Synthesis of Bimetallic Nanoparticles and Applications—An Updated Review
Abstract
:1. Introduction
2. Different Types of Bimetallic
3. Synthesis of Bimetallic Nanoparticles
3.1. Physical Method
3.1.1. Laser Ablation
3.1.2. Mechanical Grinding
3.1.3. Microwave Irradiation
3.1.4. Ball Milling
3.1.5. Electrochemical Method
3.2. Chemical Method
3.2.1. Simultaneous Reduction
Co-Reduction
Sonochemical Co-Reduction
Radiolytic Co-Reduction
Chemical Precipitation
Thermal Decomposition
Sol-Gel Method
Micro-Emulsion Method
Hydrothermal Reduction Method
3.2.2. Successive Reduction
3.3. Polymer Mediated Approach
3.4. Solid-Supported Bimetallic Nanoparticles
3.5. Biological Method
3.5.1. Microbial Synthesis Method
3.5.2. Plant Mediated Synthesis
3.5.3. Bio-Waste Approach for Synthesizing Bimetallic Nanoparticles
4. Characterization Techniques of Bimetallic Nanoparticles
5. Applications of Bimetallic Nanoparticles
5.1. Biological Applications
5.2. Agriculture
5.3. Environmental Application
5.4. Engineering Application
5.5. Bimetallic Application in Physics and Chemistry
6. Challenges and Future Prospective
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rotello, V. Nanoparticles: Building Blocks for Nanotechnology; Springer Science & Business Media: Berlin, Germany, 2004; ISBN 978-0-306-48287-8. [Google Scholar]
- Mazhar, T.; Shrivastava, V.; Tomar, R.S. Green synthesis of bimetallic nanoparticles and its applications: A review. J. Pharm. Sci. Res. 2017, 9, 102–110. [Google Scholar]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, N. Metallic Nanoparticle: A Review. BJSTR 2018, 4, 3765–3775. [Google Scholar] [CrossRef] [Green Version]
- Auffan, M.; Rose, J.; Wiesner, M.; Bottero, J.-Y.; Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential toxicity in vitro. Environ. Pollut. 2008, 157, 1127–1133. [Google Scholar] [CrossRef]
- Behera, A.; Mittu, B.; Padhi, S.; Patra, N.; Singh, J. Chapter 25—Bimetallic nanoparticles: Green synthesis, applications, and future perspectives. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Abd-Elsalam, K.A., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 639–682. ISBN 978-0-12-821354-4. [Google Scholar]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.; Zhang, Y.; Gao, H.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Li, Y. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today 2012, 7, 448–466. [Google Scholar] [CrossRef]
- Shamsiev, R.S.; Danilov, F.O.; Flid, V.R. Catalytic activity of bimetallic nanoparticles M@Pd (M = Ni, Cu, Ag, Pt, Au) in deoxygenation of carboxylic acids: A quantum chemical evaluation. Russ. Chem. Bull. 2022, 71, 220–226. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic Nanoparticles for Antimicrobial Applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Jiang, L.; Zeng, G.; Wang, D.; Tang, W.; Liang, J.; Wang, H.; He, D.; Liu, Z.; Tang, L. Bimetallic nanoparticles/metal-organic frameworks: Synthesis, applications and challenges. Appl. Mater. Today 2020, 19, 100564. [Google Scholar] [CrossRef]
- Hu, D.; Xu, H.; Yi, Z.; Chen, Z.; Ye, C.; Wu, Z.; Garces, H.F.; Yan, K. Green CO2-assisted synthesis of mono-and bimetallic Pd/Pt nanoparticles on porous carbon fabricated from sorghum for highly selective hydrogenation of furfural. ACS Sustain. Chem. Eng. 2019, 7, 15339–15345. [Google Scholar] [CrossRef]
- Stephanie, R.; Kim, M.W.; Kim, S.H.; Kim, J.-K.; Park, C.Y.; Park, T.J. Recent advances of bimetallic nanomaterials and its nanocomposites for biosensing applications. TrAC Trends Anal. Chem. 2021, 135, 116159. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2016, 2, 54–88. [Google Scholar] [CrossRef]
- Scaria, J.; Nidheesh, P.V.; Kumar, M.S. Synthesis and applications of various bimetallic nanomaterials in water and wastewater treatment. J. Environ. Manag. 2020, 259, 110011. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Basavegowda, N.; Mandal, T.K.; Baek, K.-H. Bimetallic and Trimetallic Nanoparticles for Active Food Packaging Applications: A Review. Food Bioprocess Technol. 2020, 13, 30–44. [Google Scholar] [CrossRef]
- Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef] [Green Version]
- Pissuwan, D.; Niidome, T.; Cortie, M.B. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Control. Release 2011, 149, 65–71. [Google Scholar] [CrossRef]
- Aygun, A.; Gulbagca, F.; Altuner, E.E.; Bekmezci, M.; Gur, T.; Karimi-Maleh, H.; Karimi, F.; Vasseghian, Y.; Sen, F. Highly active PdPt bimetallic nanoparticles synthesized by one-step bioreduction method: Characterizations, anticancer, antibacterial activities and evaluation of their catalytic effect for hydrogen generation. Int. J. Hydrogen Energy 2022, 48, 6666–6679. [Google Scholar] [CrossRef]
- Nasrabadi, H.T.; Abbasi, E.; Davaran, S.; Kouhi, M.; Akbarzadeh, A. Bimetallic nanoparticles: Preparation, properties, and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 376–380. [Google Scholar] [CrossRef]
- Velmurugan, P.; Mohanavel, V.; Sekar, P.; Vijayanand, S.; Chinnathambi, A.; Govindasamy, C.; Sivakumar, S. Influence of dissolved oxygen on the synthesis of Ag-Au mono and bimetallic nanostructure using Cudrania tricuspidata leaf extract and its broad-spectrum antibacterial activity. Mater. Lett. 2022, 310, 131471. [Google Scholar] [CrossRef]
- Medina-Cruz, D.; Saleh, B.; Vernet-Crua, A.; Nieto-Argüello, A.; Lomelí-Marroquín, D.; Vélez-Escamilla, L.Y.; Cholula-Díaz, J.L.; García-Martín, J.M.; Webster, T. Bimetallic Nanoparticles for Biomedical Applications: A Review. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 397–434. ISBN 978-3-030-34471-9. [Google Scholar]
- Ali, S.; Sharma, A.S.; Ahmad, W.; Zareef, M.; Hassan, M.M.; Viswadevarayalu, A.; Jiao, T.; Li, H.; Chen, Q. Noble Metals Based Bimetallic and Trimetallic Nanoparticles: Controlled Synthesis, Antimicrobial and Anticancer Applications. Crit. Rev. Anal. Chem. 2021, 51, 454–481. [Google Scholar] [CrossRef] [PubMed]
- Cruces, E.; Arancibia-Miranda, N.; Manquián-Cerda, K.; Perreault, F.; Bolan, N.; Azócar, M.I.; Cubillos, V.; Montory, J.; Rubio, M.A.; Sarkar, B. Copper/Silver Bimetallic Nanoparticles Supported on Aluminosilicate Geomaterials as Antibacterial Agents. ACS Appl. Nano Mater. 2022, 5, 1472–1483. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Cruz, A.L.; Garza-Cervantes, J.A.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synthesis and design of Ag–Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Bohara, R.A.; Rai, S.N.; Aleya, L.; Singh, M.P. Two birds with one stone: Oyster mushroom mediated bimetallic Au-Pt nanoparticles for agro-waste management and anticancer activity. Environ. Sci. Pollut. Res. 2021, 28, 13761–13775. [Google Scholar] [CrossRef] [PubMed]
- Amiripour, F.; Azizi, S.N.; Ghasemi, S. Gold-copper bimetallic nanoparticles supported on nano P zeolite modified carbon paste electrode as an efficient electrocatalyst and sensitive sensor for determination of hydrazine. Biosens. Bioelectron. 2018, 107, 111–117. [Google Scholar] [CrossRef]
- Amiripour, F.; Ghasemi, S.; Azizi, S.N. A novel non-enzymatic glucose sensor based on gold-nickel bimetallic nanoparticles doped aluminosilicate framework prepared from agro-waste material. Appl. Surf. Sci. 2021, 537, 147827. [Google Scholar] [CrossRef]
- Appa, R.M.; Raghavendra, P.; Lakshmidevi, J.; Naidu, B.R.; Sarma, L.S.; Venkateswarlu, K. Structure controlled Au@Pd NPs/rGO as robust heterogeneous catalyst for Suzuki coupling in biowaste-derived water extract of pomegranate ash. Appl. Organomet. Chem. 2021, 35, e6188. [Google Scholar] [CrossRef]
- Meena Kumari, M.; Jacob, J.; Philip, D. Green synthesis and applications of Au–Ag bimetallic nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ansari, Z.; Saha, A.; Singha, S.S.; Sen, K. Phytomediated generation of Ag, CuO and Ag-Cu nanoparticles for dimethoate sensing. J. Photochem. Photobiol. A: Chem. 2018, 367, 200–211. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Fayemi, O.E.; Botha, T.L. Green synthesis and electrochemistry of Ag, Au, and Ag–Au bimetallic nanoparticles using golden rod (Solidago canadensis) leaf extract. Appl. Phys. A 2019, 125, 42. [Google Scholar] [CrossRef]
- Wu, M.-L.; Chen, D.-H.; Huang, T.-C. Synthesis of Au/Pd Bimetallic Nanoparticles in Reverse Micelles. Langmuir 2001, 17, 3877–3883. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Ramkumar, V.S.; Archunan, G.; Chaiyasut, C.; Suganthy, N. Biogenic synthesis of silver palladium bimetallic nanoparticles from fruit extract of Terminalia chebula—In vitro evaluation of anticancer and antimicrobial activity. J. Drug Deliv. Sci. Technol. 2019, 51, 139–151. [Google Scholar] [CrossRef]
- Singh, I.; Mazhar, T.; Shrivastava, V.; Tomar, R.S. Bio-assisted synthesis of bi-metallic (Ag-Zn) nanoparticles by leaf extract of Azadirachta indica and its antimicrobial properties. Int. J. Nano Dimens. 2022, 13, 168–178. [Google Scholar] [CrossRef]
- De León-Quiroz, E.L.; García-Cerda, L.A.; Obregón, D.V.; Pedraza, A.P.; Larios-Rodríguez, E.; José-Yacaman, M. Synthesis and Characterization of Alloys and Bimetallic Nanoparticles of CuNi Prepared by Sol-Gel Method. MRS Online Proc. Libr. 2012, 1479, 9–14. [Google Scholar] [CrossRef]
- Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Synthesis and anti-bacterial activity of Cu, Ag and Cu–Ag alloy nanoparticles: A green approach. Mater. Res. Bull. 2011, 46, 384–389. [Google Scholar] [CrossRef]
- Bakina, O.V.; Glazkova, E.A.; Svarovskaya, N.V.; Rodkevich, N.G.; Lerner, M.I. «Janus»-like Cu-Fe bimetallic nanoparticles with high antibacterial activity. Mater. Lett. 2019, 242, 187–190. [Google Scholar] [CrossRef]
- Bernal, M.; Bagger, A.; Scholten, F.; Sinev, I.; Bergmann, A.; Ahmadi, M.; Rossmeisl, J.; Cuenya, B.R. CO2 electroreduction on copper-cobalt nanoparticles: Size and composition effect. Nano Energy 2018, 53, 27–36. [Google Scholar] [CrossRef]
- Wu, M.; Wu, X.; Zhang, L.; Abdelhafiz, A.; Chang, I.; Qu, C.; Jiang, Y.; Zeng, J.; Alamgir, F. Cu@Pt catalysts prepared by galvanic replacement of polyhedral copper nanoparticles for polymer electrolyte membrane fuel cells. Electrochim. Acta 2019, 306, 167–174. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Gholami, S.; Mahyari, M. Superb catalytic properties of nickel cobalt bimetallic nanoparticles immobilized on 3D nitrogen-doped graphene for thermal decomposition of ammonium perchlorate. Res. Chem. Intermed. 2019, 45, 1527–1543. [Google Scholar] [CrossRef]
