An Alternative to Chlorobenzene as a Hole Transport Materials Solvent for High-Performance Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Precursors
2.3. Device Fabrication
2.4. Measurements and Characterizations
3. Results
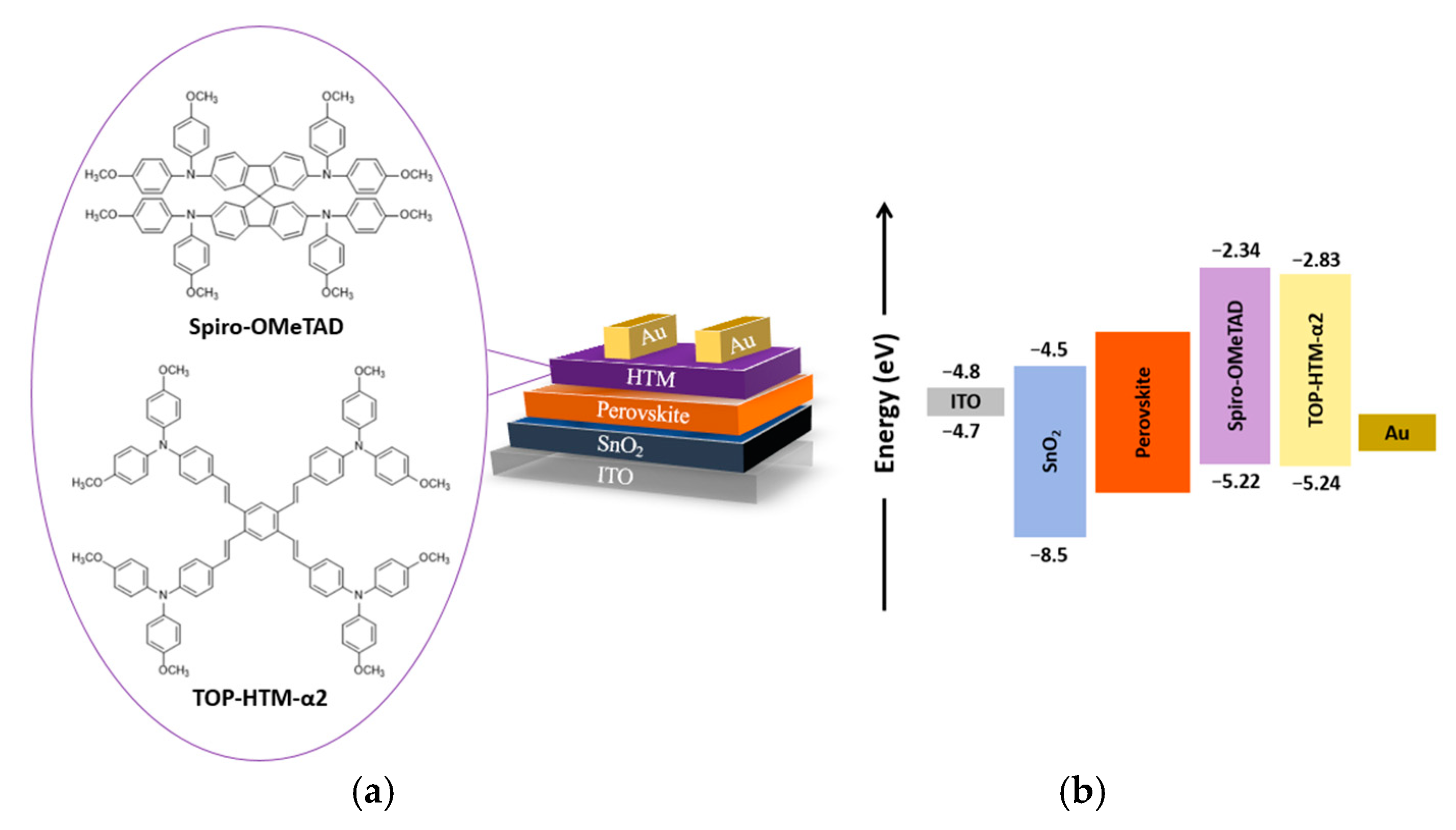
3.1. Device Characterization
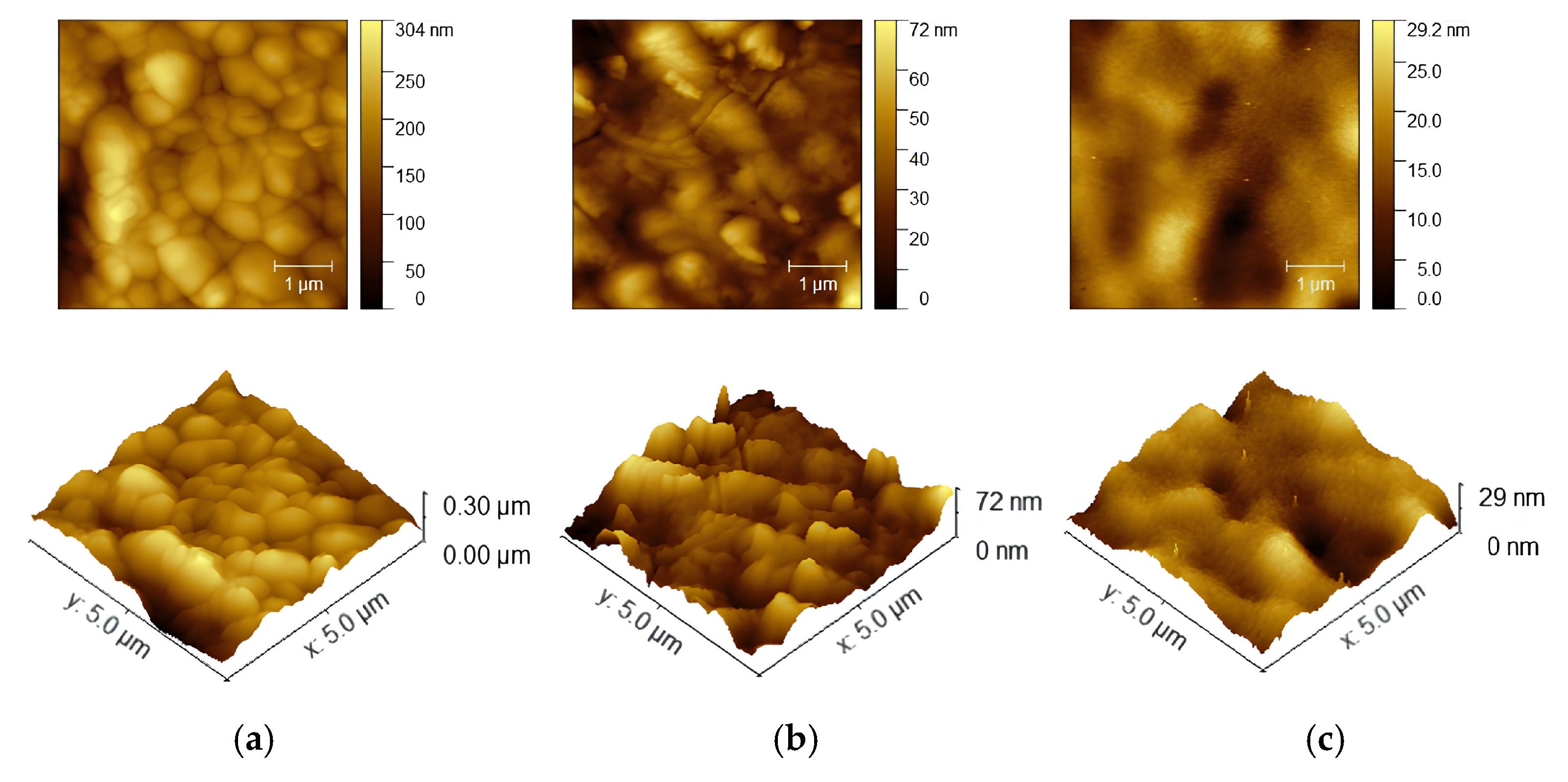
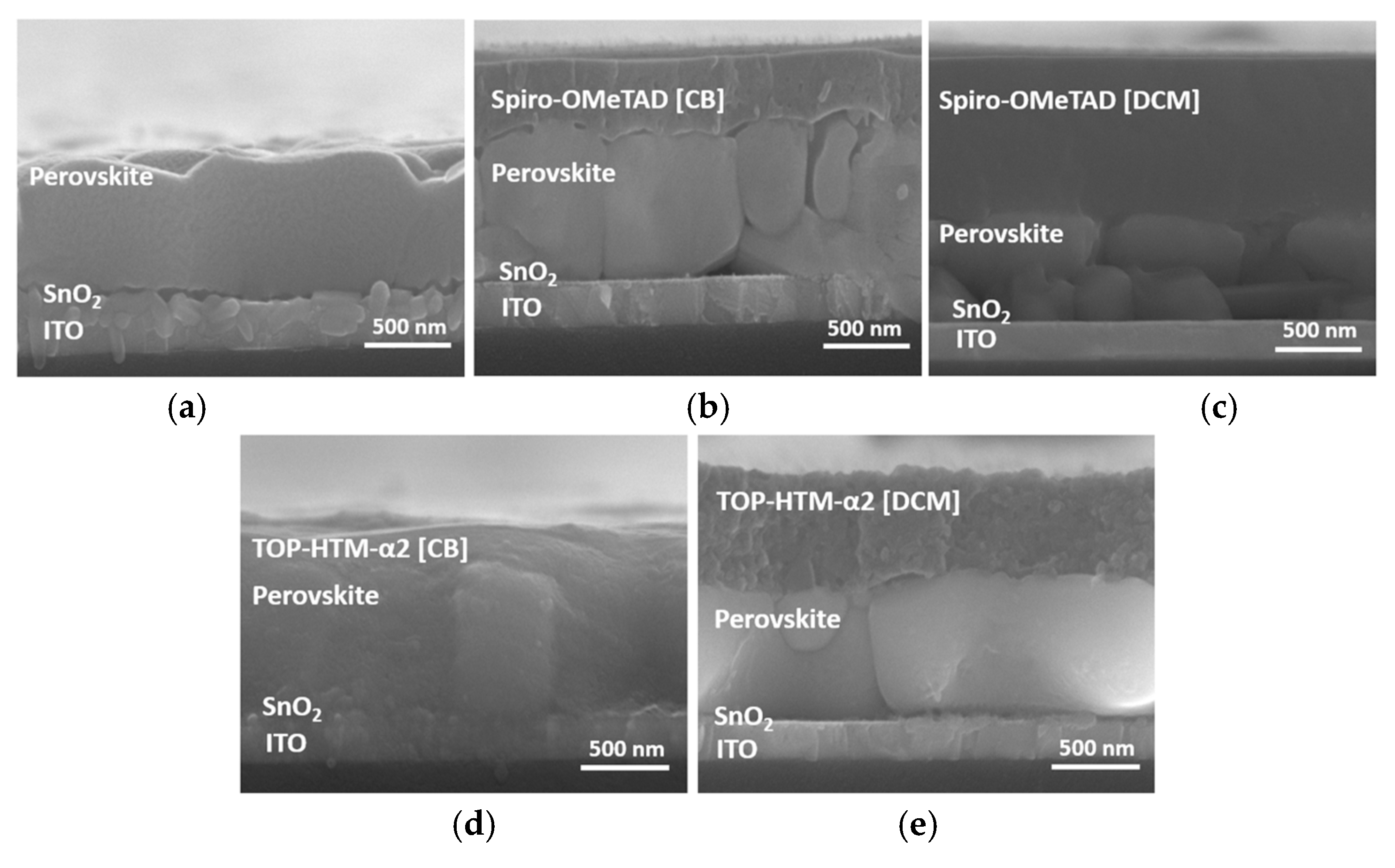
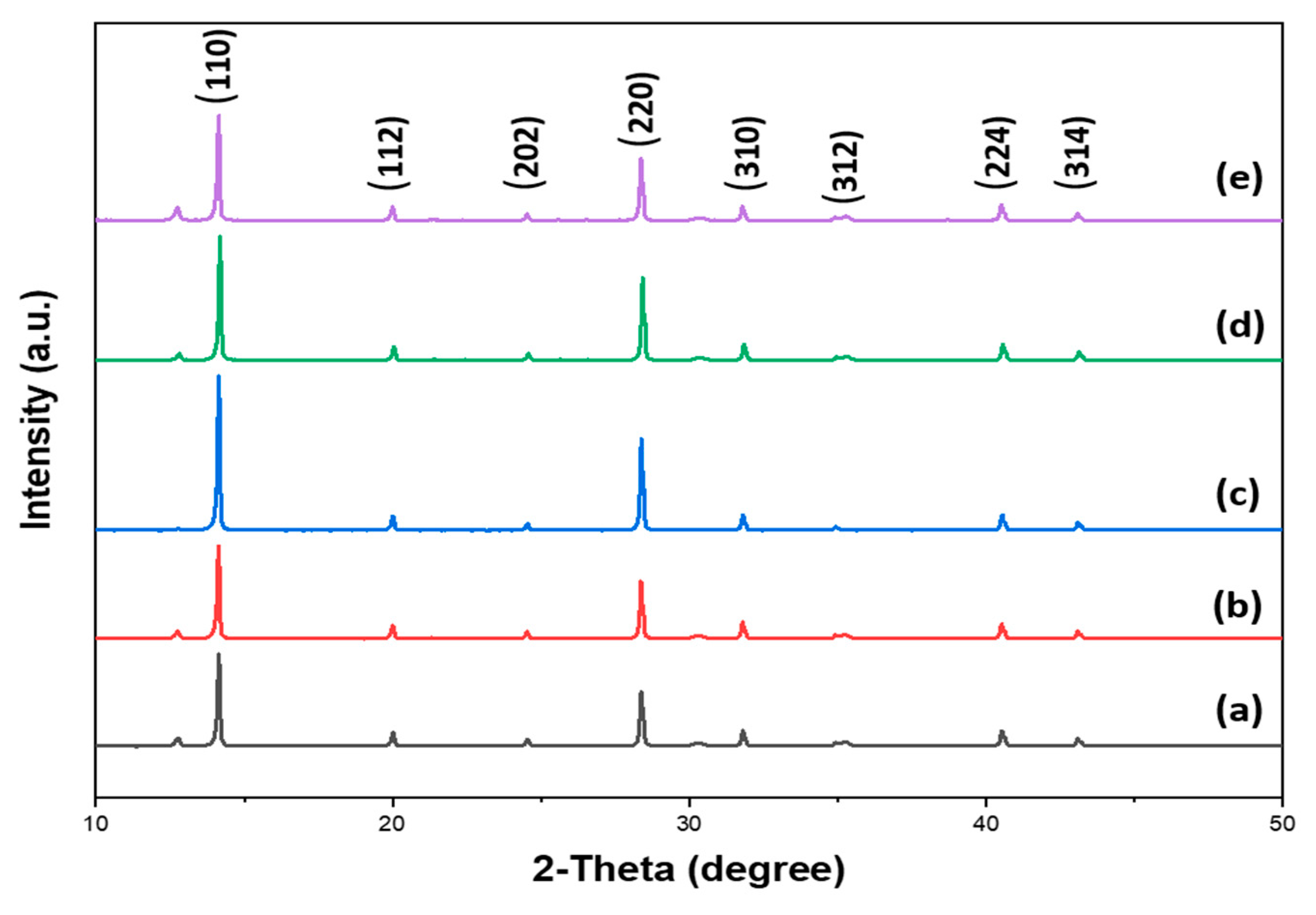
3.2. Surface Analysis
3.3. Materials Properties as HTL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mosher, D.; Boese, R.; Soukup, R. Advantages of Sun tracking for planar silicon solar cells. Sol. Energy 1977, 19, 91–97. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for Commercializing Perovskite Solar Cells. Science 2018, 361, 1214. [Google Scholar] [CrossRef] [PubMed]
- Falaras, C.; Stathatos, E. Performance Enhancement and Stability Improvement in Perovskite Solar Cells via Interface Functionalization. Electronics 2023, 12, 3319. [Google Scholar] [CrossRef]
- Jiang, Q.; Song, Z.; Bramante, R.C.; Ndione, P.F.; Tirawat, R.; Berry, J.J.; Yan, Y.; Zhu, K. Highly efficient bifacial single-junction perovskite solar cells. Joule 2023, 7, 1543–1555. [Google Scholar] [CrossRef]
