Lead-Free Organic Manganese (II) Bromide Hybrid with Highly Efficient and Stable Green Emission for UV Photodetection
Abstract
:1. Introduction
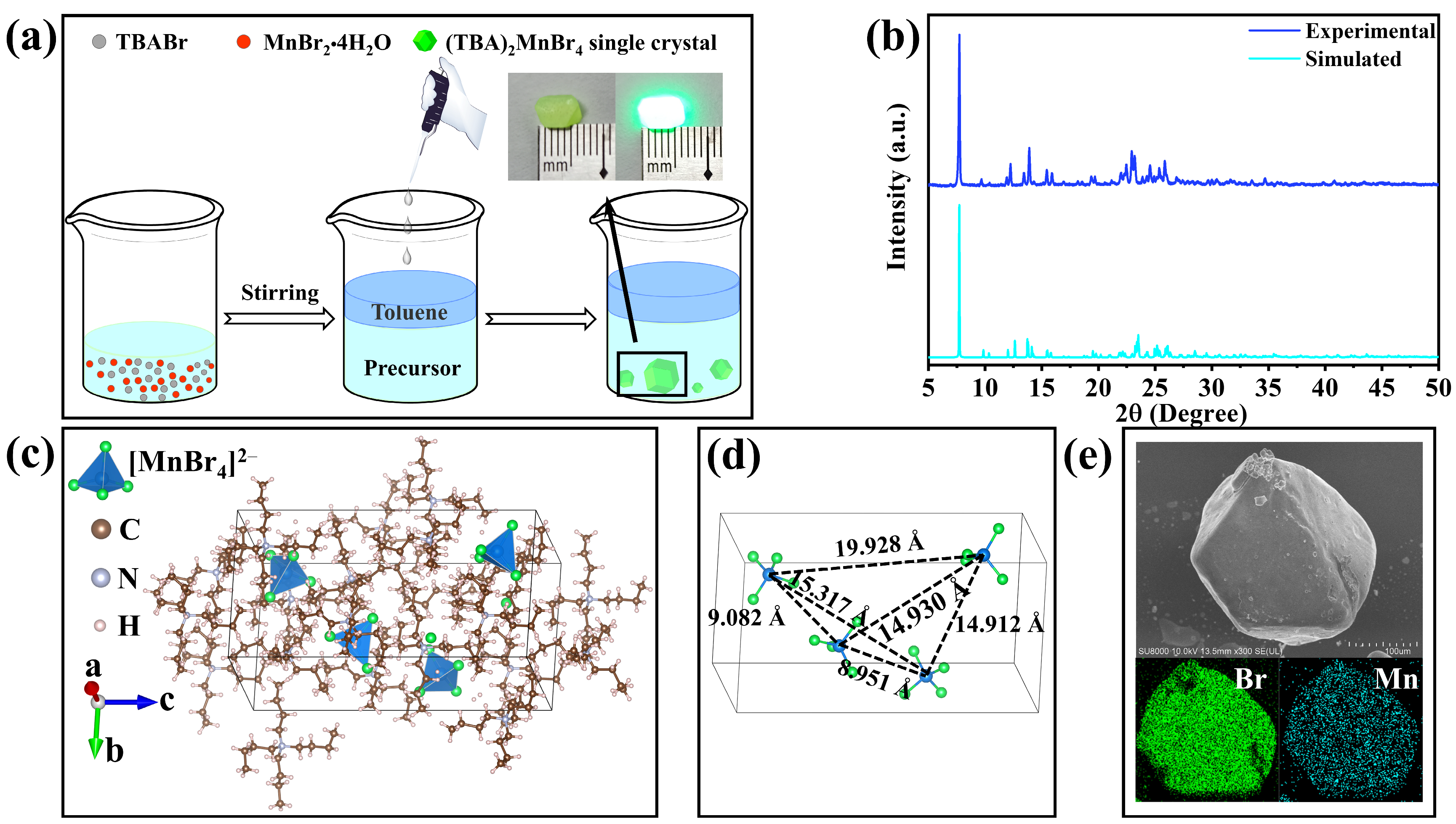
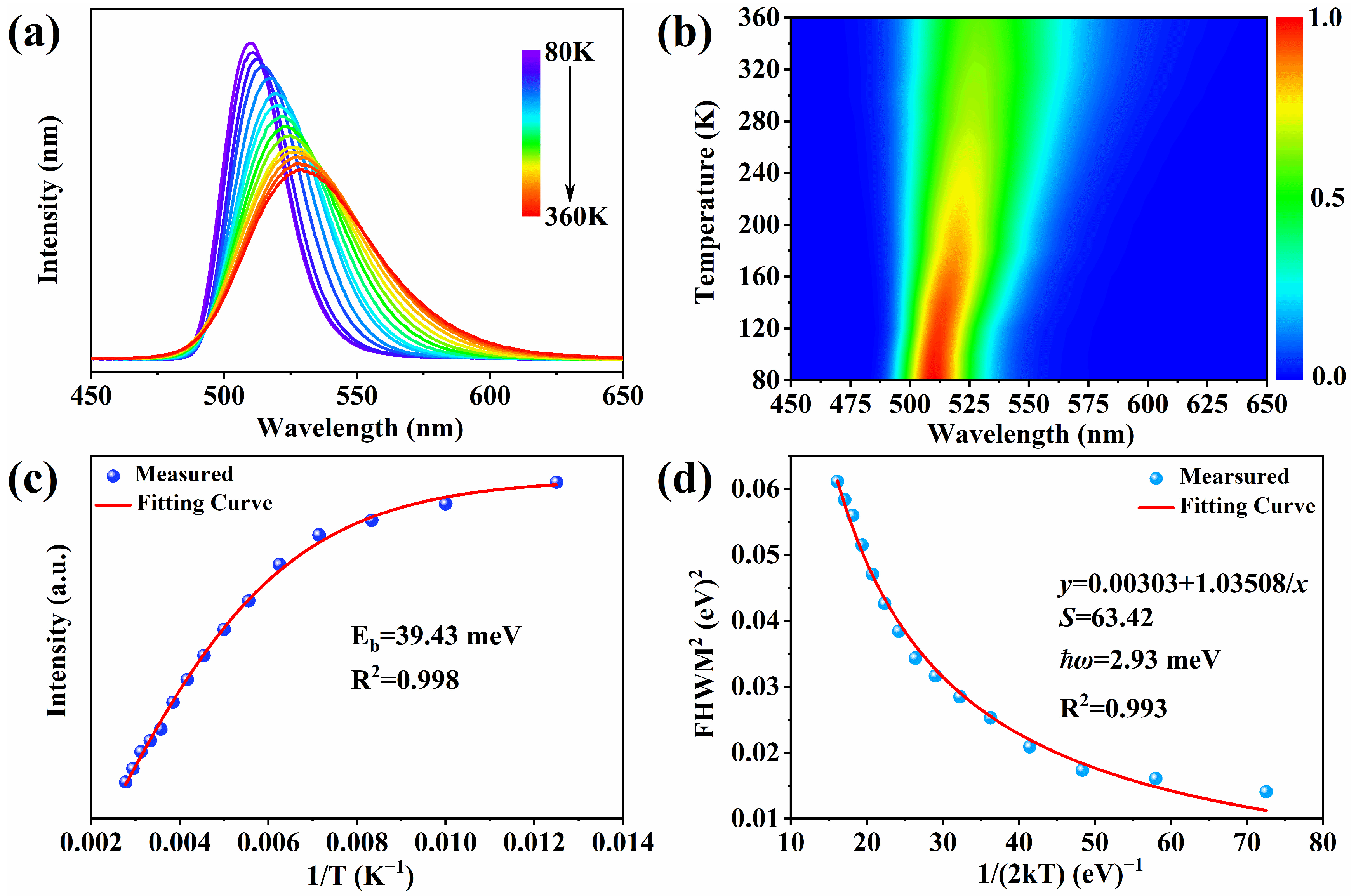
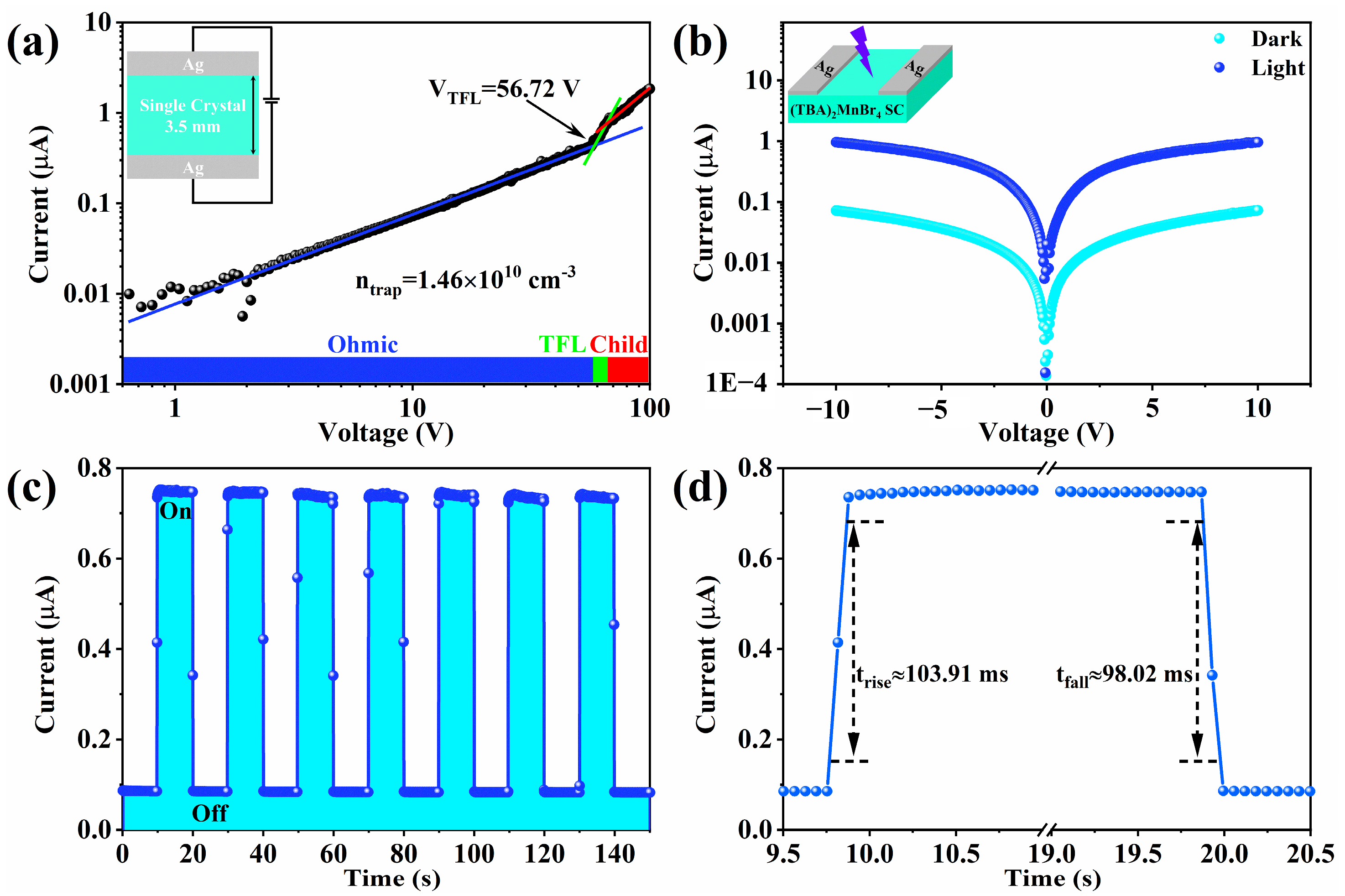
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortecchia, D.; Yin, J.; Petrozza, A.; Soci, C. White Light Emission in Low-Dimensional Perovskites. J. Mater. Chem. C 2019, 7, 4956–4969. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Zeng, R.; Liu, X.; Zou, B.; Xiang, W. High Efficiency Fluorescent Perovskite Quantum Dots Encapsulated in Superhydrophobic Silica Aerogel for Wide Color Gamut Backlight Displays. Chem. Eng. J. 2022, 433, 133195. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Ou, X.; Huang, B.; Almutlaq, J.; Zhumekenov, A.A.; Guan, X.; Han, S.; Liang, L.; Yi, Z.; et al. All-Inorganic Perovskite Nanocrystal Scintillators. Nature 2018, 561, 88–93. [Google Scholar] [CrossRef]
- Xuan, T.; Xie, R.-J. Recent Processes on Light-Emitting Lead-Free Metal Halide Perovskites. Chem. Eng. J. 2020, 393, 124757. [Google Scholar] [CrossRef]
- Bao, C.; Yang, J.; Bai, S.; Xu, W.; Yan, Z.; Xu, Q.; Liu, J.; Zhang, W.; Gao, F. High Performance and Stable All-Inorganic Metal Halide Perovskite-Based Photodetectors for Optical Communication Applications. Adv. Mater. 2018, 30, 1803422. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, F. Recent Progress on Highly Sensitive Perovskite Photodetectors. J. Mater. Chem. C 2019, 7, 1741–1791. [Google Scholar] [CrossRef]
- Afroz, M.A.; Singh, A.; Gupta, R.K.; Garai, R.; Tailor, N.K.; Choudhary, S.; Sharma, B.; Mahajan, P.; Padha, B.; Verma, S. Design Potential and Future Prospects of Lead–free Halide Perovskites in Photovoltaic Devices. J. Mater. Chem. A 2023, 11, 13133–13173. [Google Scholar] [CrossRef]
- Parikh, N.; Akin, S.; Kalam, A.; Prochowicz, D.; Yadav, P. Probing the Low-Frequency Response of Impedance Spectroscopy of Halide Perovskite Single Crystals Using Machine Learning. ACS Appl. Mater. Interfaces 2023, 15, 27801–27808. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Chu, Y.; Zhang, H.; Sun, M.; Wang, H.; Wang, S.; Zhao, G. Environmental-Friendly Lead-Free Chiral Mn-Based Metal Halides with Efficient Circularly Polarized Photoluminescence at Room Temperature. J. Alloys Compd. 2022, 910, 164892. [Google Scholar] [CrossRef]