- Foster, S.L.; Estoque, K.; Voecks, M.; Rentz, N.; Greenlee, L.F. Removal of Synthetic Azo Dye Using Bimetallic Nickel-Iron Nanoparticles. J. Nanomater. 2019, 2019, e9807605. [Google Scholar] [CrossRef]
- Pak, M.; Moshaii, A.; Nikkhah, M.; Abbasian, S.; Siampour, H. Nickel-gold bimetallic nanostructures with the improved electrochemical performance for non-enzymatic glucose determination. J. Electroanal. Chem. 2021, 900, 115729. [Google Scholar] [CrossRef]
- Sevim, M.; Kaplan, F. Ketjen Black supported monodisperse nickel–platinum alloy nanoparticles for the efficient catalyst in the hydrolytic dehydrogenation of ammonia borane. Appl. Organomet. Chem. 2021, 35, e6095. [Google Scholar] [CrossRef]
- Chen, G.; Desinan, S.; Rosei, R.; Rosei, F.; Ma, D. Synthesis of Ni–Ru Alloy Nanoparticles and Their High Catalytic Activity in Dehydrogenation of Ammonia Borane. Chem. A Eur. J. 2012, 18, 7925–7930. [Google Scholar] [CrossRef]
- Oruç, Z.; Ergüt, M.; Uzunoğlu, D.; Özer, A. Green synthesis of biomass-derived activated carbon/Fe-Zn bimetallic nanoparticles from lemon (Citrus limon (L.) Burm. f.) wastes for heterogeneous Fenton-like decolorization of Reactive Red 2. J. Environ. Chem. Eng. 2019, 7, 103231. [Google Scholar] [CrossRef]
- Chen, L.; He, B.-Y.; He, S.; Wang, T.-J.; Su, C.-L.; Jin, Y. Fe―Ti oxide nano-adsorbent synthesized by co-precipitation for fluoride removal from drinking water and its adsorption mechanism. Powder Technol. 2012, 227, 3–8. [Google Scholar] [CrossRef]
- Eslami, H.; Ehrampoush, M.H.; Esmaeili, A.; Ebrahimi, A.A.; Ghaneian, M.T.; Falahzadeh, H.; Salmani, M.H. Synthesis of mesoporous Fe-Mn bimetal oxide nanocomposite by aeration co-precipitation method: Physicochemical, structural, and optical properties. Mater. Chem. Phys. 2019, 224, 65–72. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Lerner, M.I.; Pervikov, A.V.; Kazantsev, S.O.; Fomenko, A.N. Development of Fe/Cu and Fe/Ag Bimetallic Nanoparticles for Promising Biodegradable Materials with Antimicrobial Effect. Nanotechnol Russ. 2018, 13, 18–25. [Google Scholar] [CrossRef]
- Younas, U.; Hassan, S.T.; Ali, F.; Hassan, F.; Saeed, Z.; Pervaiz, M.; Khan, S.; Jannat, F.T.; Bibi, S.; Sadiqa, A.; et al. Radical Scavenging and Catalytic Activity of Fe-Cu Bimetallic Nanoparticles Synthesized from Ixora finlaysoniana Extract. Coatings 2021, 11, 813. [Google Scholar] [CrossRef]
- Sofia Anuar, N.; Jeffrey Basirun, W.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef] [PubMed]
- Sravani, B.; Raghavendra, P.; Chandrasekhar, Y.; Veera Manohara Reddy, Y.; Sivasubramanian, R.; Venkateswarlu, K.; Madhavi, G.; Subramanyam Sarma, L. Immobilization of platinum-cobalt and platinum-nickel bimetallic nanoparticles on pomegranate peel extract-treated reduced graphene oxide as electrocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 45, 7680–7690. [Google Scholar] [CrossRef]
- Rupa Kasturi, P.; Harivignesh, R.; Lee, Y.S.; Kalai Selvan, R. Polyol assisted formaldehyde reduction of bi-metallic Pt-Pd supported agro-waste derived carbon spheres as an efficient electrocatalyst for formic acid and ethylene glycol oxidation. J. Colloid Interface Sci. 2020, 561, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Velidandi, A.; Sarvepalli, M.; Pabbathi, N.P.P.; Baadhe, R.R. Biogenic synthesis of novel platinum-palladium bimetallic nanoparticles from aqueous Annona muricata leaf extract for catalytic activity. 3 Biotech 2021, 11, 385. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Li, W.; Peng, J.; Zhang, F.; Xu, W.; Huang, Z. Enhancement of anodic oxidation of formic acid on Pd–Fe bimetallic nanoparticles by thermal treatment. Int. J. Hydrogen Energy 2021, 46, 10239–10246. [Google Scholar] [CrossRef]
- Asanova, T.I.; Asanov, I.P.; Kim, M.-G.; Gerasimov, E.Y.; Zadesenets, A.V.; Plyusnin, P.E.; Korenev, S.V. On formation mechanism of Pd–Ir bimetallic nanoparticles through thermal decomposition of [Pd(NH3)4][IrCl6]. J. Nanoparticle Res. 2013, 15, 1994. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, F.; Cheng, Y.; Yuan, Z.; Yang, F.; Liu, C.; Cao, Y.; Zhang, K.; Lu, H.; Zada, S.; et al. Pd@Au Bimetallic Nanoplates Decorated Mesoporous MnO2 for Synergistic Nucleus-Targeted NIR-II Photothermal and Hypoxia-Relieved Photodynamic Therapy. Adv. Healthc. Mater. 2020, 9, 1901528. [Google Scholar] [CrossRef]
- Gulbagça, F.; Aygun, A.; Altuner, E.E.; Bekmezci, M.; Gur, T.; Sen, F.; Karimi-Maleh, H.; Zare, N.; Karimi, F.; Vasseghian, Y. Facile bio-fabrication of Pd-Ag bimetallic nanoparticles and its performance in catalytic and pharmaceutical applications: Hydrogen production and in-vitro antibacterial, anticancer activities, and model development. Chem. Eng. Res. Des. 2022, 180, 254–264. [Google Scholar] [CrossRef]
- Sultana, S.; Devi Mech, S.; Liaquat Hussain, F.; Pahari, P.; Borah, G.; Gogoi, P.K. Green synthesis of graphene oxide (GO)-anchored Pd/Cu bimetallic nanoparticles using Ocimum sanctum as bio-reductant: An efficient heterogeneous catalyst for the Sonogashira cross-coupling reaction. RSC Adv. 2020, 10, 23108–23120. [Google Scholar] [CrossRef]
- Doğan Özcan, M.; Akay, R.G.; Çelik, C.; Akın, A.N. Preparation and characterization of bimetallic Pd–Zn nanoparticles on carbon for borohydride electrooxidation. React. Kinet. Mech. Catal. 2021, 134, 163–177. [Google Scholar] [CrossRef]
- Elsayed, K.A.; Alomari, M.; Drmosh, Q.A.; Alheshibri, M.; Al Baroot, A.; Kayed, T.S.; Manda, A.A.; Al-Alotaibi, A.L. Fabrication of ZnO-Ag bimetallic nanoparticles by laser ablation for anticancer activity. Alex. Eng. J. 2022, 61, 1449–1457. [Google Scholar] [CrossRef]
- Semaltianos, N.G. Nanoparticles by Laser Ablation. Crit. Rev. Solid State Mater. Sci. 2010, 35, 105–124. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vazquez, M.; Brabazon, D. A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid. Crystals 2023, 13, 253. [Google Scholar] [CrossRef]
- Murugadoss, A.; Kai, N.; Sakurai, H. Synthesis of bimetallic gold–silver alloy nanoclusters by simple mortar grinding. Nanoscale 2012, 4, 1280. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Ray, S.S.; Onyango, M.S.; Momba, M.N.B. Microwave-assisted synthesis, characterization and antibacterial activity of Ag/ZnO nanoparticles supported bentonite clay. J. Hazard. Mater. 2013, 262, 439–446. [Google Scholar] [CrossRef]
- Belousov, O.V.; Belousova, N.V.; Sirotina, A.V.; Solovyov, L.A.; Zhyzhaev, A.M.; Zharkov, S.M.; Mikhlin, Y.L. Formation of Bimetallic Au–Pd and Au–Pt Nanoparticles under Hydrothermal Conditions and Microwave Irradiation. Langmuir 2011, 27, 11697–11703. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Wu, S.; Fan, D. Mechanochemically synthesized Al–Fe (oxide) composite with superior reductive performance: Solid-state kinetic processes during ball milling. Chemosphere 2022, 298, 134280. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Deng, S.; Xu, J.; Zhang, W.; Wu, M.; Wang, B.; Huang, J.; Yu, G. Highly Active and Stable Ni–Fe Bimetal Prepared by Ball Milling for Catalytic Hydrodechlorination of 4-Chlorophenol. Environ. Sci. Technol. 2012, 46, 4576–4582. [Google Scholar] [CrossRef]
- Singaravelan, R.; Bangaru Sudarsan Alwar, S. Electrochemical synthesis, characterisation and phytogenic properties of silver nanoparticles. Appl. Nanosci. 2015, 5, 983–991. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, X.; Chen, Y.; Wang, Q.; Yao, Z.; Wang, L. Electrochemical synthesis of Co/Ni bimetal-organic frameworks: A high-performance SERS platform for detection of tetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121843. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Y.; Li, S.; Huang, Q.; Peng, J. Self-Supporting Bi–Sb Bimetallic Nanoleaf for Electrochemical Synthesis of Formate by Highly Selective CO2 Reduction. ACS Appl. Mater. Interfaces 2023, 15, 6942–6950. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, Z.; Lin, X. An ultrafast electrochemical synthesis of Au@ Ag core-shell nanoflowers as a SERS substrate for thiram detection in milk and juice. Food Chem. 2023, 402, 134433. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef] [Green Version]
- Mi, J.-L.; Nørby, P.; Bremholm, M.; Becker, J.; Iversen, B.B. The formation mechanism of bimetallic PtRu alloy nanoparticles in solvothermal synthesis. Nanoscale 2015, 7, 16170–16174. [Google Scholar] [CrossRef]
- Thirugnanasambandan, T. Polymers-Metal Nanocomposites. In Environmental Nanotechnology: Volume 2; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2019; pp. 213–254. ISBN 978-3-319-98708-8. [Google Scholar]
- He, Y.; Lin, H.; Dong, Y.; Li, B.; Wang, L.; Chu, S.; Luo, M.; Liu, J. Zeolite supported Fe/Ni bimetallic nanoparticles for simultaneous removal of nitrate and phosphate: Synergistic effect and mechanism. Chem. Eng. J. 2018, 347, 669–681. [Google Scholar] [CrossRef]
- Weng, X.; Cai, W.; Lan, R.; Sun, Q.; Chen, Z. Simultaneous removal of amoxicillin, ampicillin and penicillin by clay supported Fe/Ni bimetallic nanoparticles. Environ. Pollut. 2018, 236, 562–569. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Mirzajani, R.; Karimi, S. Ultrasonic assisted synthesis of magnetic Ni-Ag bimetallic nanoparticles supported on reduced graphene oxide for sonochemical simultaneous removal of sunset yellow and tartrazine dyes by response surface optimization: Application of derivative spectrophotometry. Ultrason. Sonochemistry 2019, 50, 239–250. [Google Scholar] [CrossRef]
- Kan, C.; Cai, W.; Li, C.; Zhang, L.; Hofmeister, H. Ultrasonic synthesis and optical properties of Au/Pd bimetallic nanoparticles in ethylene glycol. J. Phys. D Appl. Phys. 2003, 36, 1609–1614. [Google Scholar] [CrossRef]
- Redjala, T.; Remita, H.; Apostolescu, G.; Mostafavi, M.; Thomazeau, C.; Uzio, D. Bimetallic Au-Pd and Ag-Pd Clusters Synthesised by or Electron Beam Radiolysis and Study of the Reactivity/Structure Relationships in the Selective Hydrogenation of Buta-1,3-Diene. Oil Gas Sci. Technol. Rev. IFP 2006, 61, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Kianfar, S.; Golikand, A.N.; ZareNezhad, B. Bimetallic-metal oxide nanoparticles of Pt-M (M: W, Mo, and V) supported on reduced graphene oxide (rGO): Radiolytic synthesis and methanol oxidation electrocatalysis. J. Nanostructure Chem. 2021, 11, 287–299. [Google Scholar] [CrossRef]
