From Liquid to Solid: Cocrystallization as an Engineering Tool for the Solidification of Pyruvic Acid
Abstract
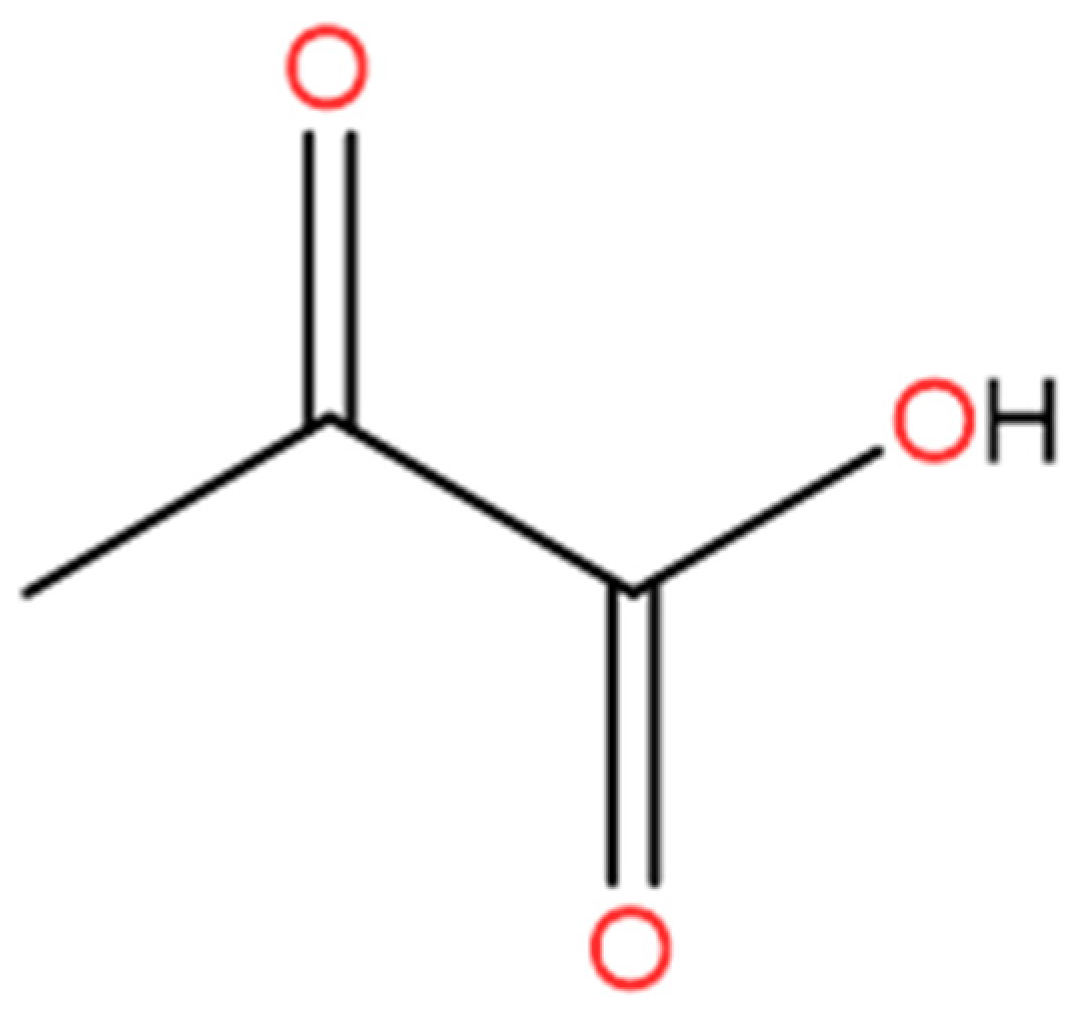
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solid Forms Screening by Grinding
2.3. Single Crystal Growth
2.4. Congruency Experiments
2.5. Powder X-ray Diffraction (PXRD)
2.6. Single Crystal Structure Determination
2.7. Thermogravimetric Analysis (TGA)
2.8. Proton Nuclear Magnetic Resonance (1H NMR)
2.9. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
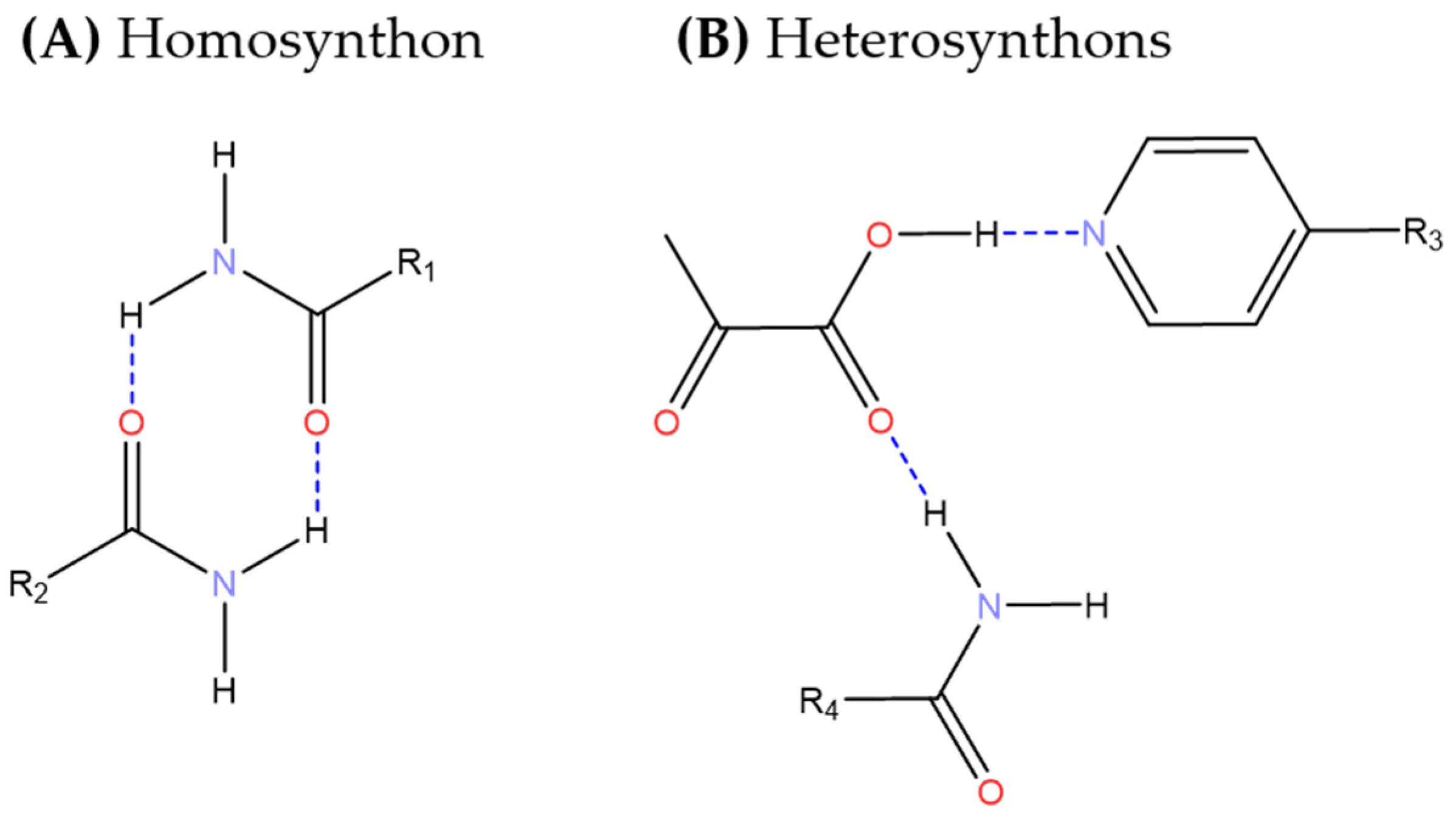
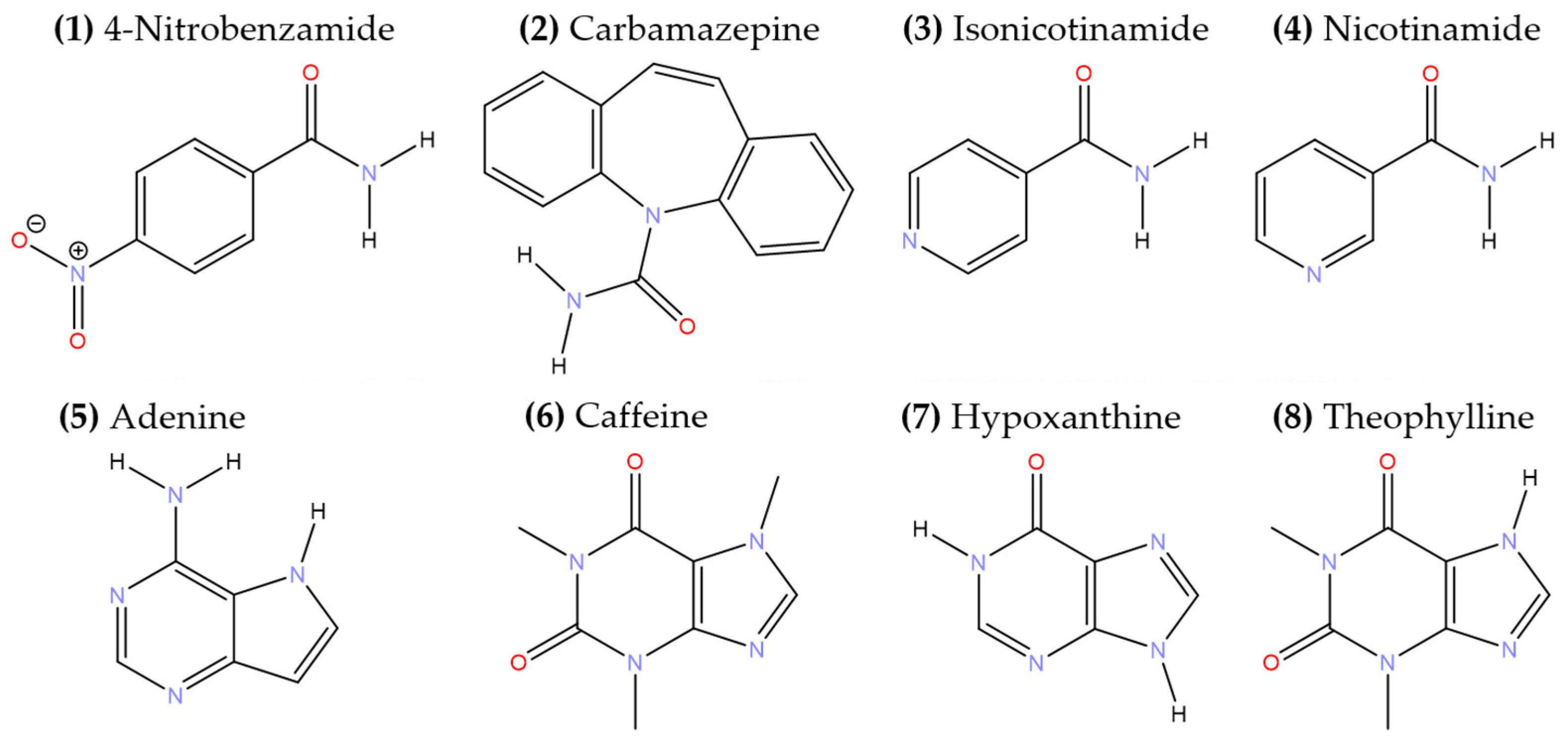
3.1. Cocrystal Screening