- He, Z.-L.; Wei, J.-H.; Luo, J.-B.; Zhang, Z.-Z.; Kuang, D.-B. Reversible Human-Temperature-Responsive Luminescence Switching in A Mn(II)-Based Metal Halide. J. Mater. Chem. C 2023, 11, 1251–1257. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, H.; Cai, W.; Lu, S.; Zhao, S.; Zang, Z.; Xie, L. Mn2+-Based Luminescent Metal Halides: Syntheses, Properties, and Applications. Adv. Opt. Mater. 2023, 11, 2202997. [Google Scholar] [CrossRef]
- Dai, G.; Ma, Z.; Qiu, Y.; Li, Z.; Fu, X.; Jiang, H.; Ma, Z. A Red-Emitting Hybrid Manganese Halide Perovskite C5H5NOMnCl2· H2O Featuring One-Dimensional Octahedron Chains. Inorg. Chem. 2022, 61, 12635–12642. [Google Scholar] [CrossRef]
- Shimono, S.; Sekine, M.; Niwa, Y.; Sagayama, H.; Araki, K.; Hata, Y.; Kishimura, H. Solid–Liquid Transitions in Mn-Based Ionic Liquids [MeIM]2[MnBr4] and [EtIM]2[MnBr4]Producing Emission Spectra with Narrow Green Bands. Mater. Res. Bull. 2023, 159, 112103. [Google Scholar] [CrossRef]
- Kou, T.; Wei, Q.; Jia, W.; Chang, T.; Peng, C.; Liang, Y.; Zou, B. Light Emission Enhancement of (C3H10N)4Pb1-xMnxBr6 Metal-Halide Powders by the Dielectric Confinement Effect of a Nanosized Water Layer. ACS Appl. Mater. Interfaces 2022, 14, 6167–6179. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, H.; Wei, Q.; Huang, T.; Yao, S.; Tian, Y.; Peng, C.; Zou, B. The Magnetic Molaron Modulated Luminescence Mands of Organic-Inorganic Hybrid Ferroelectric Anti-Perovskite (C3H9N)3Cd2Cl7 Doped with Mn2+. Mater. Today Chem. 2022, 24, 100781. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Zhou, J.; Ming, H.; Wang, C.-H.; Jing, X.; Ye, S.; Zhang, Q. Structural Design Enables Highly-Efficient Green Emission with Preferable Blue Light Excitation from Zero-Dimensional Manganese (II) Hybrids. Chem. Eng. J. 2021, 421, 129886. [Google Scholar] [CrossRef]
