Highly Porous Layered Double Hydroxide and Mixed Metal Oxide by Sacrificial Bio-Template, Egg White Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
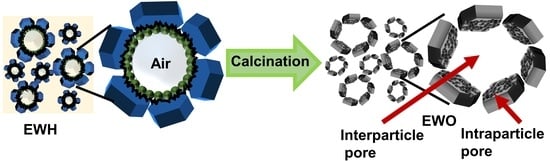
2.2. Synthesis of Egg-White-Templated LDH (EWH) and Corresponding Mixed Metal Oxide (EWO)
2.3. Synthesis of LDH and MMO without EW
2.4. Characterization
3. Results and Discussion
3.1. X-ray Diffraction Patterns
3.2. Scanning Electron Microscopy
3.3. Transmission Electron Microscopy
3.4. N2 Adsorption–Desorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Zhang, F.; Xu, S.; Evans, D.G.; Duan, X. From Layered Double Hydroxides to ZnO-Based Mixed Metal Oxides by Thermal Decomposition: Transformation Mechanism and UV-Blocking Properties of the Product. Chem. Mater. 2010, 22, 3933–3942. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, T.; Wang, C.; Ge, Z.; Liu, J.; Hao, X.; Du, N.; Xiao, H. Preparation and Photovoltaic Properties of Dye-Sensitized Solar Cells Based on Zinc Titanium Mixed Metal Oxides. Colloids Surf. A 2019, 568, 59–65. [Google Scholar] [CrossRef]
- Takemoto, M.; Tokudome, Y.; Murata, H.; Okada, K.; Takahashi, M.; Nakahira, A. Synthesis of High-Specific-Surface-Area Li-Al Mixed Metal Oxide: Through Nanoseed-Assisted Growth of Layered Double Hydroxide. Appl. Clay Sci. 2021, 203, 106006. [Google Scholar] [CrossRef]
- Abderrazek, K.; Najoua, F.S.; Srasra, E. Synthesis and Characterization of [Zn-Al] LDH: Study of the Effect of Calcination on the Photocatalytic Activity. Appl. Clay Sci. 2016, 119, 229–235. [Google Scholar] [CrossRef]
- Dou, Y.; Han, J.; Wang, T.; Wei, M.; Evans, D.G.; Duan, X. Fabrication of MMO–TiO2 One-Dimensional Photonic Crystal and Its Application as a Colorimetric Sensor. J. Mater. Chem. 2012, 22, 14001–14007. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Moloney, M.P.; Tekoriute, R.; Hardy-Dessources, A.; Nedelec, J.M.; Gun’ko, Y.K.; Kessler, V.G. Biomimetic Synthesis of Hierarchically Porous Nanostructured Metal Oxide Microparticles-Potential Scaffolds for Drug Delivery and Catalysis. Langmuir 2010, 26, 9809–9817. [Google Scholar] [CrossRef]
- Ko, S.J.; Yamaguchi, T.; Salles, F.; Oh, J.M. Systematic Utilization of Layered Double Hydroxide Nanosheets for Effective Removal of Methyl Orange from an Aqueous System by π-π Stacking-Induced Nanoconfinement. J. Environ. Manag. 2021, 277, 111455. [Google Scholar] [CrossRef]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay Minerals and Layered Double Hydroxides for Novel Biological Applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Li, E.; Xu, Z.P.; Rudolph, V. MgCoAl-LDH Derived Heterogeneous Catalysts for the Ethanol Transesterification of Canola Oil to Biodiesel. Appl. Catal. B Environ. 2009, 88, 42–49. [Google Scholar] [CrossRef]
- Védrine, J. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef]
- Kaplin, I.Y.; Lokteva, E.S.; Golubina, E.V.; Lunin, V.V. Template Synthesis of Porous Ceria-Based Catalysts for Environmental Application. Molecules 2020, 25, 4242. [Google Scholar] [CrossRef]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and Applications of Supramolecular-Templated Mesoporous Materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Lee, S.-B.; Ko, E.-H.; Park, J.Y.; Oh, J.-M. Mixed Metal Oxide by Calcination of Layered Double Hydroxide: Parameters Affecting Specific Surface Area. Nanomaterials 2021, 11, 1153. [Google Scholar] [CrossRef]