3.2. Structural and Thermal Characterization of Salt and (Salt) Cocrystals
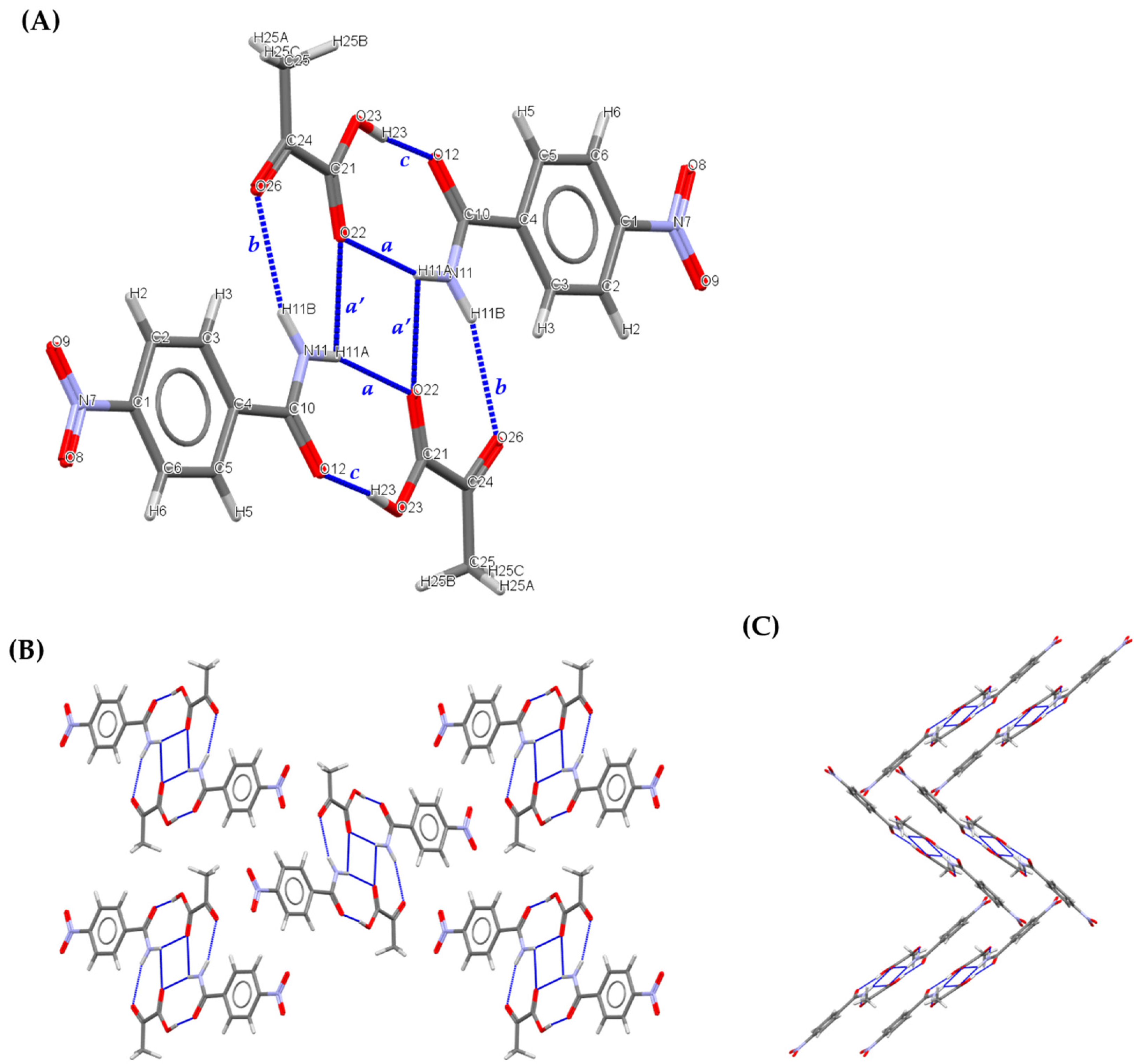
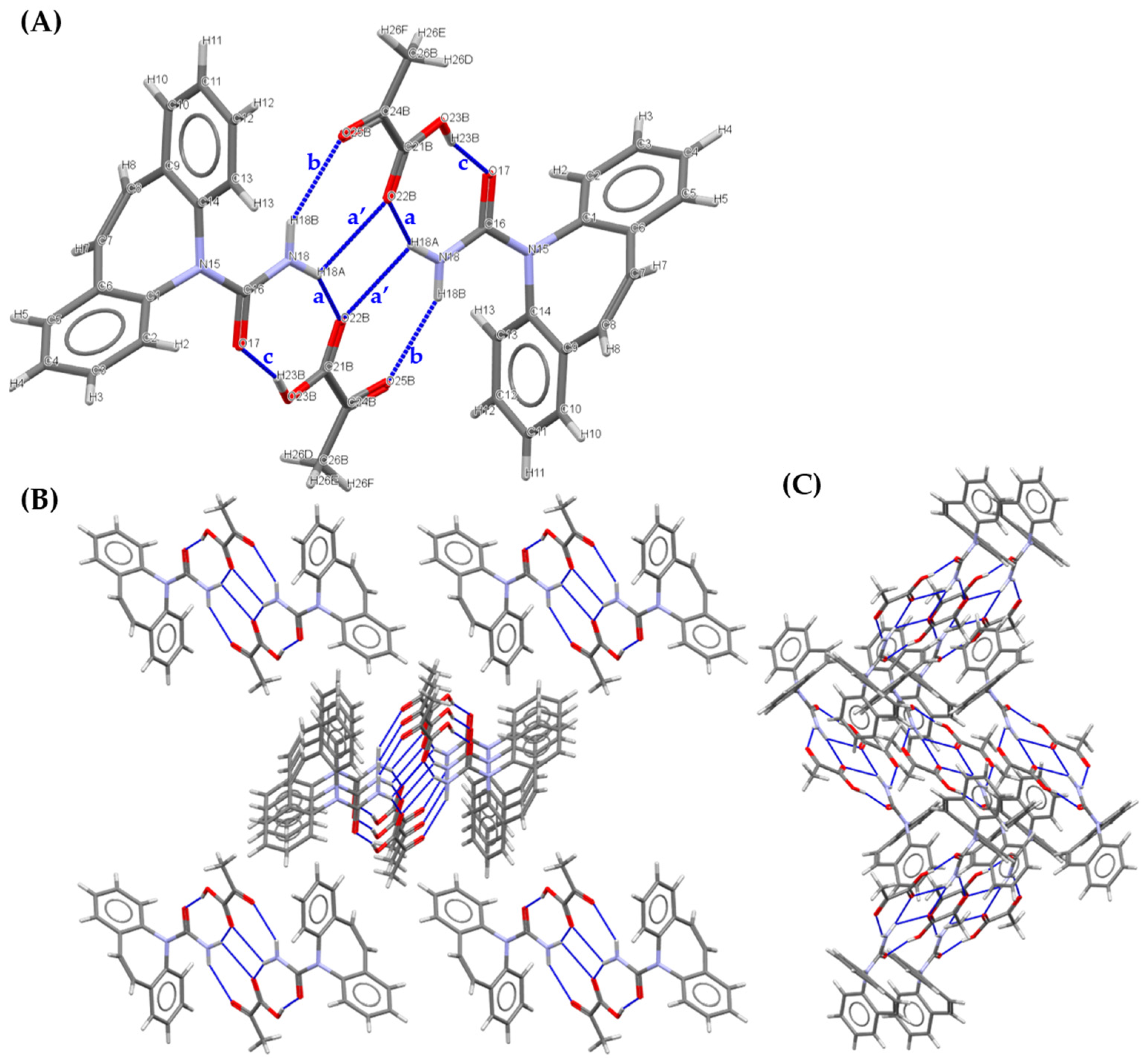
3.2.1. 1:1 Pyruvic acid-4-Nitrobenzamide Cocrystal (PANB)
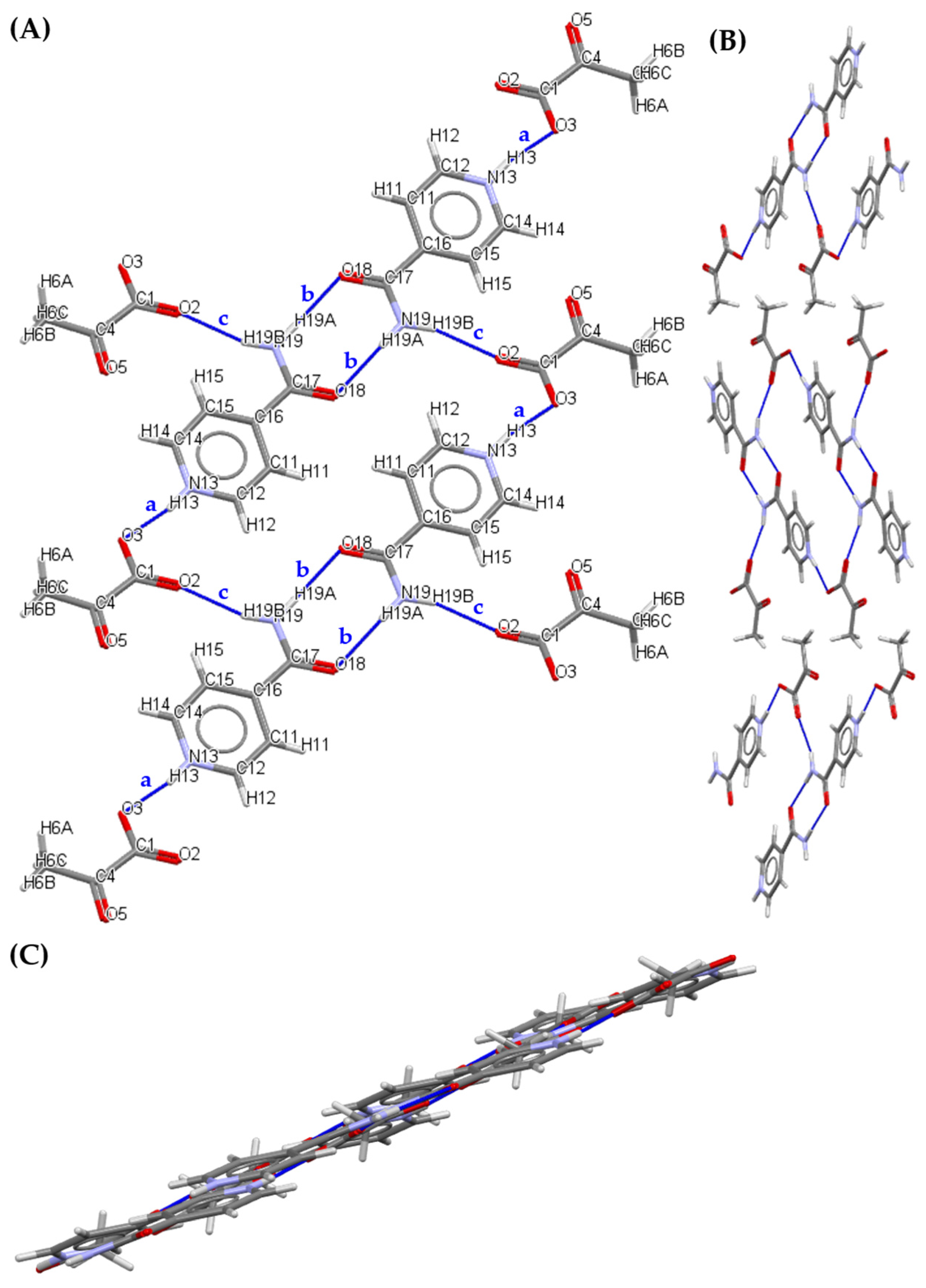
3.2.2. 1:1 Pyruvic Acid-Carbamazepine Cocrystal (PACBZ)
3.2.3. 1:1 Pyruvic Acid-Isonicotinamide Salt (PAINAM)
3.2.4. 2:3 Pyruvic Acid-Nicotinamide Salt Cocrystal (PANAM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bacchi, A.; Capucci, D.; Giannetto, M.; Mattarozzi, M.; Pelagatti, P.; Rodriguez-Hornedo, N.; Rubini, K.; Sala, A. Turning Liquid Propofol into Solid (without Freezing It): Thermodynamic Characterization of Pharmaceutical