- Zhou, G.; Ding, J.; Jiang, X.; Zhang, J.; Molokeev, M.S.; Ren, Q.; Zhou, J.; Li, S.; Zhang, X.-M. Coordination Units of Mn2+ Modulation Toward Tunable Emission in Zero-Dimensional Bromides for White Light-Emitting Diodes. J. Mater. Chem. C 2022, 10, 2095–2102. [Google Scholar] [CrossRef]
- Liu, H.L.; Ru, H.Y.; Sun, M.E.; Wang, Z.Y.; Zang, S.Q. Organic−Inorganic Manganese Bromide Hybrids with Water-Triggered Luminescence for Rewritable Paper. Adv. Opt. Mater. 2021, 10, 2101700. [Google Scholar] [CrossRef]
- Jana, A.; Sree, V.G.; Ba, Q.; Cho, S.C.; Lee, S.U.; Cho, S.; Jo, Y.; Meena, A.; Kim, H.; Im, H. Efficient Organic Manganese(II) Bromide Green-Light-Emitting Diodes Enabled by Manipulating the Hole and Electron Transport Layer. J. Mater. Chem. C 2021, 9, 11314–11323. [Google Scholar] [CrossRef]
- Xu, L.J.; Sun, C.Z.; Xiao, H.; Wu, Y.; Chen, Z.N. Green-Light-Emitting Diodes based on Tetrabromide Manganese(II) Complex through Solution Process. Adv. Mater. 2017, 29, 1605739. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, J.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Zhao, J.; Xia, Z. Lead-Free Hybrid Metal Halides with a Green-Emissive [MnBr 4] Unit as a Selective Turn-On Fluorescent Sensor for Acetone. Inorg. Chem. 2019, 58, 13464–13470. [Google Scholar] [CrossRef]
- Su, B.; Molokeev, M.S.; Xia, Z. Mn2+-Based Narrow-Vand Green-Emitting Cs3MnBr5 Phosphor and The Performance Optimization by Zn2+ Alloying. J. Mater. Chem. C 2019, 7, 11220–11226. [Google Scholar] [CrossRef]
- Chen, S.; Gao, J.; Chang, J.; Zhang, Y.; Feng, L. Organic-Inorganic Manganese (II) Halide Hybrids Based Paper Sensor for The Fluorometric Determination of Pesticide Ferbam. Sens. Actuator B-Chem. 2019, 297, 126701. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Kou, T.; Zhou, Y.; Liang, Y.; Wu, Z.; Huang, J.; Chang, T.; Peng, C.; Wei, Q.; et al. Lead-Free MnII-Based Red-Emitting Hybrid Halide (CH6N3)2MnCl4 Toward High Performance Warm WLEDs. J. Mater. Chem. C 2021, 9, 4895–4902. [Google Scholar] [CrossRef]
- Xu, L.J.; Lin, X.; He, Q.; Worku, M.; Ma, B. Highly Efficient Eco-Friendly X-Ray Scintillators Based on An Organic Manganese Halide. Nat. Commun. 2020, 11, 4329. [Google Scholar] [CrossRef]