- Yelzhanova, Z.; Nigmetova, G.; Aidarkhanov, D.; Daniyar, B.; Baptayev, B.; Balanay, M.P.; Junmabekov, A.N.; Ng, A. A Morphological Study of Solvothermally Grown SnO2 Nanostructures for Application in Perovskite Solar Cells. Nanomaterials 2022, 12, 1686. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xiang, H.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Nanostructured Inorganic Hole-Transporting Materials for Perovskite Solar Cells. Nanomaterials 2022, 12, 2592. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Li, H.; Wang, C.; Gao, Z.; Zhang, P.; Niu, X.; Li, N.; Xu, Z.; Su, Z.; et al. Stress compensation based on interfacial nanostructures for stable perovskite solar cells. Interdiscip. Mater. 2023, 2, 348–359. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, H.; Sun, J.; Lu, Y.; Luo, P.; Hu, J. Fabrication of efficient and stable wide band gap CsPbIBr2 inorganic perovskite solar cells via doping with lead chloride compound. J. Alloys Compd. 2023, 963, 171291. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, W.; Wang, H.; Cai, W.; Qaid, S.M.H.; Zang, Z. Additive Conformational Engineering to Improve the PbI2 Framework for Efficient and stable Perovskite Solar Cells. Inorg. Chem. 2023, 62, 14086–14093. [Google Scholar] [CrossRef]