Cocrystals Built with a Liquid Drug. Cryst. Growth Des. 2016, 16, 6547–6555. [Google Scholar] [CrossRef]
- McKellar, S.C.; Kennedy, A.R.; McCloy, N.C.; McBride, E.; Florence, A.J. Formulation of Liquid Propofol as a Cocrystalline Solid. Cryst. Growth Des. 2014, 14, 2422–2430. [Google Scholar] [CrossRef]
- Lopalco, A.; Deeken, R.; Douglas, J.; Denora, N.; Stella, V.J. Some Preformulation Studies of Pyruvic Acid and Other α-Keto Carboxylic Acids in Aqueous Solution: Pharmaceutical Formulation Implications for These Peroxide Scavengers. J. Pharm. Sci. 2019, 108, 3281–3288. [Google Scholar] [CrossRef]
- Cybulski, K.; Tomaszewska-Hetman, L.; Rakicka, M.; Juszczyk, P.; Rywińska, A. Production of Pyruvic Acid from Glycerol by Yarrowia Lipolytica. Folia Microbiol. 2019, 64, 809–820. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Lun, S.-Y. Biotechnological Production of Pyruvic Acid. Appl. Microbiol. Biotechnol. 2001, 57, 451–459. [Google Scholar] [CrossRef]
- Onakpoya, I.; Hunt, K.; Wider, B.; Ernst, E. Pyruvate Supplementation for Weight Loss: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Crit. Rev. Food Sci. Nutr. 2014, 54, 17–23. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Almarsson, Ö.J.; Zaworotko, M. Crystal Engineering of the Composition of Pharmaceutical Phases. Do Pharmaceutical Co-Crystals Represent a New Path to Improved Medicines? Chem. Commun. 