- Jiang, T.; Ma, W.; Zhang, H.; Tian, Y.; Lin, G.; Xiao, W.; Yu, X.; Qiu, J.; Xu, X.; Yang, Y.; et al. Highly Efficient and Tunable Emission of Lead-Free Manganese Halides toward White Light-Emitting Diode and X-Ray Scintillation Applications. Adv. Funct. Mater. 2021, 31, 2009973. [Google Scholar] [CrossRef]
- Gao, W.; Leng, M.; Hu, Z.; Li, J.; Li, D.; Liu, H.; Gao, L.; Niu, G.; Tang, J. Reversible Luminescent Humidity Chromism of Organic-Inorganic Hybrid PEA2MnBr4 Single Crystals. Dalton Trans. 2020, 49, 5662–5668. [Google Scholar] [CrossRef]
- Hu, G.; Xu, B.; Wang, A.; Guo, Y.; Wu, J.; Muhammad, F.; Meng, W.; Wang, C.; Sui, S.; Liu, Y.; et al. Stable and Bright Pyridine Manganese Halides for Efficient White Light-Emitting Diodes. Adv. Funct. Mater. 2021, 31, 2011191. [Google Scholar] [CrossRef]
- Li, C.; Luo, Z.; Liu, Y.; Wei, Y.; He, X.; Chen, Z.; Zhang, L.; Chen, Y.; Wang, W.; Liu, Y. Self-Trapped Exciton Emission with High Thermal Stability in Antimony-Doped Hybrid Manganese Chloride. Adv. Opt. Mater. 2022, 10, 2102746. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, M.; Liu, H.; Wang, Y.; Wang, K.; Yang, X.; Zou, B. Pressure-Treated Engineering to Harvest Enhanced Green Emission in Mn-Based Organic–Inorganic Metal Halides at Ambient Conditions. Adv. Funct. Mater. 2022, 32, 2109277. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, H.; Liu, W.; Zhan, K.; Liu, H.; Tang, Z.; Yang, C. High-Efficiency Narrow-Band Green-Emitting Manganese (II) Halide for Multifunctional Applications. ACS Appl. Mater. Interfaces 2023, 15, 47238–47249. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xiong, Y.; Liu, K.; He, S.; Cao, J.; Zhang, X.; Zhao, J.; Liu, Q. Efficient Narrow-Band Green Light-Emitting Hybrid Halides for Wide Color Gmut Dsplay. ACS Appl. Electron. Mater. 2022, 4, 4068–4076. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Z.; Huang, J.; Molokeev, M.S.; Xiao, Z.; Ma, C.; Xia, Z. Unraveling the Near-Unity Narrow-Band Green Emission in Zero-Dimensional Mn2+-Based Metal Halides: A Case Study of (C10H16N)2Zn1-xMnxBr4 Solid Solutions. J. Phys. Chem. Lett. 2020, 11, 5956–5962. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Sun, Y.; Xia, Z.; Xiao, Z. Photoluminescence Behavior of Zero-Dimensional Manganese Halide Tetrahedra Embedded in Conjugated Organic Matrices. J. Phys. Chem. Lett. 2021, 12, 7394–7399. [Google Scholar] [CrossRef] [PubMed]