- Lee, S.U.; Park, H.; Shin, H.; Park, N.G. Atomic layer deposition of SnO2 using hydrogen peroxide improves the efficiency and stability of perovskite solar cells. Nanoscale 2023, 15, 5044–5052. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Li, R.; Mbumba, M.T.; Akram, M.W.; Pan, J.; Cai, M.; Dai, S.; Guli, M. Low-Temperature Atomic Layer Deposition of Double-Layer Water Vapor Barrier for High Humidity Stable Perovskite Solar Cells. Adv. Opt. Mater. 2023, 11, 202300148. [Google Scholar] [CrossRef]
- Teixeira, C.; Fuentes-Pineda, R.; Andrade, L.; Mendes, A.; Forgacs, D. Fabrication or low-cost and flexible perovskite solar cells by slot-die coating for indoor applications. Mater. Adv. 2023, 4, 3863–3873. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643. [Google Scholar] [CrossRef]
- Ganesan, R.; Fu, K.; Gao, P.; Raabe, I.; Schenk, K.; Scopelliti, R.; Luo, J.; Wong, L.H.; Gratzel, M.; Nazzeruddin, M.K. A simple spiro-type hole transporting material for efficient perovskite solar cells. Energy Environ. Sci. 2015, 8, 1986–1991. [Google Scholar] [CrossRef]