- Li, Y.-J.; Qi, T.-T.; Dong, Y.-N.; Hou, W.-H.; Chu, G.-W.; Zhang, L.-L.; Sun, B.-C. Synthesized Ni/MMO Catalysts via Ultrathin Ni-Al-LDH in a Rotating Packed Bed for Hydrogenation of Maleic Anhydride. Fuel 2022, 326, 125035. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Da Costa, J.C.D. Layered Double Hydroxides for CO2 Capture: Structure Evolution and Regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [Google Scholar] [CrossRef]
- Veerabhadrappa, M.G.; Maroto-Valer, M.M.; Chen, Y.; Garcia, S. Layered Double Hydroxides-Based Mixed Metal Oxides: Development of Novel Structured Sorbents for CO2 Capture Applications. ACS Appl. Mater. Interfaces 2021, 13, 11805–11813. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xiao, H.; Liu, D.; Qin, Y.; Wu, H.; Li, H.; Du, N.; Hou, W. Preparation and Properties of Mixed Metal Oxides Based Layered Double Hydroxide as Anode Materials for Dye-Sensitized Solar Cell. Chem. Eng. J. 2014, 250, 1–5. [Google Scholar] [CrossRef]
- Chandrabose, V.; Kim, T.; Park, J.W.; Jung, S.-Y.; Oh, J.-M. Effect of Tetrahedrally Coordinated Al on the Surface Acidity of Mg-Al Binary Mixed Oxides. Molecules 2023, 28, 6072. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Chatla, A.; Zakaria, Y.; Kochkodan, V.; Shanableh, A.; Laoui, T.; Atieh, M.A. Palm Leaves Based Biochar: Advanced Material Characterization and Heavy Metal Adsorption Study. Biomass Convers. Biorefinery 2022, 1–20. [Google Scholar] [CrossRef]
- Khalil, A.K.A.; Dweiri, F.; Almanassra, I.W.; Chatla, A.; Atieh, M.A. Mg-Al Layered Double Hydroxide Doped Activated Carbon Composites for Phosphate Removal from Synthetic Water: Adsorption and Thermodynamics Studies. Sustainability 2022, 14, 6991. [Google Scholar] [CrossRef]
- Abushawish, A.; Almanassra, I.W.; Backer, S.N.; Jaber, L.; Khalil, A.K.A.; Abdelkareem, M.A.; Sayed, E.T.; Alawadhi, H.; Shanableh, A.; Atieh, M.A. High-Efficiency Removal of Hexavalent Chromium from Contaminated Water Using Nitrogen-Doped Activated Carbon: Kinetics and Isotherm Study. Mater. Chem. Phys. 2022, 291, 126758. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Li, T.; Lu, M.; Chen, Y.; Cui, Y.; Gao, J.; Qian, G. Tuning the Electronic Structure of a Metal–Organic Framework for an Efficient Oxygen Evolution Reaction by Introducing Minor Atomically Dispersed Ruthenium. Carbon Energy 2023, 5, e265. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, J.; Lv, E.; Li, T.; Xu, H.; Sun, L.; Nairan, A.; Zhang, Q. Integration of Alloy Segregation and Surface Co–O Hybridization in Carbon-Encapsulated CoNiPt Alloy Catalyst for Superior Alkaline Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2303833. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, T.; Lei, X.; Xu, S.; Zhang, F. Scalable Preparation of Alginate Templated-Layered Double Hydroxide Mesoporous Composites with Enhanced Surface Areas and Surface Acidities. J. Nanosci. Nanotechnol. 2011, 11, 3291–3297. [Google Scholar] [CrossRef]