- Barreda-Argueso, J.A.; Nataf, L.; Rodriguez-Lazcano, Y.; Aguado, F.; Gonzalez, J.; Valiente, R.; Rodriguez, F.; Wilhelm, H.; Jephcoat, A.P. Bulk and Molecular Compressibilities of Organic-Inorganic Hybrids [(CH3)4N]2MnX4 (X = Cl, Br); Role of Intermolecular Interactions. Inorg. Chem. 2014, 53, 10708–10715. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wang, J.; Hu, X.; Zhou, B.; Zheng, G.; Mo, S.; Li, S.; Long, F.; Zou, Z. Synthesis, Crystal Structure, Photoluminescence Properties of Organic-Inorganic Hybrid Materials Based on Ethylenediamine Bromide. J. Saudi Chem. Soc. 2020, 24, 52–60. [Google Scholar] [CrossRef]
- Peng, C.; Wei, Q.; Yao, S.; Meng, X.; Yu, Z.; Peng, H.; Zhong, X.; Zou, B. H2O–NH4+-Induced Emission Modulation in Sb3+-Doped (NH4)2InCl5·H2O. Inorg. Chem. 2022, 61, 12406–12414. [Google Scholar] [CrossRef]
- Jin, P.; Fu, Y.; Niu, R.; Zhang, Q.; Zhang, M.; Li, Z.; Zhang, X. Non-Destructive Detection of the Freshness of Air-Modified Mutton Based on Near-Infrared Spectroscopy. Foods 2023, 12, 2756. [Google Scholar] [CrossRef]
- Qiu, Y.; Shi, M.; Guo, X.; Li, J.; Wu, J.; Zhou, Y.; Sun, H.; Hang, Y.; Li, X.; Li, Y. Sensitivity Improvement in the Measurement of Minor Components by Spatial Confinement in Fiber-Optic Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B 2023, 209, 106800. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, W.; Gao, Z.; Tang, Z.; Lei, L.; Sun, X.; Li, Y.; Cai, H.-L.; Wu, X. New Photoluminescence Hybrid Perovskites with Ultrahigh Photoluminescence Quantum Yield and Ultrahigh Thermostability Temperature up to 600 K. Nano Energy 2020, 77, 105170. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Davydova, M.P.; Berezin, A.S.; Sukhikh, T.S.; Samsonenko, D.G. Photo- and Triboluminescent Robust 1D Polymers Made of Mn(II) Halides and Meta-Carborane Based Bis(Phosphine Oxide). Inorg. Chem. Front. 2021, 8, 2261–2270. [Google Scholar] [CrossRef]