- Hawash, Z.; Ono, L.K.; Qi, Y. Recent Advances in Spiro-MeOTAD Hole Transport Material and Its Application in Organic-Inorganic Halide Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1700623. [Google Scholar] [CrossRef]
- Shen, Y.; Deng, K.; Li, L. Spiro-OMeTAD-Based Hole Transport Layer Engineering toward Stable Perovskite Solar Cells. Small Methods 2022, 6, 2200757. [Google Scholar] [CrossRef]
- Nakka, L.; Cheng, Y.; Aberle, A.G.; Lin, F. Analytical Review of Spiro-OMeTAD Hole Transport Materials: Paths Toward Stable and Efficient Perovskite Solar Cells. Adv. Energy Sustain. Res. 2022, 3, 2200045. [Google Scholar] [CrossRef]
- Ono, L.K.; Hawash, Z.; Jaarez-Perez, E.J.; Qiu, L.; Jiang, Y.; Qi, Y. The influence of secondary solvents on the morphology of a spiro-MeOTAD hole transport layer for lead halide perovskite solar cells. Appl. Phys. 2018, 51, 294001. [Google Scholar] [CrossRef]
- Hu, M.; Wu, X.; Tan, W.L.; Tan, B.; Scully, A.D.; Ding, L.; Zhou, C.; Xiong, Y.; Huang, F.; Simonov, A.N.; et al. Solvent Engineering of a Dopant-Free Spiro-OMeTAD Hole-Transport Layer for Centimeter-Scale Perovskite Solar Cells with High Efficiency and Thermal Stability. ACS Appl. Mater. Interfaces 2020, 12, 8260–8270. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.D.; Wu, Z.; Ono, L.K.; Manzhosm, S.; Feron, K.; Motta, N.; Qi, Y.; Sonar, P. Low-Cost Alternative High-Performance Hole-Transport Material for Perovskite Solar Cells and Its Comparative Study with Conventional SPIRO-OMeTAD. Adv. Electron Mater. 2017, 3, 1700139. [Google Scholar] [CrossRef]
- Abdellah, I.M.; Chowdhury, T.H.; Lee, J.J.; Islam, A.; Nazzeruddin, M.K.; Graetzel, M.; El-Shafei, A. Facile and low-cost synthesis of a novel dopant-free hole transporting material that rivals Spiro-OMeTAD for high efficiency perovskite solar cells. Sustain. Energy Fuels 2021, 5, 199–211. [Google Scholar] [CrossRef]
- Nishimura, H.; Okada, I.; Tanabe, T.; Nakamura, T.; Murdey, R.; Wakamiya, A. Additive-Free, Cost-Effective Hole-Transporting Materials for Perovskite Solar Cells Based on Vinyl Triarylamines. ACS Appl. Mater. Interfaces 2020, 12, 32994–33003. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Mohammed, M.K.A.; Ahmed, D.S. Experimental investigation of additive free-low-cost vinyl triarylamines based hole transport material for FAPbI3-based perovskite solar cells to enhance efficiency and stability. Mater. Res. Express 2023, 10, 044003. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Liu, X.; Wang, S.; Yan, Z.; Chen, L.; Li, G.; Zhang, X.; Sun, W.; Lan, Z. High-effective SnO2-based perovskite solar cells by multifunctional molecular additive engineering. J. Alloys Compd. 2021, 886, 161352. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, S.; Su, Y.; Wang, H.; Ye, L.; Tsang, S.W.; Xie, F.; Xu, J. Enhanced efficiency of organic solar cells by mixed orthogonal solvents. Org. Electron. 2014, 15, 2007–2013. [Google Scholar] [CrossRef]
- Lim, S.B.; Ji, C.H.; Kim, K.T.; Oh, S.Y. Reduction of the Dark Current in a P3HT-Based Organic Photodiode with a Ytterbium-Fluoride Buffer Layer for Electron Transport. J. Korean Phys. Soc. 2016, 69, 421–425. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Dong, H.; Li, J.; Zhu, X.; Xu, J.; Pan, F.; Yuan, F.; Dai, J.; Jiao, B.; et al. Highly efficient and stable perovskite solar cells enabled by low-dimensional perovskitoids. Sci. Adv. 2022, 8, eabk2722. [Google Scholar] [CrossRef]
- Tien, C.H.; Lai, H.Y.; Chen, L.C. Methylammonium halide salt interfacial modification of perovskite quantum dots/triple-cation perovskites enable efficient solar cells. Sci. Rep. 2023, 13, 5387. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Y.; Zheng, K.; Pullerits, T.; Liang, Z. Insights into charge carrier dynamics in organo-metal halide perovskites: From neat films to solar cells. Chem. Soc. Rev. 2017, 46, 5714–5729. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Y.; Wang, C.; Ma, R. Interface Modification by Multifunctional Ammonium Salt toward High Performance and Stability Planar Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 11867–11876. [Google Scholar] [CrossRef]
- Le Corre, V.M.; Duijnstee, E.A.; Tambouli, O.E.; Ball, J.M.; Snaith, H.J.; Lim, J.; Koster, L.J.A. Revealing Charge Carrier Mobility and Defect Densities in Metal Halide Perovskites via Space-Charge-Limited Current Measurements. ACS Energy Lett. 2021, 6, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Sato, H.; Onkura, Y.; Ishii, A.; Miyasaka, T. Phenethylamine-Based Interfacial Dipole Engineering for High Voc Triple-Cation Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2102856. [Google Scholar] [CrossRef]
- Yi, H.; Wang, D.; Duan, L.; Haque, F.; Xu, C.; Zhang, Y.; Conibeer, G.; Uddin, A. Solution-processed WO3 and water-free PEDOT:PSS composite for hole transport layer in conventional perovskite solar cell. Electrochim. Acta 2019, 319, 349–358. [Google Scholar] [CrossRef]