- Treguer, M.; de Cointet, C.; Remita, H.; Khatouri, J.; Mostafavi, M.; Amblard, J.; Belloni, J.; de Keyzer, R. Dose Rate Effects on Radiolytic Synthesis of Gold−Silver Bimetallic Clusters in Solution. J. Phys. Chem. B 1998, 102, 4310–4321. [Google Scholar] [CrossRef]
- Doudna, C.M.; Bertino, M.F.; Blum, F.D.; Tokuhiro, A.T.; Lahiri-Dey, D.; Chattopadhyay, S.; Terry, J. Radiolytic Synthesis of Bimetallic Ag−Pt Nanoparticles with a High Aspect Ratio. J. Phys. Chem. B 2003, 107, 2966–2970. [Google Scholar] [CrossRef]
- Hai, Z.; Kolli, N.E.; Chen, J.; Remita, H. Radiolytic synthesis of Au–Cu bimetallic nanoparticles supported on TiO2: Application in photocatalysis. New J. Chem. 2014, 38, 5279–5286. [Google Scholar] [CrossRef]
- Oh, S.-D.; Kim, M.-R.; Choi, S.-H.; Chun, J.-H.; Lee, K.-P.; Gopalan, A.; Hwang, C.-G.; Sang-Ho, K.; Hoon, O.J. Radiolytic synthesis of Pd–M (M=Ag, Au, Cu, Ni and Pt) alloy nanoparticles and their use in reduction of 4-nitrophenol. J. Ind. Eng. Chem. 2008, 14, 687–692. [Google Scholar] [CrossRef]
- Yamamoto, T.A.; Nakagawa, T.; Seino, S.; Nitani, H. Bimetallic nanoparticles of PtM (M=Au, Cu, Ni) supported on iron oxide: Radiolytic synthesis and CO oxidation catalysis. Appl. Catal. A Gen. 2010, 387, 195–202. [Google Scholar] [CrossRef]
- Henglein, A. Colloidal Palladium Nanoparticles: Reduction of Pd(II) by H2; PdCoreAuShellAgShell Particles. J. Phys. Chem. B 2000, 104, 6683–6685. [Google Scholar] [CrossRef]
- Sim, K.-S.; Jeon, J.-U.; Kwen, H.-D.; Choi, S.-H. Radiolytic Preparation of MWNT-Supported Electrocatalysts with Monometallic (Pt), Bimetallic (Pt-Ru), Trimetallic (Pt-Ru-Sn and Pt-Ru-Mo), and Tetrametallic (Pt-Ru-Mo-Sn) Nanoparticles for Direct Methanol Fuel Cells. J. Nanoelectron. Optoelectron. 2011, 6, 277–282. [Google Scholar] [CrossRef]
- Lawal Usman, U.; Kumar Allam, B.; Bahadur Singh, N.; Banerjee, S. Adsorptive removal of Cr(VI) from wastewater by hexagonal boron nitride-magnetite nanocomposites: Kinetics, mechanism and LCA analysis. J. Mol. Liq. 2022, 354, 118833. [Google Scholar] [CrossRef]
- Gao, F.; Yang, C.; Tang, X.; Yi, H.; Wang, C. One-step synthesis by redox co-precipitation method for low-dimensional Me-Mn bi-metal oxides (Me=Co, Ni, Sn) as SCR DeNOx catalysts. Environ. Sci. Pollut. Res. 2022, 29, 21210–21220. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Tao, X.; Li, R.; Tian, D.; Liu, X. Gentle One-Step Co-Precipitation to Synthesize Bimetallic Cocu- Mof Immobilized Laccase for Boosting Enzyme Stability and Congo Red Removal; Social Science Research Network: Rochester, NY, USA, 2022. [Google Scholar]
- Guntlin, C.P.; Kravchyk, K.V.; Erni, R.; Kovalenko, M.V. Transition metal trifluoroacetates (M = Fe, Co, Mn) as precursors for uniform colloidal metal difluoride and phosphide nanoparticles. Sci. Rep. 2019, 9, 6613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, A.; Veser, G. Exceptional high-temperature stability through distillation-like self-stabilization in bimetallic nanoparticles. Nat. Mater. 2009, 9, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Absalan, Y.; Ryabov, M.; Kovalchukova, O. Thermal decomposition of bimetallic titanium complexes: A new method for synthesizing doped titanium nano-sized catalysts and photocatalytic application. Mater. Sci. Eng. C 2018, 97, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, R.; He, J.; Xu, P.; Li, H.; Tian, L.; Guo, C. Synthesis of bimetallic CoMn–alginate and synergistic effect on thermal decomposition of ammonium perchlorate. Mater. Res. Bull. 2019, 117, 1–8. [Google Scholar] [CrossRef]
- Ding, K.; Liu, L.; Cao, Y.; Yan, X.; Wei, H.; Guo, Z. Formic acid oxidation reaction on a PdxNiy bimetallic nanoparticle catalyst prepared by a thermal decomposition process using ionic liquids as the solvent. Int. J. Hydrogen Energy 2014, 39, 7326–7337. [Google Scholar] [CrossRef]
- Fornazier Filho, Y.; da Cruz, A.C.C.; Pedicini, R.; Salgado, J.R.C.; Rodrigues, R.V.; Luz, P.P.; Garcia-Segura, S.; Ribeiro, J. PdAg/C Electrocatalysts Synthesized by Thermal Decomposition of Polymeric Precursors Improve Catalytic Activity for Ethanol Oxidation Reaction. Catalysts 2022, 12, 96. [Google Scholar] [CrossRef]
- Rajput, N. Methods of Preparation of Nanoparticles—A Review. Int. J. Adv. Eng. Technol. 2015, 7, 1806. [Google Scholar]
- Jiang, F.; Feng, G.; Xu, C.; Qing, S.; Wu, Q.; Yu, Y.; Zhang, Q.; Jiang, W. Novel facile nonhydrolytic sol-gel synthesis of MgAl2O4 nanocrystal from bimetallic alkoxides. J. Sol-Gel Sci. Technol. 2021, 100, 555–561. [Google Scholar] [CrossRef]
- Gubanova, N.N.; Matveev, V.A.; Shilova, O.A. Bimetallic Pt/Pd nanoparticles in sol–gel-derived silica films and xerogels. J. Sol-Gel Sci. Technol. 2019, 92, 367–375. [Google Scholar] [CrossRef]
- Devarajan, S.; Bera, P.; Sampath, S. Bimetallic nanoparticles: A single step synthesis, stabilization, and characterization of Au–Ag, Au–Pd, and Au–Pt in sol–gel derived silicates. J. Colloid Interface Sci. 2005, 290, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Prakash Dwivedi, R.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Pal, A.; Shah, S.; Devi, S. Preparation of silver, gold and silver–gold bimetallic nanoparticles in w/o microemulsion containing TritonX-100. Colloids Surf. A: Physicochem. Eng. Asp. 2007, 302, 483–487. [Google Scholar] [CrossRef]
- Quinlan, F.T.; Kuther, J.; Tremel, W.; Knoll, W.; Risbud, S.; Stroeve, P. Reverse Micelle Synthesis and Characterization of ZnSe Nanoparticles. Langmuir 2000, 16, 4049–4051. [Google Scholar] [CrossRef]
- Wu, M.-L.; Chen, D.-H.; Huang, T.-C. Preparation of Au/Pt Bimetallic Nanoparticles in Water-in-Oil Microemulsions. Chem. Mater. 2001, 13, 599–606. [Google Scholar] [CrossRef]
- Chen, D.-H.; Chen, C.-J. Formation and characterization of Au–Ag bimetallic nanoparticles in water-in-oil microemulsions. J. Mater. Chem. 2002, 12, 1557–1562. [Google Scholar] [CrossRef]
- Szumełda, T.; Drelinkiewicz, A.; Kosydar, R.; Gurgul, J.; Duraczyńska, D. Synthesis of carbon-supported bimetallic palladium–iridium catalysts by microemulsion: Characterization and electrocatalytic properties. J. Mater. Sci. 2021, 56, 392–414. [Google Scholar] [CrossRef]
- Ahmadi Khoshooei, M.; Scott, C.E.; Carbognani, L.; Pereira-Almao, P. Ultrasound-assisted bimetallic NiMo nanocatalyst preparation using microemulsions for in-situ upgrading application: Impact on particle size. Catal. Today 2021, 365, 132–141. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramanujachary, K.V.; Lofland, S.E.; Furiato, A.; Gupta, G.; Shivaprasad, S.M.; Ganguli, A.K. Bimetallic Cu–Ni nanoparticles of varying composition (CuNi3, CuNi, Cu3Ni). Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 206–212. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, K.-Y. Water-in-Oil Microemulsion Synthesis of Platinum−Ruthenium Nanoparticles, Their Characterization and Electrocatalytic Properties. Chem. Mater. 2003, 15, 451–459. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, P.; Liu, M. Sunlight-driven plasmonic photocatalysts based on Ag/AgCl nanostructures synthesized via an oil-in-water medium: Enhanced catalytic performance by morphology selection. J. Mater. Chem. 2011, 21, 16413–16419. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, S.; Liu, L.; Hu, J.; Cui, W. Oil-in-water self-assembled Ag@AgCl QDs sensitized Bi2WO6: Enhanced photocatalytic degradation under visible light irradiation. Appl. Catal. B Environ. 2015, 164, 192–203. [Google Scholar] [CrossRef]
- Lin, S.; Liu, L.; Hu, J.; Liang, Y.; Cui, W. Nano Ag@AgBr surface-sensitized Bi2WO6 photocatalyst: Oil-in-water synthesis and enhanced photocatalytic degradation. Appl. Surf. Sci. 2015, 324, 20–29. [Google Scholar] [CrossRef]
- Pemartin, K.; Solans, C.; Alvarez-Quintana, J.; Sanchez-Dominguez, M. Synthesis of Mn–Zn ferrite nanoparticles by the oil-in-water microemulsion reaction method. Colloids Surf. A Physicochem. Eng. Asp. 2014, 451, 161–171. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, M.; Morales-Mendoza, G.; Rodriguez-Vargas, M.J.; Ibarra-Malo, C.C.; Rodriguez-Rodriguez, A.A.; Vela-Gonzalez, A.V.; Perez-Garcia, S.A.; Gomez, R. Synthesis of Zn-doped TiO2 nanoparticles by the novel oil-in-water (O/W) microemulsion method and their use for the photocatalytic degradation of phenol. J. Environ. Chem. Eng. 2015, 3, 3037–3047. [Google Scholar] [CrossRef]
- Pemartin-Biernath, K.; Vela-González, A.V.; Moreno-Trejo, M.B.; Leyva-Porras, C.; Castañeda-Reyna, I.E.; Juárez-Ramírez, I.; Solans, C.; Sánchez-Domínguez, M. Synthesis of Mixed Cu/Ce Oxide Nanoparticles by the Oil-in-Water Microemulsion Reaction Method. Materials 2016, 9, 480. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.-M.; Zhang, R.-H.; Chen, D.; Hu, Q.-Y.; Zhang, X.; Yang, C.-Y.; Zhou, X.-W. Hydrothermal synthesis of PdAu nanocatalysts with variable atom ratio for methanol oxidation. Electrochim. Acta 2018, 259, 284–292. [Google Scholar] [CrossRef]
- Kang, X.; Fu, G.; Song, Z.; Huo, G.; Si, F.; Deng, X.; Fu, X.-Z.; Luo, J.-L. Microwave-assisted hydrothermal synthesis of MOFs-derived bimetallic CuCo-N/C electrocatalyst for efficient oxygen reduction reaction. J. Alloys Compd. 2019, 795, 462–470. [Google Scholar] [CrossRef]
- Mohamed Saeed, G.H.; Radiman, S.; Gasaymeh, S.S.; Lim, H.N.; Huang, N.M. Mild Hydrothermal Synthesis of Ni–Cu Nanoparticles. J. Nanomater. 2010, 2010, e184137. [Google Scholar] [CrossRef] [Green Version]
- Seethapathy, V.; Sudarsan, P.; Pandey, A.K.; Pandiyan, A.; Kumar, T.H.V.; Sanjeevi, K.; Sundramoorthy, A.K.; Moorthy, S.B.K. Synergistic effect of bimetallic Cu:Ni nanoparticles for the efficient catalytic conversion of 4-nitrophenol. New J. Chem. 2019, 43, 3180–3187. [Google Scholar] [CrossRef]
- Wen, Z.; Ke, J.; Xu, J.; Guo, S.; Zhang, Y.; Chen, R. One-step facile hydrothermal synthesis of flowerlike Ce/Fe bimetallic oxides for efficient As(V) and Cr(VI) remediation: Performance and mechanism. Chem. Eng. J. 2018, 343, 416–426. [Google Scholar] [CrossRef]
- Wang, B.; Huang, X.; Zhu, Z.; Huang, H.; Dai, J. Hydrothermal synthesis of cobalt–nickel bimetallic phosphides. Appl. Nanosci. 2012, 2, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.-C.; Feng, J.-J.; Lin, X.-X.; Zhang, L.; Yuan, J.; Zhang, Q.-L.; Wang, A.-J. One-step hydrothermal synthesis of three-dimensional nitrogen-doped reduced graphene oxide hydrogels anchored PtPd alloyed nanoparticles for ethylene glycol oxidation and hydrogen evolution reactions. Electrochim. Acta 2019, 293, 504–513. [Google Scholar] [CrossRef]