- Sugano, S. Multiplets of Transition-Metal Ions in Crystals; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Jing, Y.; Liu, Y.; Jiang, X.; Molokeev, M.S.; Lin, Z.; Xia, Z. Sb3+ Dopant and Halogen Substitution Triggered Highly Efficient and Tunable Emission in Lead-Free Metal Halide Single Crystals. Chem. Mater. 2020, 32, 5327–5334. [Google Scholar] [CrossRef]
- Gong, X.; Voznyy, O.; Jain, A.; Liu, W.; Sabatini, R.; Piontkowski, Z.; Walters, G.; Bappi, G.; Nokhrin, S.; Bushuyev, O.; et al. Electron-Phonon Interaction in Efficient Perovskite Blue Emitters. Nat. Mater. 2018, 17, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, C.; Li, B.; Lin, C.; Li, Y.; Wang, L.; Xie, R.-J. Large-Scale Room-Temperature Synthesis of High-Efficiency Lead-Free Perovskite Derivative (NH4)2SnCl6:Te Phosphor for Warm wLEDs. Chem. Eng. J. 2021, 420, 129740. [Google Scholar] [CrossRef]
- Kong, L.; Liu, Y.; Dong, L.; Zhang, L.; Qiao, L.; Wang, W.; You, H. Enhanced Red Luminescence in CaAl12O19:Mn4+ via Doping Ga3+ for Plant Growth Lighting. Dalton Trans. 2020, 49, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Li, T.; Huang, F.; Li, Z.; Zhou, Y.; Lin, T.; Ouyang, Y.; Tao, X.; Pan, C. Highly-Efficient All-Inorganic Lead-Free 1D CsCu2I3 Single Crystal for White-Light Emitting Diodes and UV Photodetection. Nano Energy 2021, 81, 105570. [Google Scholar] [CrossRef]
- Zhong, J.; Han, M.; Li, C.; Li, R.; He, H. Facile and Scalable Fabrication Process of Electroluminescent Filament with High Luminescent Efficiency. Mater. Lett. 2023, 350, 134868. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Z.; Molokeev, M.S.; Xiao, Z.; Xia, Z.; Zhang, X.-M. Manipulation of Cl/Br Transmutation in Zero-Dimensional Mn2+-Based Metal Halides Toward Tunable Photoluminescence and Thermal Quenching Behaviors. J. Mater. Chem. C 2021, 9, 2047–2053. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Reddeppa, M.; Park, B.-G.; Oh, J.-E.; Kim, S.-G.; Kim, M.-D. Efficient Charge Separation in Polypyrrole/GaN-Nanorod-Based Hybrid Heterojunctions for High-Performance Self-Powered UV Photodetection. Phys. Status Solidi RRL 2021, 15, 2000518. [Google Scholar] [CrossRef]