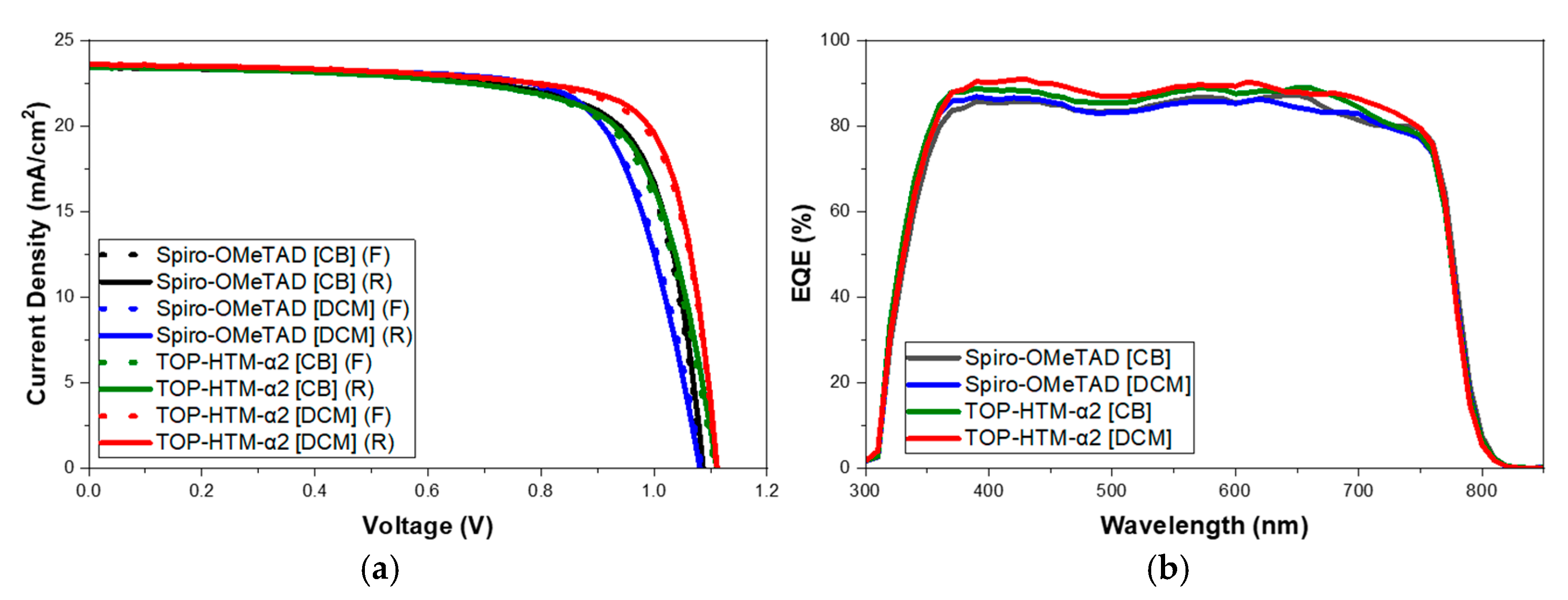
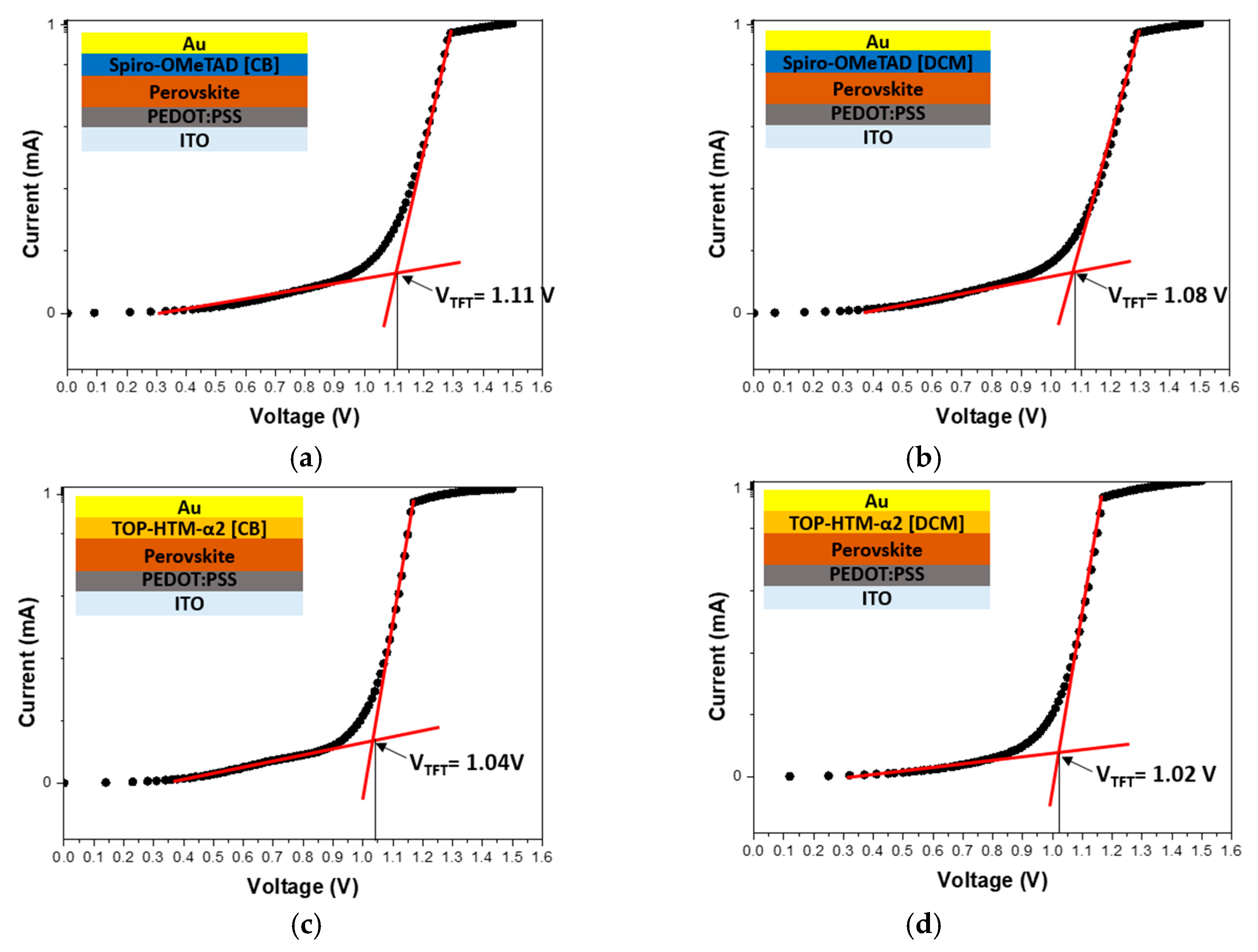
| Sample | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Spiro-OMeTAD [CB] | 23.42 | 1.086 | 75.06 | 18.85 |
| Spiro-OMeTAD [DCM] | 23.54 | 1.081 | 72.88 | 18.55 |
| TOP-HTM-α2 [CB] | 23.42 | 1.110 | 71.77 | 18.67 |
| TOP-HTM-α2 [DCM] | 23.58 | 1.111 | 76.97 | 20.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Lim, S.B.; Kim, J.Y.; Lee, S.; Oh, S.Y.; Kim, G.M. An Alternative to Chlorobenzene as a Hole Transport Materials Solvent for High-Performance Perovskite Solar Cells. Crystals 2023, 13, 1667. https://doi.org/10.3390/cryst13121667
Lee SH, Lim SB, Kim JY, Lee S, Oh SY, Kim GM. An Alternative to Chlorobenzene as a Hole Transport Materials Solvent for High-Performance Perovskite Solar Cells. Crystals. 2023; 13(12):1667. https://doi.org/10.3390/cryst13121667
Chicago/Turabian StyleLee, Seung Ho, Seong Bin Lim, Jin Young Kim, Seri Lee, Se Young Oh, and Gyu Min Kim. 2023. "An Alternative to Chlorobenzene as a Hole Transport Materials Solvent for High-Performance Perovskite Solar Cells" Crystals 13, no. 12: 1667. https://doi.org/10.3390/cryst13121667