- Jingyu, S.; Jianshu, H.; Yanxia, C.; Xiaogang, Z. Hydrothermal Synthesis of Pt-Ru/MWCNTs and its Electrocatalytic Properties for Oxidation of Methanol. Int. J. Electrochem. Sci. 2007, 2, 8. [Google Scholar]
- Zheng, J.; Sun, L.; Jiao, C.; Shao, Q.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. Hydrothermally synthesized Ti/Zr bimetallic MOFs derived N self-doped TiO2/ZrO2 composite catalysts with enhanced photocatalytic degradation of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126629. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, T.; Wang, X.; Cheng, Z.; Xu, J. Coal mine gases sensors with dual selectivity at variable temperatures based on a W18O49 ultra-fine nanowires/Pd@Au bimetallic nanoparticles composite. Sens. Actuators B Chem. 2022, 354, 131004. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Mao, X.; Fu, W.; Liu, C. Seed-mediated growth of bimetallic nanoparticles as an effective strategy for sensitive detection of vitamin C. Sens. Actuators B Chem. 2016, 231, 95–101. [Google Scholar] [CrossRef]
- Zhan, F.; Yin, J.; Zhou, J.; Jiao, T.; Zhang, L.; Xia, M.; Bai, Z.; Peng, Q. Facile Preparation and Highly Efficient Catalytic Performances of Pd-Cu Bimetallic Catalyst Synthesized via Seed-Mediated Method. Nanomaterials 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Leonardi, A.; Chen, J.; Cao, M.; Li, N.; Su, D.; Zhang, Q.; Engel, M.; Ye, X. Imaging the kinetics of anisotropic dissolution of bimetallic core–shell nanocubes using graphene liquid cells. Nat. Commun. 2020, 11, 3041. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Asakura, K.; Toshima, N. Catalytic activity and structural analysis of polymer-protected gold/palladium bimetallic clusters prepared by the successive reduction of hydrogen tetrachloroaurate(III) and palladium dichloride. J. Phys. Chem. 1993, 97, 5103–5114. [Google Scholar] [CrossRef]
- Ferrer, D.; Torres-Castro, A.; Gao, X.; Sepúlveda-Guzmán, S.; Ortiz-Méndez, U.; José-Yacamán, M. Three-Layer Core/Shell Structure in Au−Pd Bimetallic Nanoparticles. Nano Lett. 2007, 7, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Haldar, K.K.; Kundu, S.; Patra, A. Core-Size-Dependent Catalytic Properties of Bimetallic Au/Ag Core–Shell Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 21946–21953. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-H.; Kim, K.Y.; Nam, H.; Kim, H.; Yoon, J.; Lee, J.-H.; Kim, H.-K.; Ahn, J.-P.; Lee, S.Y.; Lee, K.-Y.; et al. Facile Direct Seed-Mediated Growth of AuPt Bimetallic Shell on the Surface of Pd Nanocubes and Application for Direct H2O2 Synthesis. Catalysts 2020, 10, 650. [Google Scholar] [CrossRef]
- Wang, L.; Yamauchi, Y. Autoprogrammed Synthesis of Triple-Layered Au@Pd@Pt Core−Shell Nanoparticles Consisting of a Au@Pd Bimetallic Core and Nanoporous Pt Shell. J. Am. Chem. Soc. 2010, 132, 13636–13638. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Chaudhary, A.; Srinivasan, S.; Nandi, C.K. Polymer Stabilized Bimetallic Alloy Nanoparticles: Synthesis and Catalytic Application. Colloid Interface Sci. Commun. 2018, 24, 62–67. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X. Synthesis of polymer-stabilized monometallic Cu and bimetallic Cu/Ag nanoparticles and their surface-enhanced Raman scattering properties. J. Mol. Struct. 2013, 1035, 471–475. [Google Scholar] [CrossRef]
- Mondal, A.; Arora, M.; Kumar Dubey, B.; Mumford, K. Comparative assessment of the characteristics and Cr(VI) removal activity of the bimetallic Fe/Cu nanoparticles pre- and post-coated with carboxymethyl cellulose. Chem. Eng. J. 2022, 444, 136343. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, D.; Xu, Y.; Zhong, N. Polyethylene glycol-stabilized bimetallic nickel–zero valent iron nanoparticles for efficient removal of Cr(VI). New J. Chem. 2021, 45, 13969–13978. [Google Scholar] [CrossRef]
- Al-shaikh, H.; Lasri, J.; Knight, J.G. Bimetallic Ru:Co Mesoporous Nanoparticles Stabilized by PEG and Imidazolium Ionic Liquid Based [KIT-6] as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura Cross-Couplings in H2O:EtOH Solution. Catal. Lett. 2022, 152, 3761–3771. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, M. Novel core–shell structured Paclitaxel-loaded PLGA@Ag–Au nanoparticles. Mater. Lett. 2013, 92, 350–353. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, D.; Lai, C.; Xu, P.; Zeng, G.; Wan, J.; Tang, L.; Dong, H.; Huang, B.; Hu, T. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total Environ. 2018, 644, 1181–1189. [Google Scholar] [CrossRef]
- Weng, X.; Lin, S.; Zhong, Y.; Chen, Z. Chitosan stabilized bimetallic Fe/Ni nanoparticles used to remove mixed contaminants-amoxicillin and Cd (II) from aqueous solutions. Chem. Eng. J. 2013, 229, 27–34. [Google Scholar] [CrossRef]
- Thi, P.P.N.; Nguyen, M.T.; Hai, N.D. Role of Collagen Concentration in Stability of Star-Shaped Silver@Gold Nanoparticles. J. Nano Res. 2016, 40, 113–119. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Li, L.; Wang, X.; Haller, G.L.; Yang, Y. Carbon nanotube-supported Pt-based bimetallic catalysts prepared by a microwave-assisted polyol reduction method and their catalytic applications in the selective hydrogenation. J. Catal. 2010, 276, 314–326. [Google Scholar] [CrossRef]
- Yang, X.; Chen, D.; Liao, S.; Song, H.; Li, Y.; Fu, Z.; Su, Y. High-performance Pd–Au bimetallic catalyst with mesoporous silica nanoparticles as support and its catalysis of cinnamaldehyde hydrogenation. J. Catal. 2012, 291, 36–43. [Google Scholar] [CrossRef]
- Li, C.; Xu, G.; Zhai, Y.; Liu, X.; Ma, Y.; Zhang, Y. Hydrogenation of biomass-derived ethyl levulinate into γ-valerolactone by activated carbon supported bimetallic Ni and Fe catalysts. Fuel 2017, 203, 23–31. [Google Scholar] [CrossRef]
- Buxaderas, E.; Graziano-Mayer, M.; Volpe, M.A.; Radivoy, G. Bimetallic Cu-Pd Nanoparticles Supported on Bio-silica as an Efficient Catalyst for Selective Aerobic Oxidation of Benzylic Alcohols. Synthesis 2017, 49, 1387–1393. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Kaur, G.; Singh, P.P.; Kaushal, S. Supported bimetallic nanoparticles as anode catalysts for direct methanol fuel cells: A review. Int. J. Hydrogen Energy 2021, 46, 15820–15849. [Google Scholar] [CrossRef]
- Yang, M.; Guo, Z.; Li, L.-N.; Huang, Y.-Y.; Liu, J.-H.; Zhou, Q.; Chen, X.; Huang, X.-J. Electrochemical determination of arsenic(III) with ultra-high anti-interference performance using Au–Cu bimetallic nanoparticles. Sens. Actuators B Chem. 2016, 231, 70–78. [Google Scholar] [CrossRef]
- Tuo, Y.; Liu, G.; Dong, B.; Yu, H.; Zhou, J.; Wang, J.; Jin, R. Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol. Environ. Sci. Pollut. Res. 2017, 24, 5249–5258. [Google Scholar] [CrossRef]
- Han, R.; Song, X.; Wang, Q.; Qi, Y.; Deng, G.; Zhang, A.; Wang, Q.; Chang, F.; Wu, C.; Cheng, Y. Microbial synthesis of graphene-supported highly-dispersed Pd-Ag bimetallic nanoparticles and its catalytic activity. J. Chem. Technol. Biotechnol. 2019, 94, 3375–3383. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Hong, Z.; Cai, K.; Zhao, F.; Han, H. Microbial synthesis of highly dispersed PdAu alloy for enhanced electrocatalysis. Sci. Adv. 2016, 2, e1600858. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Xiao, Y.; Xu, M.; Cui, H.; Tan, L.; Feng, N.; Liu, X.; Qiu, G.; Dong, H.; Xie, J. Microbial synthesis of Pd–Pt alloy nanoparticles using Shewanella oneidensis MR-1 with enhanced catalytic activity for nitrophenol and azo dyes reduction. Nanotechnology 2018, 30, 065607. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Xie, J.; Deng, Y.; Wu, L.; He, Z.; Van Nostrand, J.; Luo, Y.; Zhou, J. Interactive effects of clipping practice and experimental warming on soil microbial communities involved in nitrogen cycling in a tallgrass prairie. In Proceedings of the 97th ESA Annual Convention 2012, Portland, OR, USA, 5–10 August 2012. [Google Scholar]
- Deplanche, K.; Merroun, M.L.; Casadesus, M.; Tran, D.T.; Mikheenko, I.P.; Bennett, J.A.; Zhu, J.; Jones, I.P.; Attard, G.A.; Wood, J.; et al. Microbial synthesis of core/shell gold/palladium nanoparticles for applications in green chemistry. J. R. Soc. Interface 2012, 9, 1705–1712. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, X.; Xu, W.; Zhang, R.; Wu, G. Biosynthesis of Bimetallic Au–Ag Nanoparticles Using Escherichia coli and its Biomedical Applications. ACS Biomater. Sci. Eng. 2020, 6, 680–689. [Google Scholar] [CrossRef]
- Gomez-Bolivar, J.; Mikheenko, I.P.; Orozco, R.L.; Sharma, S.; Banerjee, D.; Walker, M.; Hand, R.A.; Merroun, M.L.; Macaskie, L.E. Synthesis of Pd/Ru Bimetallic Nanoparticles by Escherichia coli and Potential as a Catalyst for Upgrading 5-Hydroxymethyl Furfural Into Liquid Fuel Precursors. Front. Microbiol. 2019, 10, 1276. [Google Scholar] [CrossRef] [Green Version]
- Nair, B.; Pradeep, T. Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactobacillus Strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Ameen, F. Optimization of the Synthesis of Fungus-Mediated Bi-Metallic Ag-Cu Nanoparticles. Appl. Sci. 2022, 12, 1384. [Google Scholar] [CrossRef]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B: Biointerfaces 2011, 83, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular Biosynthesis of Bimetallic Au–Ag Alloy Nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Boroumand Moghaddam, A.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef]
- Zheng, D.; Hu, C.; Gan, T.; Dang, X.; Hu, S. Preparation and application of a novel vanillin sensor based on biosynthesis of Au–Ag alloy nanoparticles. Sens. Actuators B Chem. 2010, 148, 247–252. [Google Scholar] [CrossRef]
- Ramakritinan, C.M.; Kaarunya, E.; Shankar, S.; Kumaraguru, A.K. Antibacterial Effects of Ag, Au and Bimetallic (Ag-Au) Nanoparticles Synthesized from Red Algae. Solid State Phenom. 2013, 201, 211–230. [Google Scholar] [CrossRef]
- Thangavelu, R.M.; Ganapathy, R.; Ramasamy, P.; Krishnan, K. Fabrication of virus metal hybrid nanomaterials: An ideal reference for bio semiconductor. Arab. J. Chem. 2020, 13, 2750–2765. [Google Scholar] [CrossRef]