- Vuong, V.-H.; Pammi, S.; Ippili, S.; Jella, V.; Thi, T.N.; Pasupuleti, K.S.; Kim, M.-D.; Jeong, M.J.; Jeong, J.-R.; Chang, H.S. Flexible, Stable, and Self-Powered Photodetectors Embedded with Chemical Vapor Deposited Lead-Free Bismuth Mixed Halide Perovskite Films. Chem. Eng. J. 2023, 458, 141473. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Li, D.; Chen, P.; Pi, L.; Zhou, X.; Zhai, T. Van der Waals Integration Based on Two-Dimensional Materials for High-Performance Infrared Photodetectors. Adv. Funct. Mater. 2021, 31, 2103106. [Google Scholar] [CrossRef]
- Balderas-Aguilar, J.U.; Falcony, C.; Garduño-Wilchis, I.A.; Aguilar-Frutis, M.; Hernandez-Como, N.; Martínez-Merlín, I.E.; García-Hipólito, M.; Alonso-Huitrón, J.C. All-Green Cs4CuSb2Cl12 Perovskite Films Deposited in Situ by AACVD and Their Doping with F-Ions for Photodetectors and Memdiodes. J. Mater. Chem. C 2023, 11, 16214–16224. [Google Scholar] [CrossRef]
- Afzal, A.M.; Javed, Y.; Akhtar Shad, N.; Iqbal, M.Z.; Dastgeer, G.; Munir Sajid, M.; Mumtaz, S. Tunneling-Based Rectification and Photoresponsivity in Black Phosphorus/Hexagonal Boron Nitride/Rhenium Diselenide Van der Waals Heterojunction Diode. Nanoscale 2020, 12, 3455–3468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhao, K.; Yang, Z.; Feng, J.; Zhang, X.; Wang, K.; Meng, L.; Ye, H.; Liu, M.; et al. A 1300 mm2 Ultrahigh-Performance Digital Imaging Assembly using High-Quality Perovskite Single Crystals. Adv. Mater. 2018, 30, 1707314. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhou, D.; Ding, N.; Sun, R.; Xu, W.; Wang, N.; Xu, S.; Liu, D.; Song, H. Broadband Ultraviolet Photodetectors Based on Cerium Doped Lead-Free Cs3MnBr5 Metal Halide Nanocrystals. ACS Sustain. Chem. Eng. 2021, 9, 4980–4987. [Google Scholar] [CrossRef]
- Luo, J.B.; Wei, J.H.; Zhang, Z.Z.; He, Z.L.; Kuang, D.B. A Melt-Quenched Luminescent Glass of an Organic–Inorganic Manganese Halide as a Large-Area Scintillator for Radiation Detection. Angew. Chem. Int. Ed. 2023, 135, e202216504. [Google Scholar] [CrossRef]