- Swain, N.; Saravanakumar, B.; Kundu, M.; Schmidt-Mende, L.; Ramadoss, A. Recent Trends in Template Assisted 3D Porous Materials for Electrochemical Supercapacitors. J. Mater. Chem. A 2021, 9, 25286–25324. [Google Scholar] [CrossRef]
- Li, B.; He, J.; Evans, D.G.; Duan, X. Morphology and Size Control of Ni-Al Layered Double Hydroxides Using Chitosan as Template. J. Phys. Chem. Solids 2006, 67, 1067–1070. [Google Scholar] [CrossRef]
- Yan, X.; Sahimi, M.; Tsotsis, T.T. Fabrication of High-Surface Area Nanoporous SiOC Ceramics Using Pre-Ceramic Polymer Precursors and a Sacrificial Template: Precursor Effects. Microporous Mesoporous Mater. 2017, 241, 338–345. [Google Scholar] [CrossRef]
- Coelho, A.; Perrone, O.M.; Gomes, E.; Da-Silva, R.; Thoméo, J.C.; Boscolo, M. Mixed Metal Oxides from Sucrose and Cornstarch Templated Hydrotalcite-like LDHs as Catalysts for Ethyl Biodiesel Synthesis. Appl. Catal. A Gen. 2017, 532, 32–39. [Google Scholar] [CrossRef]
- Su, H.; Jing, L.; Shi, K.; Yao, C.; Fu, H. Synthesis of Large Surface Area LaFeO3 Nanoparticles by SBA-16 Template Method as High Active Visible Photocatalysts. J. Nanoparticle Res. 2010, 12, 967–974. [Google Scholar] [CrossRef]
- Pahalagedara, M.N.; Pahalagedara, L.R.; Kuo, C.H.; Dharmarathna, S.; Suib, S.L. Ordered Mesoporous Mixed Metal Oxides: Remarkable Effect of Pore Size on Catalytic Activity. Langmuir 2014, 30, 8228–8237. [Google Scholar] [CrossRef]
- Xie, J.; Yamaguchi, T.; Oh, J.-M. Synthesis of a Mesoporous Mg–Al–Mixed Metal Oxide with P123 Template for Effective Removal of Congo Red via Aggregation-Driven Adsorption. J. Solid State Chem. 2021, 293, 121758. [Google Scholar] [CrossRef]
- An, W.; Ma, J.; Xu, Q. Bio-Template Synthesis of MgAl Layered Double Hydroxide with Enhanced Flame Retardant Property for Leather Finishes. Appl. Surf. Sci. 2021, 551, 149409. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, M.; Lu, J.; Wang, Z.L.; Duan, X. Biotemplated Hierarchical Nanostructure of Layered Double Hydroxides with Improved Photocatalysis Performance. ACS Nano 2009, 3, 4009–4016. [Google Scholar] [CrossRef]
- Sobhana, S.S.L.; Bogati, D.R.; Reza, M.; Gustafsson, J.; Fardim, P. Cellulose Biotemplates for Layered Double Hydroxides Networks. Microporous Mesoporous Mater. 2016, 225, 66–73. [Google Scholar] [CrossRef]
- Gabal, M.A. Magnetic Properties of NiCuZn Ferrite Nanoparticles Synthesized Using Egg-White. Mater. Res. Bull. 2010, 45, 589–593. [Google Scholar] [CrossRef]
- Lai, B.H.; Yeh, C.C.; Chen, D.H. Surface Modification of Iron Oxide Nanoparticles with Polyarginine as a Highly Positively Charged Magnetic Nano-Adsorbent for Fast and Effective Recovery of Acid Proteins. Process Biochem. 2012, 47, 799–805. [Google Scholar] [CrossRef]
- Moshafi, M.H.; Ranjbar, M.; Ilbeigi, G. Biotemplate of Albumen for Synthesized Iron Oxide Quantum Dots Nanoparticles (QDNPs) and Investigation of Antibacterial Effect against Pathogenic Microbial Strains. Int. J. Nanomed. 2019, 14, 3273–3282. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Luna-Lama, F.; Caballero, Á.; Muñoz-Batista, M.J.; Luque, R. Versatile Protein-Templated TiO2 Nanocomposite for Energy Storage and Catalytic Applications. ACS Sustain. Chem. Eng. 2019, 7, 5329–5337. [Google Scholar] [CrossRef]