- Raju, D.; Mendapara, R.; Mehta, U.J. Protein mediated synthesis of Au–Ag bimetallic nanoparticles. Mater. Lett. 2014, 124, 271–274. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Jai, J.; Arham, N.A.; Hadi, A. A short review on the synthesis of bimetallic nanoparticles using plant extract. In Proceedings of the 2013 IEEE International Conference on Control System, Computing and Engineering, Penang, Malaysia, 29 November–1 December 2013; pp. 334–339. [Google Scholar]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Lateef, A.; Ojo, S.A.; Folarin, B.I.; Gueguim-Kana, E.B.; Beukes, L.S. Kolanut (Cola nitida) Mediated Synthesis of Silver–Gold Alloy Nanoparticles: Antifungal, Catalytic, Larvicidal and Thrombolytic Applications. J. Clust. Sci. 2016, 27, 1561–1577. [Google Scholar] [CrossRef]
- Providence, B.A.; Chinyere, A.A.; Ayi, A.A.; Charles, O.O.; Elijah, T.A.; Ayomide, H.L. Green synthesis of silver monometallic and copper-silver bimetallic nanoparticles using Kigelia africana fruit extract and evaluation of their antimicrobial activities. Int. J. Phys. Sci. 2018, 13, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Mohanlall, V.; Biyela, B. Biocatalytic and biological activities of Kigelia africana mediated silver monometallic and copper-silver bimetallic nanoparticles. Indian J. Biochem. Biophys. (IJBB) 2022, 59, 94–102. [Google Scholar]
- Jacob, J.; Mukherjee, T.; Kapoor, S. A simple approach for facile synthesis of Ag, anisotropic Au and bimetallic (Ag/Au) nanoparticles using cruciferous vegetable extracts. Mater. Sci. Eng. C 2012, 32, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Sreenivasulu, K.; Gangadhara, S.; Venkateswarlu, P. Bio inspired green synthesis of Ni/Fe3O4 magnetic nanoparticles using Moringa oleifera leaves extract: A magnetically recoverable catalyst for organic dye degradation in aqueous solution. J. Alloys Compd. 2017, 700, 252–258. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. One-step green synthesis of bimetallic Fe/Pd nanoparticles used to degrade Orange II. J. Hazard. Mater. 2016, 303, 145–153. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Mondal, S.; Roy, N.; Laskar, R.A.; Sk, I.; Basu, S.; Mandal, D.; Begum, N.A. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ.) leaves. Colloids Surf. B Biointerfaces 2011, 82, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sheny, D.S.; Mathew, J.; Philip, D. Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Membr. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Haddad, J.; Alzaabi, F.; Pal, P.; Rambabu, K.; Banat, F. Green synthesis of bimetallic copper–silver nanoparticles and their application in catalytic and antibacterial activities. Clean Technol. Environ. Policy 2020, 22, 269–277. [Google Scholar] [CrossRef]
- Mamatha, G.; Varada Rajulu, A.; Madhukar, K. In Situ Generation of Bimetallic Nanoparticles in Cotton Fabric Using Aloe Vera Leaf Extract, as a Reducing Agent. J. Nat. Fibers 2020, 17, 1121–1129. [Google Scholar] [CrossRef]
- Alti, D.; Veeramohan Rao, M.; Rao, D.N.; Maurya, R.; Kalangi, S.K. Gold–Silver Bimetallic Nanoparticles Reduced with Herbal Leaf Extracts Induce ROS-Mediated Death in Both Promastigote and Amastigote Stages of Leishmania donovani. ACS Omega 2020, 5, 16238–16245. [Google Scholar] [CrossRef]
- Sasireka, K.S.; Lalitha, P. Biogenic synthesis of bimetallic nanoparticles and their applications. Rev. Inorg. Chem. 2021, 41, 223–244. [Google Scholar] [CrossRef]
- Wicaksono, W.P.; Kadja, G.T.M.; Amalia, D.; Uyun, L.; Rini, W.P.; Hidayat, A.; Fahmi, R.L.; Nasriyanti, D.; Leun, S.G.V.; Ariyanta, H.A.; et al. A green synthesis of gold–palladium core–shell nanoparticles using orange peel extract through two-step reduction method and its formaldehyde colorimetric sensing performance. Nano-Struct. Nano-Objects 2020, 24, 100535. [Google Scholar] [CrossRef]
- Ravikumar, K.V.G.; Sudakaran, S.V.; Ravichandran, K.; Pulimi, M.; Natarajan, C.; Mukherjee, A. Green synthesis of NiFe nano particles using Punica granatum peel extract for tetracycline removal. J. Clean. Prod. 2019, 210, 767–776. [Google Scholar] [CrossRef]
- Kang, C.-W.; Kolya, H. Green Synthesis of Ag-Au Bimetallic Nanocomposites Using Waste Tea Leaves Extract for Degradation Congo Red and 4-Nitrophenol. Sustainability 2021, 13, 3318. [Google Scholar] [CrossRef]
- Adebayo, A.E.; Oke, A.M.; Lateef, A.; Oyatokun, A.A.; Abisoye, O.D.; Adiji, I.P.; Fagbenro, D.O.; Amusan, T.V.; Badmus, J.A.; Asafa, T.B.; et al. Biosynthesis of silver, gold and silver–gold alloy nanoparticles using Persea americana fruit peel aqueous extract for their biomedical properties. Nanotechnol. Environ. Eng. 2019, 4, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Cui, X.; Liu, W.; Zou, B.; Liao, W. Cobalt/calcium bimetallic oxides based on bio-waste eggshells for the efficient degradation of norfloxacin by peroxymonosulfate activation. J. Colloid Interface Sci. 2022, 621, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Prashanna Suvaitha, S.; Sridhar, P.; Divya, T.; Palani, P.; Venkatachalam, K. Bio-waste eggshell membrane assisted synthesis of NiO/ZnO nanocomposite and its characterization: Evaluation of antibacterial and antifungal activity. Inorg. Chim. Acta 2022, 536, 120892. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, A.K.; Sharma, S.; Khan, I.; Sharma, D.K.; Shamsi, A.; Santhosh Kumar, T.R.; Seervi, M. Biosynthesized composites of Au-Ag nanoparticles using Trapa peel extract induced ROS-mediated p53 independent apoptosis in cancer cells. Drug Chem. Toxicol. 2019, 42, 43–53. [Google Scholar] [CrossRef]
- Sharma, M.; Yadav, S.; Ganesh, N.; Srivastava, M.M.; Srivastava, S. Biofabrication and characterization of flavonoid-loaded Ag, Au, Au–Ag bimetallic nanoparticles using seed extract of the plant Madhuca longifolia for the enhancement in wound healing bio-efficacy. Prog. Biomater. 2019, 8, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhruval, S.R.; Pai, N.; Dhanwant, S.S.; Hussein, B.; Nayak, S.; Rao, C.V.; Kumar, A.; Janakaraj, M. Rapid synthesis of antimicrobial Fe/Cu alloy nanoparticles using Waste Silkworm Cocoon extract for cement mortar applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 025006. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Garcıa, R.; Perez, R. Dynamic atomic force microscopy methods. Surf. Sci. Rep. 2002, 47, 197–301. [Google Scholar] [CrossRef]
- Falke, S.; Betzel, C. Dynamic Light Scattering (DLS). In Radiation in Bioanalysis: Spectroscopic Techniques and Theoretical Methods; Pereira, A.S., Tavares, P., Limão-Vieira, P., Eds.; Bioanalysis; Springer International Publishing: Cham, Switzerland, 2019; pp. 173–193. ISBN 978-3-030-28247-9. [Google Scholar]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanoparticle Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Inkson, B.J. 2—Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Hübschen, G., Altpeter, I., Tschuncky, R., Herrmann, H.-G., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 17–43. ISBN 978-0-08-100040-3. [Google Scholar]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists; Jones & Bartlett Learning: Burlington, MA, USA, 1999; ISBN 978-0-7637-0192-5. [Google Scholar]
- Trache, A.; Meininger, G.A. Atomic force microscopy (AFM). Curr. Protoc. Microbiol. 2008, 8, 2C2. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Chapter 6—Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications. In Interface Science and Technology; Nasrollahzadeh, M., Sajadi, S.M., Sajjadi, M., Issaabadi, Z., Atarod, M., Eds.; An Introduction to Green Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 199–322. [Google Scholar]
- Kohli, R.; Mittal, K.L. (Eds.) Chapter 3—Methods for Assessing Surface Cleanliness. In Developments in Surface Contamination and Cleaning, Volume 12; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–105. ISBN 978-0-12-816081-7. [Google Scholar]
- Sima, F.; Ristoscu, C.; Duta, L.; Gallet, O.; Anselme, K.; Mihailescu, I.N. 3—Laser thin films deposition and characterization for biomedical applications. In Laser Surface Modification of Biomaterials; Vilar, R., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 77–125. ISBN 978-0-08-100883-6. [Google Scholar]
- Patil, S.P.; Burungale, V.V. Physical and chemical properties of nanomaterials. In Nanomedicines for Breast Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–31. ISBN 978-0-12-820016-2. [Google Scholar]
- Kumar, A.; Dixit, C.K. 3—Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 43–58. ISBN 978-0-08-100557-6. [Google Scholar]
- Jayakrishnan, P.; Ramesan, M.T. Synthesis, Characterization, Electrical Conductivity and Material Properties of Magnetite/Polyindole/Poly(vinyl alcohol) Blend Nanocomposites. J. Inorg. Organomet. Polym. Mater. 2017, 27, 323–333. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Zhong, X.; Yan, K.; Li, Y.; Xu, Z. Li and Na storage behaviours of MgFe2O4 nanoparticles as anode materials for lithium ion and sodium ion batteries. Int. J. Electrochem. Sci. 2019, 14, 1725–1732. [Google Scholar] [CrossRef]
- Thabet Mohamed, A.; Salem, N. Optimizing Dielectric Characteristics of Electrical Materials Using Multi-Nanoparticles Technique. In Proceedings of the 2017 Nineteenth International Middle East Power Systems Conference (MEPCON), Cairo, Egypt, 19–21 December 2017. [Google Scholar]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Characterization and optical studies of PVP-capped silver nanoparticles. J. Nanostructure Chem. 2017, 7, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Tsoulos, T.V.; Han, L.; Weir, J.; Xin, H.L.; Fabris, L. A closer look at the physical and optical properties of gold nanostars: An experimental and computational study. Nanoscale 2017, 9, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Anancharoenwong, E.; Chueangchayaphan, W.; Rakkapao, N.; Marthosa, S.; Chaisrikhwun, B. Thermo-mechanical and antimicrobial properties of natural rubber-based polyurethane nanocomposites for biomedical applications. Polym. Bull. 2021, 78, 833–848. [Google Scholar] [CrossRef]
- Burgaz, E. Thermomechanical Analysis of Polymer Nanocomposites. In Polymer Nanocomposites: Electrical and Thermal Properties; Huang, X., Zhi, C., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 191–242. ISBN 978-3-319-28238-1. [Google Scholar]