- Zhang, W.; Sui, P.; Zheng, W.; Li, L.; Wang, S.; Huang, P.; Zhang, W.; Zhang, Q.; Yu, Y.; Chen, X. Pseudo-2D Layered Organic-Inorganic Manganese Bromide with a Near-Unity Photoluminescence Quantum Yield for White Light-Emitting Diode and X-Ray Scintillator. Angew. Chem. Int. Ed. 2023, 135, e202309230. [Google Scholar] [CrossRef]
- Shao, W.; Zhu, G.; Wang, X.; Zhang, Z.; Lv, H.; Deng, W.; Zhang, X.; Liang, H. Highly Efficient, Flexible, and Eco-Friendly Manganese (II) Halide Nanocrystal Membrane with Low Light Scattering for High-Resolution X-ray Imaging. ACS Appl. Mater. Interfaces 2023, 15, 932–941. [Google Scholar] [CrossRef]
- Li, B.; Xu, Y.; Zhang, X.; Han, K.; Jin, J.; Xia, Z. Zero-Dimensional Luminescent Metal Halide Hybrids Enabling Bulk Transparent Medium as Large-Area X-Ray Scintillators. Adv. Opt. Mater. 2022, 10, 2102793. [Google Scholar] [CrossRef]
- Li, C.; Wu, D.; Wang, Q.; Bai, Y.; He, P.; An, K.; Tang, X. Lead-Free Scintillators Based on Pyridine Manganese Halide for X-ray Imaging. J. Lumin. 2022, 250, 119130. [Google Scholar] [CrossRef]
- Kong, Q.; Meng, X.; Ji, S.; Wang, Q.; Yang, B.; Bai, T.; Wang, X.; Wang, Z.; Zhang, R.; Zheng, D. Highly Reversible Cesium Manganese Iodine for Sensitive Water Detection and X-ray Imaging. ACS Mater. Lett. 2022, 4, 1734–1741. [Google Scholar] [CrossRef]
- Sree, V.G.; Jana, A.; Cho, S.C.; Lee, S.U.; Cho, S.; Sohn, J.I.; Im, H. Green and Warm-White Light-Emitting Diodes Enabled by Zero-Dimensional Green-Emitting Mn(II) Bromide Complex with Record High Efficiency. Chem. Eng. J. 2023, 474, 145936. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wei, Q.; Duan, L.; Peng, C. Lead-Free Organic Manganese (II) Bromide Hybrid with Highly Efficient and Stable Green Emission for UV Photodetection. Crystals 2023, 13, 1678. https://doi.org/10.3390/cryst13121678
Tian Y, Wei Q, Duan L, Peng C. Lead-Free Organic Manganese (II) Bromide Hybrid with Highly Efficient and Stable Green Emission for UV Photodetection. Crystals. 2023; 13(12):1678. https://doi.org/10.3390/cryst13121678
Chicago/Turabian StyleTian, Ye, Qilin Wei, Lian Duan, and Chengyu Peng. 2023. "Lead-Free Organic Manganese (II) Bromide Hybrid with Highly Efficient and Stable Green Emission for UV Photodetection" Crystals 13, no. 12: 1678. https://doi.org/10.3390/cryst13121678