2004, No. 17, 1889–1896. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical Cocrystals: An Overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef]
- Koranne, S.; Krzyzaniak, J.F.; Luthra, S.; Arora, K.K.; Suryanarayanan, R. Role of Coformer and Excipient Properties on the Solid-State Stability of Theophylline Cocrystals. Cryst. Growth Des. 2019, 19, 868–875. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef]
- Friščić, T.; Jones, W. Benefits of Cocrystallisation in Pharmaceutical Materials Science: An Update. J. Pharm. Pharmacol. 2010, 62, 1547–1559. [Google Scholar] [CrossRef]
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Elder, D.P.; Holm, R.; de Diego, H.L. Use of Pharmaceutical Salts and Cocrystals to Address the Issue of Poor Solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical Cocrystals: Along the Path to Improved Medicines. Chem. Commun. 2015, 52, 640–655. [Google Scholar] [CrossRef]
- Harmsen, B. Co-Crystal Applications: Stability and Separation. A Case Study of Ibuprofen: Levetiracetam. Doctoral Thesis, UCL–Université Catholique de Louvain, Ottignies-Louvain-la-Neuve, Belgium, 2018. Available online: https://dial.uclouvain.be/pr/boreal/object/boreal:202803 (accessed on 23 April 2023).