- Kadam, A.N.; Salunkhe, T.T.; Kim, H.; Lee, S.-W. Biogenic Synthesis of Mesoporous N–S–C Tri-Doped TiO2 Photocatalyst via Ultrasonic-Assisted Derivatization of Biotemplate from Expired Egg White Protein. Appl. Surf. Sci. 2020, 518, 146194. [Google Scholar] [CrossRef]
- Maensiri, S.; Masingboon, C.; Laokul, P.; Jareonboon, W.; Promarak, V.; Anderson, P.L.; Seraphin, S. Egg White Synthesis and Photoluminescence of Platelike Clusters of CeO2 Nanoparticles. Cryst. Growth Des. 2007, 7, 950–955. [Google Scholar] [CrossRef]
- Stadelman, W.J.; Newkirk, D.; Newby, L. Egg Science and Technology; Stadelman, W.J., Cotterill, O.J., Eds.; Avi Publishing: Westport, CT, USA, 1986. [Google Scholar]
- Drakos, A.; Kiosseoglou, V. Stability of Acidic Egg White Protein Emulsions Containing Xanthan Gum. J. Agric. Food Chem. 2006, 54, 10164–10169. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kim, B.K.; Kim, H.J.; Oh, J.M. Development of Mesopore Structure of Mixed Metal Oxide through Albumin-Templated Coprecipitation and Reconstruction of Layered Double Hydroxide. Nanomaterials 2021, 11, 620. [Google Scholar] [CrossRef]
- Kooli, F.; Chisem, I.C.; Vucelic, M.; Jones, W. Synthesis and Properties of Terephthalate and Benzoate Intercalates of Mg−Al Layered Double Hydroxides Possessing Varying Layer Charge. Chem. Mater. 1996, 8, 1969–1977. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001; p. 388. [Google Scholar]
- Yao, W.; Wang, X.; Liang, Y.; Yu, S.; Gu, P.; Sun, Y.; Xu, C.; Chen, J.; Hayat, T.; Alsaedi, A.; et al. Synthesis of Novel Flower-like Layered Double Oxides/Carbon Dots Nanocomposites for U(VI) and 241Am(III) Efficient Removal: Batch and EXAFS Studies. Chem. Eng. J. 2018, 332, 775–786. [Google Scholar] [CrossRef]
- Huang, P.P.; Cao, C.Y.; Wei, F.; Sun, Y.B.; Song, W.G. MgAl Layered Double Hydroxides with Chloride and Carbonate Ions as Interlayer Anions for Removal of Arsenic and Fluoride Ions in Water. RSC Adv. 2015, 5, 10412–10417. [Google Scholar] [CrossRef]
- Bîrjega, R.; Pavel, O.D.; Costentin, G.; Che, M.; Angelescu, E. Rare-Earth Elements Modified Hydrotalcites and Corresponding Mesoporous Mixed Oxides as Basic Solid Catalysts. Appl. Catal. A Gen. 2005, 288, 185–193. [Google Scholar] [CrossRef]
- Kim, B.K.; Gwak, G.H.; Okada, T.; Oh, J.M. Effect of Particle Size and Local Disorder on Specific Surface Area of Layered Double Hydroxides upon Calcination-Reconstruction. J. Solid State Chem. 2018, 263, 60–64. [Google Scholar] [CrossRef]
- Gago, S.; Pillinger, M.; Valente, A.A.; Santos, T.M.; Rocha, J.; Gonçalves, I.S. Immobilization of Oxomolybdenum Species in a Layered Double Hydroxide Pillared by 2,2′-Bipyridine-5,5′-Dicarboxylate Anions. Inorg. Chem. 2004, 43, 5422–5431. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Kim, B.-K.; Hirata, S.; Inada, M.; Oh, J.-M. Particle Size Effect of Layered Double Hydroxide on the Porosity of Calcined Metal Oxide. Appl. Clay Sci. 2020, 195, 105701. [Google Scholar] [CrossRef]
