Google Scholar] [CrossRef]
- Pagoria, P.; Zhang, M.X.; Zuckerman, N.; Lee, G.; Mitchell, A.; DeHope, A.; Gash, A.; Coon, C.; Gallagher, P. Synthetic Studies of 2,6-Diamino-3,5-Dinitropyrazine-1-Oxide (LLM-105) from Discovery to Multi-Kilogram Scale. Propell. Explos. Pyrot. 2018, 43, 15–27. [Google Scholar] [CrossRef]
- Tran, T.; Pagoria, P.; Hoffman, D.; Cutting, J.; Lee, R.; Simpson, R. Characterization of 2,6-Diamino-3,5-Dinitropyrazine-1-Oxide (LLM-105) as An Insensitive High Explosive Material; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2002. [Google Scholar]
- Liu, J.J.; Liu, Z.L.; Cheng, J. Synthesis, crystal structure and catalytic properties of two energetic complexes containing 2,6-diamino-3,5-dinitropyrazine-l-oxide. Chin. J. Inorg. Chem. 2014, 30, 696–704. [Google Scholar]
- Shi, Q.; Liu, Z.L.; Cheng, J.; Zhao, F.; Xu, S.; Shen, P. Synthesis of an energetic Pb(Ⅱ) complex of LLM-105 and its catalytic effect on the thermal decomposition behavior of AP. Explos. Mater. 2015, 44, 19–23. [Google Scholar]
- Vo, T.T.; Parrish, D.A.; Shreeve, J.M. 1,1-Diamino-2,2-dintroethene (FOX-7) in Copper and Nickel Diamine Complexes and Copper FOX-7. Inorg. Chem. 2012, 51, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Fleck, M.; Layek, M.; Saha, R.; Bandyopadhyay, D. Synthetic aspects, crystal structures and antibacterial activities of manganese (III) and cobalt (III) complexes containing a tetradentate Schiff base. Transit. Met. Chem. 2013, 38, 715–724. [Google Scholar] [CrossRef]
- Manrique, E.; Poater, A.; Fontrodona, X.; Solà, M.; Rodríguez, M.; Romero, I. Reusable manganese compounds containing pyrazole-based ligands for olefin epoxidation reactions. Dalton Trans. 2015, 44, 17529–17543. [Google Scholar] [CrossRef] [PubMed]
- Paine, T.K.; Weyhermüller, T.; Bothe, E.; Wieghardt, K.; Chaudhuri, P. Manganese complexes of mixed O, X, O-donor ligands (X = S or Se): Synthesis, characterization and catalytic reactivity. Dalton Trans. 2003, 32, 3136–3144. [Google Scholar] [CrossRef]
- Fegy, K.; Sanz, N.; Luneau, D.; Belorizky, E.; Rey, P. Proximate nitroxide ligands in the coordination spheres of manganese (II) and nickel (II) ions. Precursors for high-dimensional molecular magnetic materials. Inorg. Chem. 1998, 37, 4518–4523. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Fokin, S.V.; Romanenko, G.V.; Ovcharenko, V.I. Nitronyl nitroxides containing tetrazole substituents and metal complexes with spin-labeled tetrazole. Polyhedron 2003, 22, 1965–1972. [Google Scholar] [CrossRef]
- Fegy, K.; Luneau, D.; Ohm, T.; Paulsen, C.; Rey, P. Two-Dimensional Nitroxide-Based Molecular Magnetic Materials. Angew. Chem. Int. Ed. 1998, 37, 1270–1273. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, B.; Li, X.; Hao, W.; Huang, T.; Lei, B.; Guo, Z.; Shen, J.; Peng, R. Study of H2AzTO-based energetic metal-organic frameworks for catalyzing the thermal decomposition of ammonium perchlorate. Chem. Eng. J. 2021, 404, 126287. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. Axial substitution of a precursor resulted in two high-energy copper (ii) complexes with superior detonation performances. Dalton Trans. 2017, 46, 12893–12900. [Google Scholar] [CrossRef]






| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Formula | C11H13N13NiO11 | C14H20CoN14 O12 | C33H39Mn2N39O33 | C21H25Cu3N21O18 |
| MW/(g/mol) | 562.03 | 635.37 | 1619.91 | 1050.27 |
| Crystal system | monoclinic | monoclinic | trigonal | monoclinic |
| Space group | P 21/c | C 2/c | R -3 | C 2/c |
| a/Å | 15.2819(2) | 16.80800(10) | 15.7601(3) | 19.7761(4) |
| b/Å | 13.0345(2) | 6.999 | 15.7601(3) | 19.5785(3) |
| c/Å | 9.9895(2) | 20.96050(10) | 24.0861(8) | 9.6564(2) |
| a/o | 90 | 90 | 90 | 90 |
| b/o | 92.204(2) | 105.0760(10) | 90 | 96.095(2) |
| g/o | 90 | 90 | 120 | 90 |
| Cell vol /Å3 | 1988.36(6) | 2380.91(2) | 5181.0(3) | 3717.69(12) |
| rcalc/(g/cm3) | 1.878 | 1.773 | 1.558 | 1.877 |
| Z | 4 | 4 | 3 | 4 |
| Temperature/K | 294 | 294 | 294 | 294 |
| F(000) | 1144 | 1300 | 2472 | 2116 |
| R1 [I > 2s(I)] | 0.0469 | 0.0337 | 0.0748 | 0.0538 |
| wR2 [I > 2s(I)] | 0.1444 | 0.0991 | 0.2363 | 0.1526 |
| R1 (all data) | 0.0510 | 0.0358 | 0.0778 | 0.0643 |
| wR2 (all data) | 0.1484 | 0.0998 | 0.2419 | 0.1613 |
| GOF | 1.074 | 1.090 | 1.092 | 1.055 |
| CCDC number | 2274630 | 2274631 | 2274633 | 2274632 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Complex/hartree | −2158.31 | −2380.3 | −6845.13 | −4059.35 |
| NiO (s)/hartree | −268.57 | |||
| CoO (s)/hartree | −241.89 | |||
| MnO (s)/hartree | −196.91 | |||
| CuO (s)/hartree | −272.48 | |||
| H2O (g)/hartree | −76.38 | −76.38 | −76.38 | −76.38 |
| CO2 (g)/hartree | −188.18 | −188.18 | −188.18 | −188.18 |
| C (s)/hartree | −37.74 | −37.74 | −37.74 | −37.74 |
| N2 (g)/hartree | −109.45 | −109.45 | −109.45 | −109.45 |
| ∆Edet/hartree | 3.44 | 4.61 | 717.18 | 157.34 |
| ∆Edet/(kcal/g) | 3.84 | 4.55 | 277.78 | 93.99 |
| ∆Hdet/(kcal/g) | 4.37 | 5.17 | 313.09 | 105.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Huang, H.; Guo, J.; Yan, M.; Wei, L.; Liu, Y.; Huang, S.; Peng, R.; Jin, B. Construction of Energetic Complexes Based on LLM-105 and Transition Metal Cations (Ni, Co, Mn, and Cu). Crystals 2023, 13, 1587. https://doi.org/10.3390/cryst13111587
Xiao Y, Huang H, Guo J, Yan M, Wei L, Liu Y, Huang S, Peng R, Jin B. Construction of Energetic Complexes Based on LLM-105 and Transition Metal Cations (Ni, Co, Mn, and Cu). Crystals. 2023; 13(11):1587. https://doi.org/10.3390/cryst13111587
Chicago/Turabian StyleXiao, Yiyi, Hui Huang, Jinkun Guo, Mi Yan, Liyuan Wei, Yu Liu, Shiliang Huang, Rufang Peng, and Bo Jin. 2023. "Construction of Energetic Complexes Based on LLM-105 and Transition Metal Cations (Ni, Co, Mn, and Cu)" Crystals 13, no. 11: 1587. https://doi.org/10.3390/cryst13111587