- Cuenot, S.; Frétigny, C.; Demoustier-Champagne, S.; Nysten, B. Surface tension effect on the mechanical properties of nanomaterials measured by atomic force microscopy. Phys. Rev. B 2004, 69, 165410. [Google Scholar] [CrossRef] [Green Version]
- Peymanfar, R.; Khodamoradipoor, N. Preparation and Characterization of Copper Chromium Oxide Nanoparticles Using Modified Sol-Gel Route and Evaluation of Their Microwave Absorption Properties. Phys. Status Solidi (A) 2019, 216, 1900057. [Google Scholar] [CrossRef]
- Škrátek, M.; Dvurečenskij, A.; Kluknavský, M.; Barta, A.; Bališ, P.; Mičurová, A.; Cigáň, A.; Eckstein-Andicsová, A.; Maňka, J.; Bernátová, I. Sensitive SQUID Bio-Magnetometry for Determination and Differentiation of Biogenic Iron and Iron Oxide Nanoparticles in the Biological Samples. Nanomaterials 2020, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Zhang, Y.; Voit, W.; Rao, K.V.; Muhammed, M. Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles. J. Magn. Magn. Mater. 2001, 225, 30–36. [Google Scholar] [CrossRef]
- Kunc, F.; Balhara, V.; Sun, Y.; Daroszewska, M.; Jakubek, Z.J.; Hill, M.; Brinkmann, A.; Johnston, L.J. Quantification of surface functional groups on silica nanoparticles: Comparison of thermogravimetric analysis and quantitative NMR. Analyst 2019, 144, 5589–5599. [Google Scholar] [CrossRef]
- Seifi, H.; Gholami, T.; Seifi, S.; Ghoreishi, S.M.; Salavati-Niasari, M. A review on current trends in thermal analysis and hyphenated techniques in the investigation of physical, mechanical and chemical properties of nanomaterials. J. Anal. Appl. Pyrolysis 2020, 149, 104840. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W. Comparison of Chemical and Biological Properties of Metal Nanoparticles (Au, Ag), with Metal Oxide Nanoparticles (ZnO-NPs) and their Applications. Adv. J. Chem. Sect. A 2020, 3, 192–210. [Google Scholar] [CrossRef]
- Pereira, T.M.; Polez, V.L.P.; Sousa, M.H.; Silva, L.P. Modulating physical, chemical, and biological properties of silver nanoparticles obtained by green synthesis using different parts of the tree Handroanthus heptaphyllus (Vell.) Mattos. Colloid Interface Sci. Commun. 2020, 34, 100224. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Litti, L.; Meneghetti, M.; Zuccolotto, G.; Rosato, A.; Nicolato, E.; Marzola, P.; Fracasso, G.; Anselmi, C.; et al. Magneto-Plasmonic Au-Fe Alloy Nanoparticles Designed for Multimodal SERS-MRI-CT Imaging. Small 2014, 10, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, P.; Lin, L.; Gao, X.; Zhou, Y.; Feng, J.; Zhang, H. Ultra-small bimetallic phosphides for dual-modal MRI imaging guided photothermal ablation of tumors. Dalton Trans. 2022, 51, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.; Park, O.K.; Kim, J.; Han, S.I.; Yoo, T.Y.; Lee, N.; Kim, Y.G.; Kim, H.; Lim, C.; Bae, J.-S.; et al. Enhanced Chemodynamic Therapy by Cu–Fe Peroxide Nanoparticles: Tumor Microenvironment-Mediated Synergistic Fenton Reaction. ACS Nano 2022, 16, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, D.A.; Tang, N.; Pakeltis, G.; Emery, R.; Ivanov, I.N.; Gilbert, D.A.; Rack, P.D. Magnetic and Optical Properties of Au–Co Solid Solution and Phase-Separated Thin Films and Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 15047–15058. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Velu, P.; Liu, X.; Vijayalakshmi, A. Enhanced green mediated synthesis of optimized Ag-Cu bimetallic nanoparticles using Leucas aspera and its application in Anti-cancer activity against alveolar cancer. Mater. Lett. 2022, 313, 131645. [Google Scholar] [CrossRef]
- Koyyati, R.; Kudle, K.R.; Nagati, V.; Merugu, R.; Padigya, P.R.M. Extracellular Synthesis of Mono and Bimetallic Nanocomposites from Novel Strains of Rhodopseudomonas palustris and Evaluation of Their Biomedical Applications. Macromol. Symp. 2022, 402, 2100378. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Iku, S.I.I.; Ntwasa, M.; Nkambule, T.T.I.; Mamba, B.B.; Msagati, T.A.M. Doxorubicin conjugated hydrophilic AuPt bimetallic nanoparticles fabricated from Phragmites australis: Characterization and cytotoxic activity against human cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101749. [Google Scholar] [CrossRef]
- He, F.; Ji, H.; Feng, L.; Wang, Z.; Sun, Q.; Zhong, C.; Yang, D.; Gai, S.; Yang, P.; Lin, J. Construction of thiol-capped ultrasmall Au–Bi bimetallic nanoparticles for X-ray CT imaging and enhanced antitumor therapy efficiency. Biomaterials 2021, 264, 120453. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.G.; Nand, D.; Sivadas, S.; Alfarhan, A.; Muthusamy, K. CuO/NiO bimetallic nanocomposite: A facile DNA assisted synthetic approach and evaluation of bio efficacy. J. King Saud Univ. Sci. 2022, 34, 101718. [Google Scholar] [CrossRef]
- Ghosh, S.; Rana, D.; Sarkar, P.; Roy, S.; Kumar, A.; Naskar, J.; Kole, R.K. Ecological safety with multifunctional applications of biogenic mono and bimetallic (Au–Ag) alloy nanoparticles. Chemosphere 2022, 288, 132585. [Google Scholar] [CrossRef]
- Zhou, F.; Zhu, Y.; Yang, L.; Yang, D.-Q.; Sacher, E. Ag NP catalysis of Cu ions in the preparation of AgCu NPs and the mechanism of their enhanced antibacterial efficacy. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 632, 127831. [Google Scholar] [CrossRef]
- Formaggio, D.M.D.; de Oliveira Neto, X.A.; Rodrigues, L.D.A.; de Andrade, V.M.; Nunes, B.C.; Lopes-Ferreira, M.; Ferreira, F.G.; Wachesk, C.C.; Camargo, E.R.; Conceição, K.; et al. In vivo toxicity and antimicrobial activity of AuPt bimetallic nanoparticles. J. Nanoparticle Res. 2019, 21, 244. [Google Scholar] [CrossRef]
- Singh, A.K. Flower extract-mediated green synthesis of bimetallic CuZn oxide nanoparticles and its antimicrobial efficacy in hydrocolloid films. Bioresour. Technol. Rep. 2022, 18, 101034. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, H.; He, R.; Wang, N.; Zhao, Q.; Yang, L.; Qu, Z.; Sun, L.; Chen, S.; Ma, J.; et al. Hydro electroactive Cu/Zn coated cotton fiber nonwovens for antibacterial and antiviral applications. Int. J. Biol. Macromol. 2022, 207, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M.; Kotb, H.M.; Mushtaq, S.; Waheed-Ur-Rehman, M.; Maghanga, C.M.; Alam, M.W. Green Synthesis of Mn + Cu Bimetallic Nanoparticles Using Vinca rosea Extract and Their Antioxidant, Antibacterial, and Catalytic Activities. Crystals 2022, 12, 72. [Google Scholar] [CrossRef]
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Kitture, R.; Kale, S.; Bellare, J.; Chopade, B.A. Novel platinum–palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activities. Int. J. Nanomed. 2015, 10, 7477–7490. [Google Scholar] [CrossRef] [Green Version]
- Bhanja, S.K.; Samanta, S.K.; Mondal, B.; Jana, S.; Ray, J.; Pandey, A.; Tripathy, T. Green synthesis of Ag@Au bimetallic composite nanoparticles using a polysaccharide extracted from Ramaria botrytis mushroom and performance in catalytic reduction of 4-nitrophenol and antioxidant, antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100341. [Google Scholar] [CrossRef]
- Malapermal, V.; Mbatha, J.N.; Gengan, R.M.; Anand, K. Biosynthesis of bimetallic Au-Ag nanoparticles using Ocimum basilicum (L.) with antidiabetic and antimicrobial properties. Adv. Mater. Lett. 2015, 6, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Sher, N.; Ahmed, M.; Mushtaq, N. Enhancing Antioxidant, Antidiabetic, and Antialzheimer Performance of Hippeastrum Hybridum (L.) Using Silver, Gold, and Silver/Gold Bimetallic Nanoparticles; Social Science Research Network: Rochester, NY, USA, 2022. [Google Scholar]
- D’Souza, J.N.; Nagaraja, G.K.; Prabhu, A.; Navada, K.M.; Kouser, S.; Manasa, D.J. AgVI and Ag/ZnOVI nanostructures from Vateria indica (L.) exert antioxidant, antidiabetic, anti-inflammatory and cytotoxic efficacy on triple negative breast cancer cells in vitro. Int. J. Pharm. 2022, 615, 121450. [Google Scholar] [CrossRef] [PubMed]
- Bakur, A.; Elshaarani, T.; Niu, Y.; Chen, Q. Comparative study of antidiabetic, bactericidal, and antitumor activities of MEL@AgNPs, MEL@ZnONPs, and Ag–ZnO/MEL/GA nanocomposites prepared by using MEL and gum arabic. RSC Adv. 2019, 9, 9745–9754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imraish, A.; Abu Thiab, T.; Al-Awaida, W.; Al-Ameer, H.J.; Bustanji, Y.; Hammad, H.; Alsharif, M.; Al-Hunaiti, A. In vitro anti-inflammatory and antioxidant activities of ZnFe2O4 and CrFe2O4 nanoparticles synthesized using Boswellia carteri resin. J. Food Biochem. 2021, 45, e13730. [Google Scholar] [CrossRef] [PubMed]
- Antonoglou, O.; Lafazanis, K.; Mourdikoudis, S.; Vourlias, G.; Lialiaris, T.; Pantazaki, A.; Dendrinou-Samara, C. Biological relevance of CuFeO2 nanoparticles: Antibacterial and anti-inflammatory activity, genotoxicity, DNA and protein interactions. Mater. Sci. Eng. C 2019, 99, 264–274. [Google Scholar] [CrossRef]
- Orlowski, P.; Zmigrodzka, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Pajak, B.; Slonska, A.; Cymerys, J.; Celichowski, G.; Grobelny, J.; Krzyzowska, M. Polyphenol-Conjugated Bimetallic Au@AgNPs for Improved Wound Healing. Int. J. Nanomed. 2020, 15, 4969–4990. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Nkambule, T.T.I.; Mamba, B.B.; Msagati, T.A.M. The stimuli-responsive properties of doxorubicin adsorbed onto bimetallic Au@Pd nanodendrites and its potential application as drug delivery platform. Mater. Sci. Eng. C 2020, 110, 110696. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, A.P.; Theodosiou, M.; Sakellis, E.; Boukos, N.; Papanastasiou, G.; Wang, C.; Tavares, A.; Corral, C.A.; Gournis, D.; Chalmpes, N.; et al. Bimetallic gold-platinum nanoparticles as a drug delivery system coated with a new drug to target glioblastoma. Colloids Surf. B: Biointerfaces 2022, 214, 112463. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Bao, Z.; Sun, M.; Wang, X.; Zhang, H.; Chen, W.; Sui, Y.; Li, J.; Zhuang, Y.; Wang, D. NIR Stimulus-Responsive PdPt Bimetallic Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy. Pharmaceutics 2020, 12, 675. [Google Scholar] [CrossRef]
- De França Bettencourt, G.M.; Degenhardt, J.; Zevallos Torres, L.A.; de Andrade Tanobe, V.O.; Soccol, C.R. Green biosynthesis of single and bimetallic nanoparticles of iron and manganese using bacterial auxin complex to act as plant bio-fertilizer. Biocatal. Agric. Biotechnol. 2020, 30, 101822. [Google Scholar] [CrossRef]