- Tanaka, R.; Ogino, I.; Mukai, S.R. Synthesis of Mg-Al Mixed Oxides with Markedly High Surface Areas from Layered Double Hydroxides with Organic Sulfonates. ACS Omega 2018, 3, 16916–16923. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiang, Y.; Li, L.; Huang, X.; Zhuang, Z.; Yu, Y. Thin CuOx-Based Nanosheets for Efficient Phenol Removal Benefitting from Structural Memory and Ion Exchange of Layered Double Oxides. J. Mater. Chem. A 2018, 6, 4167–4178. [Google Scholar] [CrossRef]
- Lin, S.T.; Tran, H.N.; Chao, H.P.; Lee, J.F. Layered Double Hydroxides Intercalated with Sulfur-Containing Organic Solutes for Efficient Removal of Cationic and Oxyanionic Metal Ions. Appl. Clay Sci. 2018, 162, 443–453. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Utilization of Calcined Ni-Al Layered Double Hydroxide (LDH) as an Adsorbent for Removal of Methyl Orange Dye from Aqueous Solution. Environ. Prog. Sustain. Energy 2014, 33, 154–159. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, D.Y.; Gwak, G.H.; Han, Y.S.; Oh, J.M. Zn-Fe Mixed Metal Oxides from Metal Hydroxide Precursor: Effect of Calcination Temperature on Phase Evolution, Porosity, and Catalytic Acidity. J. Solid State Chem. 2019, 269, 454–458. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Ng, D.H.L. Synthesis of a 3D Hierarchical Structure of γ-AlO(OH)/Mg-Al-LDH/C and Its Performance in Organic Dyes and Antibiotics Adsorption. J. Mater. Chem. A 2015, 3, 21106–21115. [Google Scholar] [CrossRef]
- Gonzalez Rodriguez, P.; de Ruiter, M.; Wijnands, T.; ten Elshof, J.E. Porous Layered Double Hydroxides Synthesized Using Oxygen Generated by Decomposition of Hydrogen Peroxide. Sci. Rep. 2017, 7, 481. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Avilés, Y.; Borau, V.; Luque, J.M.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Thermal Decomposition of Mg/Al and Mg/Ga Layered-Double Hydroxides: A Spectroscopic Study. J. Mater. Chem. 1999, 9, 1603–1607. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Vikraman, D.; Bose, R.; Hussain, S.; Santhoshkumar, P.; Manikandan, R.; Jung, J.; Alameri, S.; Alfantazi, A.; Kim, H.-S. Unveiling the Redox Electrochemical Kinetics of Interconnected Wrinkled Microspheres of Binary Cu2−xSe/Ni1−xSe as Battery-Type Electrode for Advanced Supercapatteries. J. Colloid Interface Sci. 2024, 654, 1098–1110. [Google Scholar] [CrossRef]






| Parameters | LDH | MMO | EWH | EWO |
|---|---|---|---|---|
| SBET (m2/g) | 29 | 62 | 144 | 248 |
| Pore volume (cm3/g) | 0.2 | 0.2 | 0.6 | 0.6 |
| Mean pore diameter (nm) | 24 | 14 | 16 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrabose, V.; Park, J.w.; Jung, S.Y.; Wang, K.K.; Oh, J.-M. Highly Porous Layered Double Hydroxide and Mixed Metal Oxide by Sacrificial Bio-Template, Egg White Foam. Crystals 2023, 13, 1603. https://doi.org/10.3390/cryst13111603
Chandrabose V, Park Jw, Jung SY, Wang KK, Oh J-M. Highly Porous Layered Double Hydroxide and Mixed Metal Oxide by Sacrificial Bio-Template, Egg White Foam. Crystals. 2023; 13(11):1603. https://doi.org/10.3390/cryst13111603
Chicago/Turabian StyleChandrabose, Vidya, Ji won Park, Sang Yong Jung, Kang Kyun Wang, and Jae-Min Oh. 2023. "Highly Porous Layered Double Hydroxide and Mixed Metal Oxide by Sacrificial Bio-Template, Egg White Foam" Crystals 13, no. 11: 1603. https://doi.org/10.3390/cryst13111603