- Tului, V.; Janmohammadi, M.; Abbasi, A.; Vahdati-Khajeh, S.; Nouraein, M. Influence of iron, zinc and bimetallic Zn-Fe nanoparticles on growth and biochemical characteristics in chickpea (Cicer arietinum) cultivars. Poljopr. I Sumar. 2021, 67, 179–193. [Google Scholar]
- Kumar, R.; Ashfaq, M.; Verma, N. Synthesis of novel PVA–starch formulation-supported Cu–Zn nanoparticle carrying carbon nanofibers as a nanofertilizer: Controlled release of micronutrients. J. Mater. Sci. 2018, 53, 7150–7164. [Google Scholar] [CrossRef]
- Mendez-Trujillo, V.; Valdez-Salas, B.; Carrillo-Beltran, M.; Curiel-Alvarez, M.A.; Tzintzun-Camacho, O.; Ceceña-Duran, C.; Gonzalez-Mendoza, D. Green synthesis of bimetallic nanoparticles from Prosopis juliflora (Sw) DC., and its effect against cotton mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Phyton 2019, 88, 269. [Google Scholar] [CrossRef]
- Kumaravel, J.; Lalitha, K.; Arunthirumeni, M.; Shivakumar, M.S. Mycosynthesis of bimetallic zinc oxide and titanium dioxide nanoparticles for control of Spodoptera frugiperda. Pestic. Biochem. Physiol. 2021, 178, 104910. [Google Scholar] [CrossRef] [PubMed]
- Lamayi, D.W.; Abba, E.; Shehu, Z.; Adam, M.M. Nanoinsecticidal efficacy of Ag/Ni bimetallic nanoparticles (BMNPs) on lymphatic filariasis vector. Asian J. Res. Infect. Dis. 2020, 5, 14–21. [Google Scholar] [CrossRef]
- Minal, S.P.; Prakash, S. Laboratory analysis of Au–Pd bimetallic nanoparticles synthesized with Citrus limon leaf extract and its efficacy on mosquito larvae and non-target organisms. Sci. Rep. 2020, 10, 21610. [Google Scholar] [CrossRef] [PubMed]
- Metak, A.M. Effects of nanocomposite based nano-silver and nano-titanium dioxide on food packaging materials. Int. J. Appl. Sci. Technol. 2015, 5, 26–40. [Google Scholar]
- Panea, B.; Ripoll, G.; González, J.; Fernández-Cuello, Á.; Albertí, P. Effect of nanocomposite packaging containing different proportions of ZnO and Ag on chicken breast meat quality. J. Food Eng. 2014, 123, 104–112. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active Chicken Meat Packaging Based on Polylactide Films and Bimetallic Ag–Cu Nanoparticles and Essential Oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Bher, A.; Jacob, H.; Auras, R. Compression molded LLDPE films loaded with bimetallic (Ag-Cu) nanoparticles and cinnamon essential oil for chicken meat packaging applications. LWT 2018, 93, 329–338. [Google Scholar] [CrossRef]
- Ge, L.; Wang, W.; Peng, Z.; Tan, F.; Wang, X.; Chen, J.; Qiao, X. Facile fabrication of Fe@MgO magnetic nanocomposites for efficient removal of heavy metal ion and dye from water. Powder Technol. 2018, 326, 393–401. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S.; Zhu, J.; Wang, K.; Gao, M.; Wang, X.; Xu, Q. Au-Ag bimetallic nanoparticles decorated multi-amino cyclophosphazene hybrid microspheres as enhanced activity catalysts for the reduction of 4-nitrophenol. Mater. Chem. Phys. 2018, 207, 315–324. [Google Scholar] [CrossRef]
- Tripathy, T.; Kolya, H.; Jana, S.; Senapati, M. Green synthesis of Ag-Au bimetallic nanocomposites using a biodegradable synthetic graft copolymer; hydroxyethyl starch-g-poly (acrylamide-co-acrylic acid) and evaluation of their catalytic activities. Eur. Polym. J. 2017, 87, 113–123. [Google Scholar] [CrossRef]
- Zhao, B.; Mele, G.; Pio, I.; Li, J.; Palmisano, L.; Vasapollo, G. Degradation of 4-nitrophenol (4-NP) using Fe–TiO2 as a heterogeneous photo-Fenton catalyst. J. Hazard. Mater. 2010, 176, 569–574. [Google Scholar] [CrossRef]
- Kharlamova, T.S.; Salina, M.V.; Svetlichnyi, V.A.; Salaev, M.A.; Stadnichenko, A.I.; Mamontov, G.V. CeO2-supported Pt–Ag bimetallic catalysts for 4-nitrophenol reduction. Catal. Today 2022, 384–386, 12–24. [Google Scholar] [CrossRef]
- Omiri, J.; Snoussi, Y.; Bhakta, A.K.; Truong, S.; Ammar, S.; Khalil, A.M.; Jouini, M.; Chehimi, M.M. Citric-Acid-Assisted Preparation of Biochar Loaded with Copper/Nickel Bimetallic Nanoparticles for Dye Degradation. Colloids Interfaces 2022, 6, 18. [Google Scholar] [CrossRef]
- Younas, U.; Gulzar, A.; Ali, F.; Pervaiz, M.; Ali, Z.; Khan, S.; Saeed, Z.; Ahmed, M.; Alothman, A.A. Antioxidant and Organic Dye Removal Potential of Cu-Ni Bimetallic Nanoparticles Synthesized Using Gazania rigens Extract. Water 2021, 13, 2653. [Google Scholar] [CrossRef]
- Parveen, M.F.; Ranchani, A.A.J.; Parthasarathy, V.; Anbarasan, R. Synthesis, characterization and catalytic applications of CuO–NiO bimetallic oxide nanoparticles towards the reduction of hazardous pollutants, derivative preparation and cross linking reaction. Appl. Nanosci. 2022, 12, 1643–1656. [Google Scholar] [CrossRef]
- Cerrón-Calle, G.A.; Fajardo, A.S.; Sánchez-Sánchez, C.M.; Garcia-Segura, S. Highly reactive Cu-Pt bimetallic 3D-electrocatalyst for selective nitrate reduction to ammonia. Appl. Catal. B: Environ. 2022, 302, 120844. [Google Scholar] [CrossRef]
- Mansouriieh, N.; Sohrabi, M.R.; Khosravi, M. Adsorption kinetics and thermodynamics of organophosphorus profenofos pesticide onto Fe/Ni bimetallic nanoparticles. Int. J. Environ. Sci. Technol. 2016, 13, 1393–1404. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Jin, X.; Khan, N.I.; Owens, G.; Chen, Z. Bimetallic Fe/Ni nanoparticles derived from green synthesis for the removal of arsenic (V) in mine wastewater. J. Environ. Manag. 2022, 301, 113838. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, T.; Ouda, M.; Krishnamoorthy, R.; Hai, A.; Gnanasundaram, N.; Hasan, S.W.; Banat, F. Surface-engineered polyethersulfone membranes with inherent Fe–Mn bimetallic oxides for improved permeability and antifouling capability. Environ. Res. 2022, 204, 112390. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, T.; Wu, L.; Wang, Y. Enhancing the permeation and antifouling performance of PVDF hybrid membranes by incorporating Co–Fe hydroxide nanoparticles in reverse microemulsion. J. Environ. Chem. Eng. 2021, 9, 106556. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Asadpour-Zeynali, K.; Khataee, A.; Mokhtarzadeh, A. Bimetallic Fe/Mn MOFs/MβCD/AuNPs stabilized on MWCNTs for developing a label-free DNA-based genosensing bio-assay applied in the determination of Salmonella typhimurium in milk samples. Chemosphere 2022, 287, 132373. [Google Scholar] [CrossRef] [PubMed]
- Sravani, B.; Kiranmai, S.; Rajasekhara Reddy, G.; Park, J.P.; VeeraManohara Reddy, Y.; Madhavi, G. Highly sensitive detection of anti-cancer drug based on bimetallic reduced graphene oxide nanocomposite. Chemosphere 2022, 287, 132281. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Akhtar, M.; Zulfiqar, S.; Hanif, F.; Alsafari, I.A.; Agboola, P.O.; Haider, S.; Warsi, M.F.; Shakir, I. Thiamine-functionalized silver–copper bimetallic nanoparticles-based electrochemical sensor for sensitive detection of anti-inflammatory drug 4-aminoantipyrine. Chem. Pap. 2022, 76, 2721–2731. [Google Scholar] [CrossRef]
- Tang, J.; Hu, T.; Li, N.; Zhu, Y.; Li, J.; Zheng, S.; Guo, J. Ag doped Co/Ni bimetallic organic framework for determination of luteolin. Microchem. J. 2022, 179, 107461. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Khoshnavaz, Y.; Tuzen, M.; Erk, N. Voltammetric sensor based on bimetallic nanocomposite for determination of favipiravir as an antiviral drug. Microchim. Acta 2021, 188, 434. [Google Scholar] [CrossRef]
- Eteya, M.M.; Rounaghi, G.H.; Deiminiat, B. Fabrication of a new electrochemical sensor based on AuPt bimetallic nanoparticles decorated multi-walled carbon nanotubes for determination of diclofenac. Microchem. J. 2019, 144, 254–260. [Google Scholar] [CrossRef]
- Huan, K.; Li, Y.; Deng, D.; Wang, H.; Wang, D.; Li, M.; Luo, L. Composite-controlled electrospinning of CuSn bimetallic nanoparticles/carbon nanofibers for electrochemical glucose sensor. Appl. Surf. Sci. 2022, 573, 151528. [Google Scholar] [CrossRef]
- Aarthi, A.; Bindhu, M.R.; Umadevi, M.; Parimaladevi, R.; Sathe, G.V.; Al-Mohaimeed, A.M.; Elshikh, M.S.; Balasubramanian, B. Evaluating the detection efficacy of advanced bimetallic plasmonic nanoparticles for heavy metals, hazardous materials and pesticides of leachate in contaminated groundwater. Environ. Res. 2021, 201, 111590. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Lai, J.; Qiu, P.; Wang, X. An ultrasensitive fluorescent sensor for organophosphorus pesticides detection based on RB-Ag/Au bimetallic nanoparticles. Sens. Actuators B Chem. 2018, 263, 517–523. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, Y.; Jiang, C.; Shao, Y.; Barceló, D.; Ying, Y.; Ping, J. Self-reduction bimetallic nanoparticles on ultrathin MXene nanosheets as functional platform for pesticide sensing. J. Hazard. Mater. 2020, 384, 121358. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Rao, G.R.; Sharma, M.K.; Bhattacharya, B.K.; Rao, V.K.; Vijayaraghavan, R. Immobilization of acetylcholineesterase–choline oxidase on a gold–platinum bimetallic nanoparticles modified glassy carbon electrode for the sensitive detection of organophosphate pesticides, carbamates and nerve agents. Biosens. Bioelectron. 2009, 25, 832–838. [Google Scholar] [CrossRef]
- Sabeeh, H.; Aadil, M.; Zulfiqar, S.; Rasheed, A.; Al-Khalli, N.F.; Agboola, P.O.; Haider, S.; Warsi, M.F.; Shakir, I. Hydrothermal synthesis of CuS nanochips and their nanohybrids with CNTs for electrochemical energy storage applications. Ceram. Int. 2021, 47, 13613–13621. [Google Scholar] [CrossRef]
- Ramesh, S.; Karuppasamy, K.; Vikraman, D.; Santhoshkumar, P.; Bathula, C.; Palem, R.R.; Kathalingam, A.; Kim, H.-S.; Kim, J.-H.; Kim, H.S. Sheet-like morphology CuCo2O4 bimetallic nanoparticles adorned on graphene oxide composites for symmetrical energy storage applications. J. Alloys Compd. 2022, 892, 162182. [Google Scholar] [CrossRef]
- Bhiradi, I.; Hiremath, S.S. Energy storage and photosensitivity of in-situ formed silver-copper (Ag-Cu) heterogeneous nanoparticles generated using multi-tool micro electro discharge machining process. J. Alloys Compd. 2022, 897, 162950. [Google Scholar] [CrossRef]
- Lim, G.J.H.; Liu, X.; Guan, C.; Wang, J. Co/Zn bimetallic oxides derived from metal organic frameworks for high performance electrochemical energy storage. Electrochim. Acta 2018, 291, 177–187. [Google Scholar] [CrossRef]
- Zamani Meymian, M.R.; Ghaffarinejad, A.; Fazli, R.; Kosari Mehr, A. Fabrication and characterization of bimetallic nickel-molybdenum nano-coatings for mild steel corrosion protection in 3.5% NaCl solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124617. [Google Scholar] [CrossRef]
- Nazhirah, S.N.M.; Ghoshal, S.K.; Arifin, R.; Hamzah, K. Effects of bimetallic nanoparticles Ag and TiO2 embedment on tellurite zinc–silicate glass: Self-cleaning characteristics. Surf. Interfaces 2021, 25, 101236. [Google Scholar] [CrossRef]
- Sharma, M.K.; Buchner, R.D.; Scharmach, W.J.; Papavassiliou, V.; Swihart, M.T. Creating Conductive Copper–Silver Bimetallic Nanostructured Coatings Using a High Temperature Reducing Jet Aerosol Reactor. Aerosol Sci. Technol. 2013, 47, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Tiri, R.N.E.; Gulbagca, F.; Aygun, A.; Cherif, A.; Sen, F. Biosynthesis of Ag–Pt bimetallic nanoparticles using propolis extract: Antibacterial effects and catalytic activity on NaBH4 hydrolysis. Environ. Res. 2022, 206, 112622. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, Z.; Jiang, Y.; Han, H.; Wu, T.; Wu, L.; Liu, J.; Wang, Z.; Wang, F. Platinum−Copper Bimetallic Nanoparticles Supported on TiO2 as Catalysts for Photo−thermal Catalytic Toluene Combustion. ACS Appl. Nano Mater. 2022, 5, 1845–1854. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, D.; Lu, G.; Cai, C. Supported Pd–Au bimetallic nanoparticles as an efficient catalyst for the hydrodeoxygenation of vanillin with formic acid at room temperature. Green Chem. 2022, 24, 1096–1102. [Google Scholar] [CrossRef]
- Karanjit, S.; Jinasan, A.; Samsook, E.; Dhital, R.N.; Motomiya, K.; Sato, Y.; Tohji, K.; Sakurai, H. Significant stabilization of palladium by gold in the bimetallic nanocatalyst leading to an enhanced activity in the hydrodechlorination of aryl chlorides. Chem. Commun. 2015, 51, 12724–12727. [Google Scholar] [CrossRef] [Green Version]
- Karanjit, S.; Tamura, A.; Kashihara, M.; Ushiyama, K.; Shrestha, L.K.; Ariga, K.; Nakayama, A.; Namba, K. Hydrotalcite-Supported Ag/Pd Bimetallic Nanoclusters Catalyzed Oxidation and One-Pot Aldol Reaction in Water. Catalysts 2020, 10, 1120. [Google Scholar] [CrossRef]
- Brett, G.L.; He, Q.; Hammond, C.; Miedziak, P.J.; Dimitratos, N.; Sankar, M.; Herzing, A.A.; Conte, M.; Lopez-Sanchez, J.A.; Kiely, C.J.; et al. Selective Oxidation of Glycerol by Highly Active Bimetallic Catalysts at Ambient Temperature under Base-Free Conditions. Angew. Chem. 2011, 123, 10318–10321. [Google Scholar] [CrossRef] [Green Version]
- García, T.; Agouram, S.; Dejoz, A.; Sánchez-Royo, J.F.; Torrente-Murciano, L.; Solsona, B. Enhanced H2O2 production over Au-rich bimetallic Au–Pd nanoparticles on ordered mesoporous carbons. Catal. Today 2015, 248, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-Free Oxidation of Primary Alcohols to Aldehydes Using Au-Pd/TiO2 Catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, Y.; He, M.; Zhou, L.; Xu, T.; Yuan, R.; Lin, S.; Xiang, W.; Liang, X. Optical properties of bimetallic Au-Cu nanocrystals embedded in glass. Mater. Res. Bull. 2018, 98, 94–102. [Google Scholar] [CrossRef]
- Tao, C.; Zou, X.; Du, K.; Zhou, G.; Yan, H.; Yuan, X.; Zhang, L. Fabrication of robust, self-cleaning, broadband TiO2SiO2 double-layer antireflective coatings with closed-pore structure through a surface sol-gel process. J. Alloys Compd. 2018, 747, 43–49. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, J.; Yan, L.; Zhang, X. Sol-gel preparation of self-cleaning SiO2-TiO2/SiO2-TiO2 double-layer antireflective coating for solar glass. Results Phys. 2018, 8, 532–536. [Google Scholar] [CrossRef]
- Gu, C.; Chen, L.; Liu, Y.; Zhang, X.; Liu, J.; Zhang, Q.; Wang, C.; Ma, L. One-pot conversion of biomass-derived levulinic acid to furanic biofuel 2-methyltetrahydrofuran over bimetallic NiCo/γ-Al2O3 catalysts. Mol. Catal. 2022, 524, 112317. [Google Scholar] [CrossRef]
- Singh, R.; Nawale, L.; Arkile, M.; Wadhwani, S.; Shedbalkar, U.; Chopade, S.; Sarkar, D.; Chopade, B.A. Phytogenic silver, gold, and bimetallic nanoparticles as novel antitubercular agents. Int. J. Nanomed. 2016, 11, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Albee, B.; Alemayehu, M.; Diaz, R.; Ingham, L.; Kamal, S.; Rodriguez, M.; Whaley Bishnoi, S. Comparative toxicity study of Ag, Au, and Ag–Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 2010, 398, 689–700. [Google Scholar] [CrossRef] [PubMed]
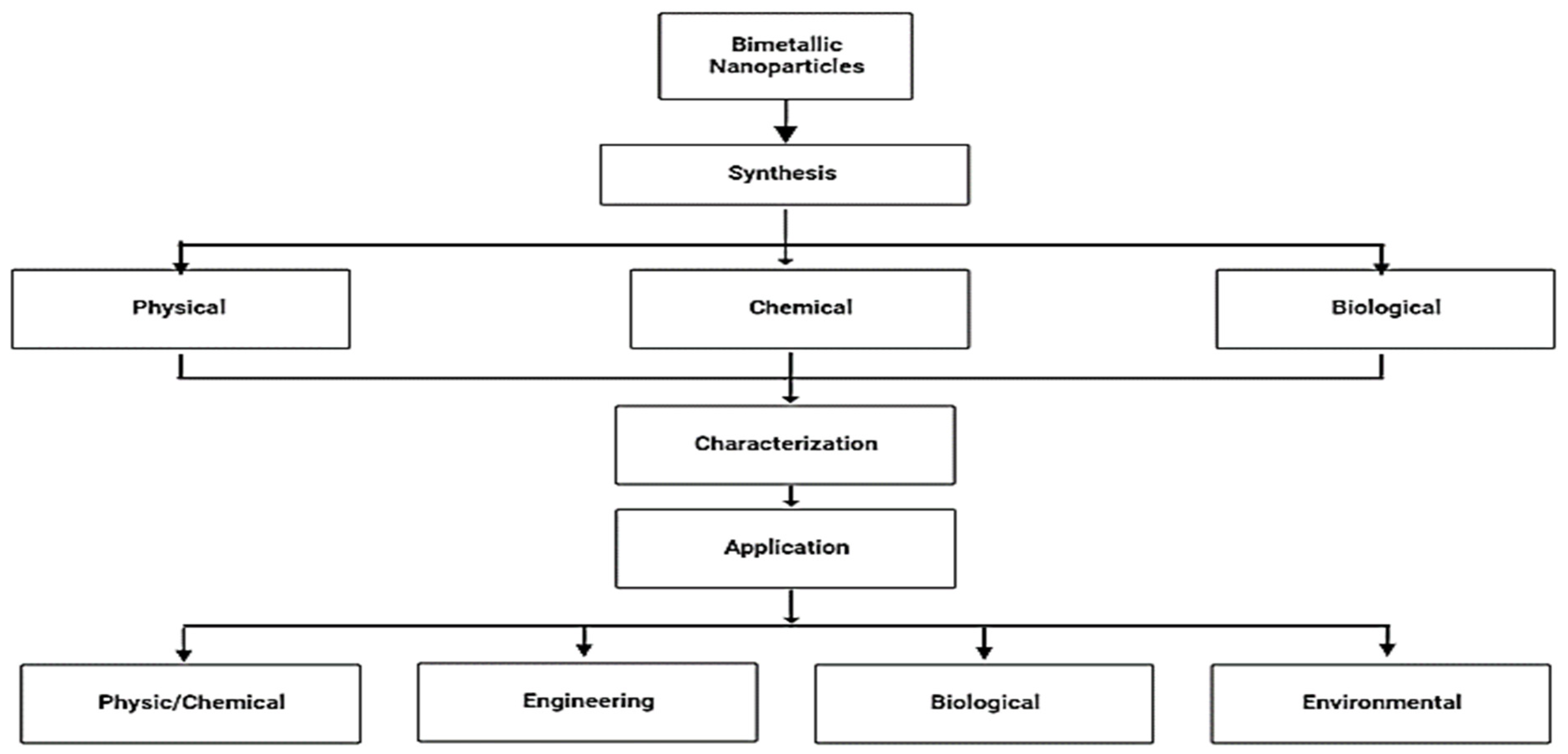



| Forms of Bimetallic Nanoparticles | Type of Bimetallic Nanoparticle | Structure of the Bimetallic Nanoparticle | Method of Synthesis | References |
|---|---|---|---|---|
| Gold based | Au-Pt | Cubic crystal structure | Green synthesis | [29] |
| Au-Cu | Porus structure | Galvanic replacement | [30] | |
| Au-Ni | Porus structure | Chemical synthesis | [31] | |
| Au–Pd | Core-shell | Chemical Synthesis | [32] | |
| Au-Ag | Alloy & Core-shell | Green synthesis | [33] | |
| Silver based | Ag-Cu | Alloy | Green synthesis | [34] |
| Ag-Au | Alloy | Green synthesis | [35] | |
| Ag-Fe | Spherical shape | Green synthesis | [36] | |
| Ag-Pd | Cubic crystalline structure | Green synthesis | [37] | |
| Ag-Zn | Wurtzite hexagonal | Green synthesis | [38] | |
| Copper based | Cu-Ni | Alloy | Sol-gel | [39] |
| Cu-Ag | Alloy | Green synthesis | [40] | |
| Cu-Fe | Classified Janus | Electrical explosion | [41] | |
| Cu-Co | Spherical | Inverse micellar encapsulation | [42] | |
| Cu-Pt | Alloy & core | Hydrothermal method | [43] | |
| Nickel based | Ni-Co | Porous | Chemical co-reduction | [44] |
| Ni-Fe | Alloy & core shell | Chemical synthesis | [45] | |
| Ni-Au | - | Physical Synthesis | [46] | |
| Ni-Pt | Alloy | Co-reduction | [47] | |
| Ni-Ru | Alloy | Chemical synthesis | [48] | |
| Iron based | Fe-Zn | Spherical | Green synthesis | [49] |
| Fe-Ti | Nano-Sphere | Chemical synthesis | [50] | |
| Fe-Mn | Spheroid-like | Chemical synthesis | [51] | |
| Fe-Ag | Janus structure | Physical Synthesis | [52] | |
| Fe-Cu | Crystalline | Green synthesis | [53] | |
| Platinum based | Pt-Ag | face-centred cubic structure | Chemical synthesis | [54] |
| Pt-Co | face-centred cubic structure | Chemical reduction | [55] | |
| Pt-Ni | face-centred cubic structure | Chemical reduction | [56] | |
| Pt-Pd | Carbon sphere | Chemical reduction | [57] | |
| Pt-Pd | Crystalline | Green synthesis | [58] | |
| Pt-Fe | Face-cantered cubic | Chemical synthesis | [59] | |
| Palladium based | Pd-Au | Hexagonal nanoplate | DNA ligand | [60] |
| Pd-Ag | Spherical | Green synthesis | [61] | |
| Pd-Cu | Crystalline alloy | Green synthesis | [62] | |
| Pd-Zn | Spherical | Chemical synthesis | [63] |
| Properties | Features | Techniques | Reference |
|---|---|---|---|
| Morphology | shape, size, structure | DLS, TEM/SEM, STM, XPS, AFM | [198,199,200,201,202,203,204] |
| Topology | Chemical bonding/composition, physical properties, crystallographic structures | XRD, TEM, zeta potential, Brunauer Emmett Teller (BET) | [204,205,206] |
| Chemical properties | Surface energy, chemical potential, oxidation process, catalysis, functionalization of nanostructures | UV-vis, EDXS, FTIR | [207,208] |
| Electrical properties | Conductivity, resistivity, dielectric permittivity | AC/DC conductivity, electrokinetics (zeta potential/cyclic volumetry studies) | [209,210,211] |
| Optical properties | Absorption, reflectance, luminescence, phosphorescence | Microscopy, FTIR, UV-vis, DPCS, Raman spectroscopy, surface plasmon resonance. | [212,213] |
| Mechanical properties | Hardness, plasticity, young modulus | AFM, thermomechanical analysis | [214,215,216] |
| Magnetic properties | Magnetism | VSM, SQUID, MFM | [217,218,219] |
| Thermal properties | Heat flux, change of temperature, volatiles | DSC, EGA, TGA, DTA | [220,221] |
| Biological properties | Anti-microbial, biodegradability, toxicity | In vitro cell viability, in vivo microbial colony viability | [222,223] |
| Application | Bimetallic Nanoparticle | Reference |
|---|---|---|
| Antimicrobial agents | Pd–Pt | [21] |
| Ag–Fe | [28] | |
| CuO–NiO | [232] | |
| Ag–Au | [23,169,174,233] | |
| Cu–Ag | [176] | |
| Ag–Cu | [234] | |
| Au–Pt | [235] | |
| Cu–Zn | [236,237] | |
| Cu–Ni | [237] | |
| Antioxidant | CuO/NiO | [232] |
| Ag–Cu | [228] | |
| Au–Ag | [233] | |
| Mn–Cu | [238] | |
| Pt–Pd | [239] | |
| Ag–Au | [240] | |
| Antidiabetic | Au–Ag | [241] |
| Ag–Au | [242] | |
| Ag/ZnOVI | [243] | |
| Ag–ZnO | [244] | |
| Anti-Alzheimer | Ag–Au | [242] |
| Anti-inflammatory | Ag/ZnOVI | [243] |
| Zn–Fe2O4, | [245] | |
| CuFeO2, | [246] | |
| Au@Ag | [247] | |
| Drug delivery | Au@Pd | [248] |
| Au–Pt | [249] | |
| Pd–Pt | [250] |
| Types of BMNP | Light Source | Compound/Dosage | Degradation%/Activation Energy/Conversion Rate | Reference |
|---|---|---|---|---|
| Mn–Cu | visible light | 240 µg/mL of methyl red | 78.54 ± 0.16% degradation | [238] |
| 240 µg/mL of eriochrome black | 87.67 ± 0.06% degradation | |||
| 240 µg/mL of methyl orange decayed | 69.79 ± 0.36% degradation | |||
| Ag–Pt | -- | -- | 25.61 kJ/mol activation energy | [293] |
| Pd–Pt | -- | -- | 13.93 kJ/mol activation energy | [21] |
| Pd–Ag | -- | NaBH4 | 27.01 kJ/mol activation energy | [61] |
| Pt–Cu | Sun light | Toluene conversion | 55% conversion rate | [294] |
| Pd–Au | -- | HDO of vanillin at room temp. | 99% conversion rate | [295] |
| Au–Pd | -- | Hydrodechlorination of aryl chlorides | 95% conversion rate | [296] |
| Ag–Pd | -- | Alcohol oxidation | up to 97% yield | [297] |
| Au–Pd, | -- | Oxidation of glycerol | 42.9% yield | [298] |
| Au–Pt | -- | Oxidation of glycerol | 29.4% yield | [298] |
| Au–Pd | -- | H2O2 production | 99% yield | [299] |
| Au/Pd–TiO2 | -- | Alcohols oxidation to aldehydes | 83.3% conversion rate | [300] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, D.S.; Roy, A. Synthesis of Bimetallic Nanoparticles and Applications—An Updated Review. Crystals 2023, 13, 637. https://doi.org/10.3390/cryst13040637
Idris DS, Roy A. Synthesis of Bimetallic Nanoparticles and Applications—An Updated Review. Crystals. 2023; 13(4):637. https://doi.org/10.3390/cryst13040637
Chicago/Turabian StyleIdris, Dahir Sagir, and Arpita Roy. 2023. "Synthesis of Bimetallic Nanoparticles and Applications—An Updated Review" Crystals 13, no. 4: 637. https://doi.org/10.3390/cryst13040637