- Bolla, G.; Nangia, A. Pharmaceutical Cocrystals: Walking the Talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef] [PubMed]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Pharmaceutical Cocrystallization: Engineering a Remedy for Caffeine Hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Physical Stability Enhancement of Theophylline via Cocrystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Duggirala, N.K.; Smith, A.J.; Wojtas, Ł.; Shytle, R.D.; Zaworotko, M.J. Physical Stability Enhancement and Pharmacokinetics of a Lithium Ionic Cocrystal with Glucose. Cryst. Growth Des. 2014, 14, 6135–6142. [Google Scholar] [CrossRef]
- de Maere d’Aertrycke, J.B.; Robeyns, K.; Willocq, J.; Leyssens, T. Cocrystallization as a Tool to Solve Deliquescence Issues: The Case of l-Lactic Acid. J. Cryst. Growth 2017, 472, 3–10. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysalisPro Software System, Version 1.171.38.41l.; Rigaku Oxford Diffraction Ltd.: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G.M. XS, version 2013/1; Georg-August-Universität Göttingen: Göttingen, Germany, 2013; b) GM Sheldrick. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Cryst D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Grell, J.; Bernstein, J.; Tinhofer, G. Graph-Set Analysis of Hydrogen-Bond Patterns: Some Mathematical Concepts. Acta Crystallogr. B 2000, 56 Pt 1, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Shattock, T.R.; Arora, K.K.; Vishweshwar, P.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Carboxylic Acid···Pyridine Hydrogen Bonds in Cocrystals That Also Contain a Hydroxyl Moiety. Cryst. Growth Des. 2008, 8, 4533–4545. [Google Scholar] [CrossRef]
- Allen, F.H.; Motherwell, W.D.S.; Raithby, P.R.; Shields, G.P.; Taylor, R. Systematic Analysis of the Probabilities of Formation of Bimolecular Hydrogen-Bonded Ring Motifs in Organic Crystal Structures. New J. Chem. 1999, 23, 25–34. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.-J.; Ulrich, J. N-H⋯O, O-H⋯O Hydrogen Bonded Supramolecular Formation in the Cocrystal of Salicylic Acid with N-Containing Bases. Cryst. Res. Technol. 2016, 51, 197–206. [Google Scholar] [CrossRef]
- Infantes, L.; Motherwell, W.D.S. The Probable Number of Hydrogen-Bonded Contacts for Chemical Groups in Organic Crystal Structures. Chem. Commun. 2004, 10, 1166–1167. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or Salt: Does It Really Matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef]
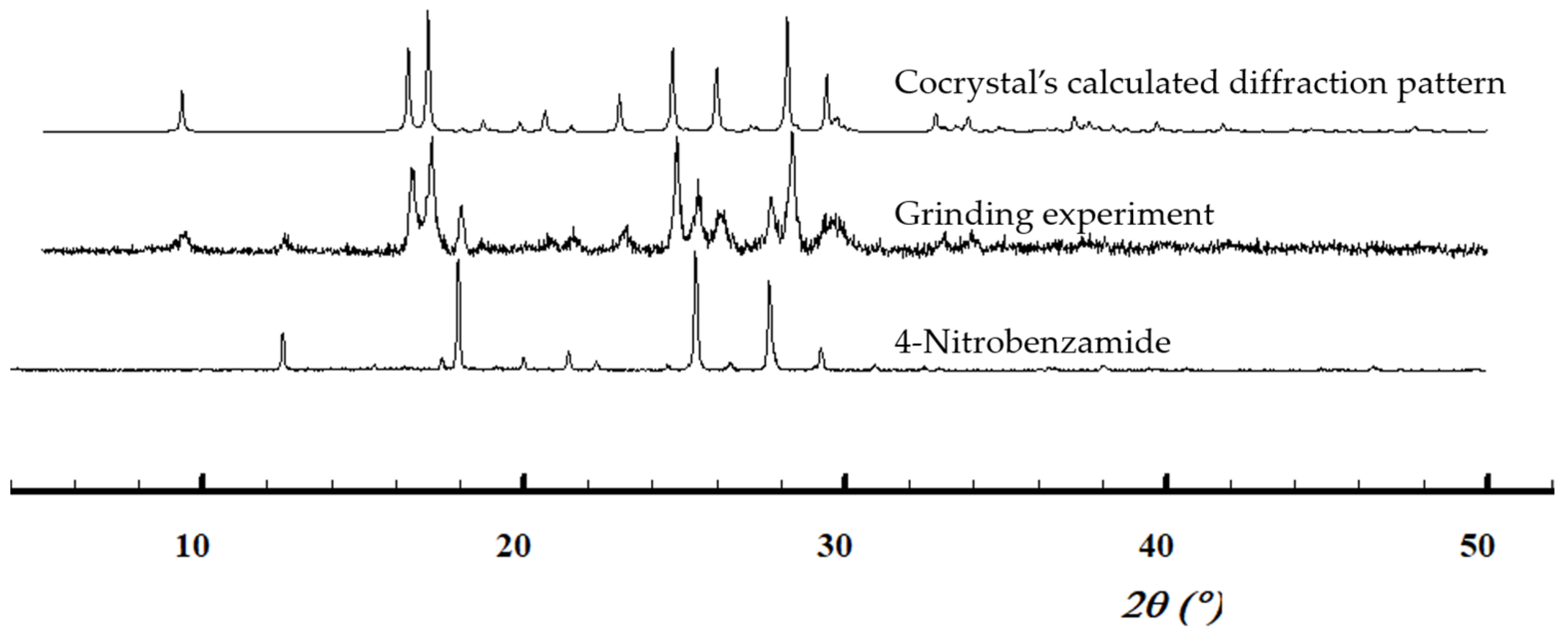
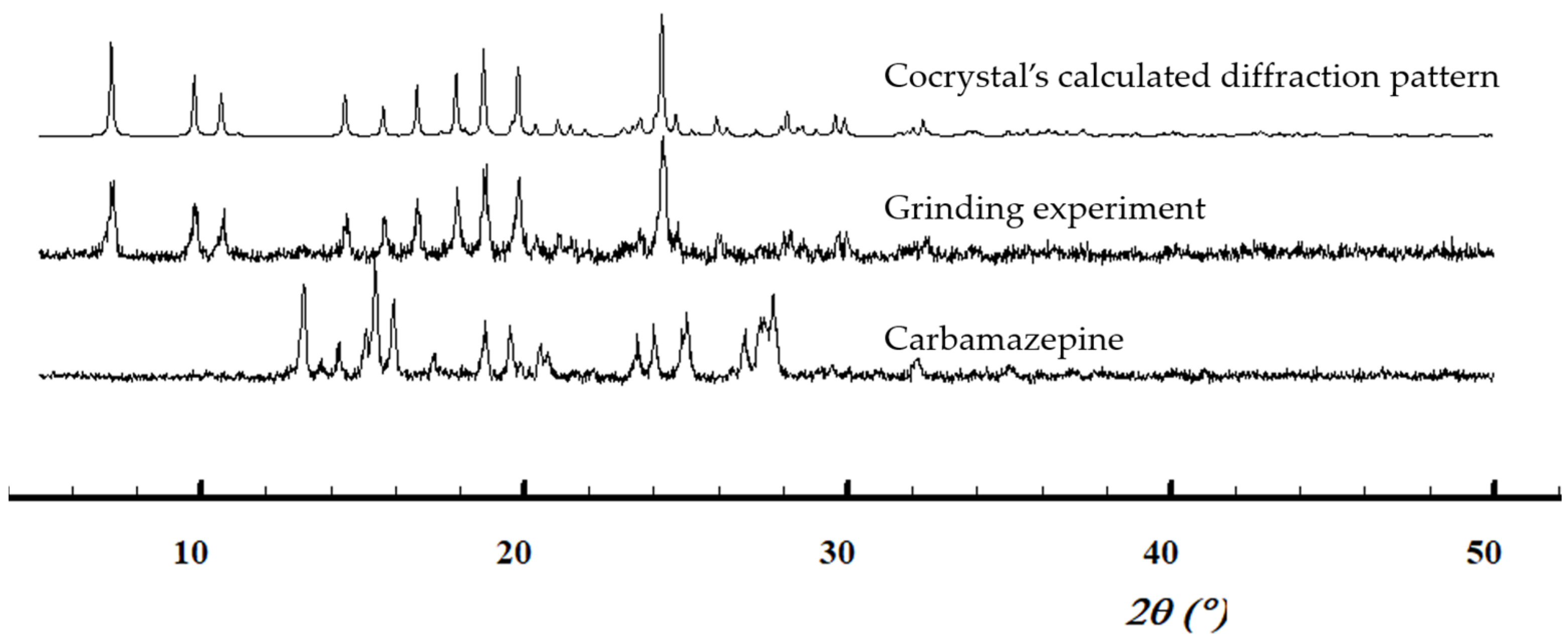

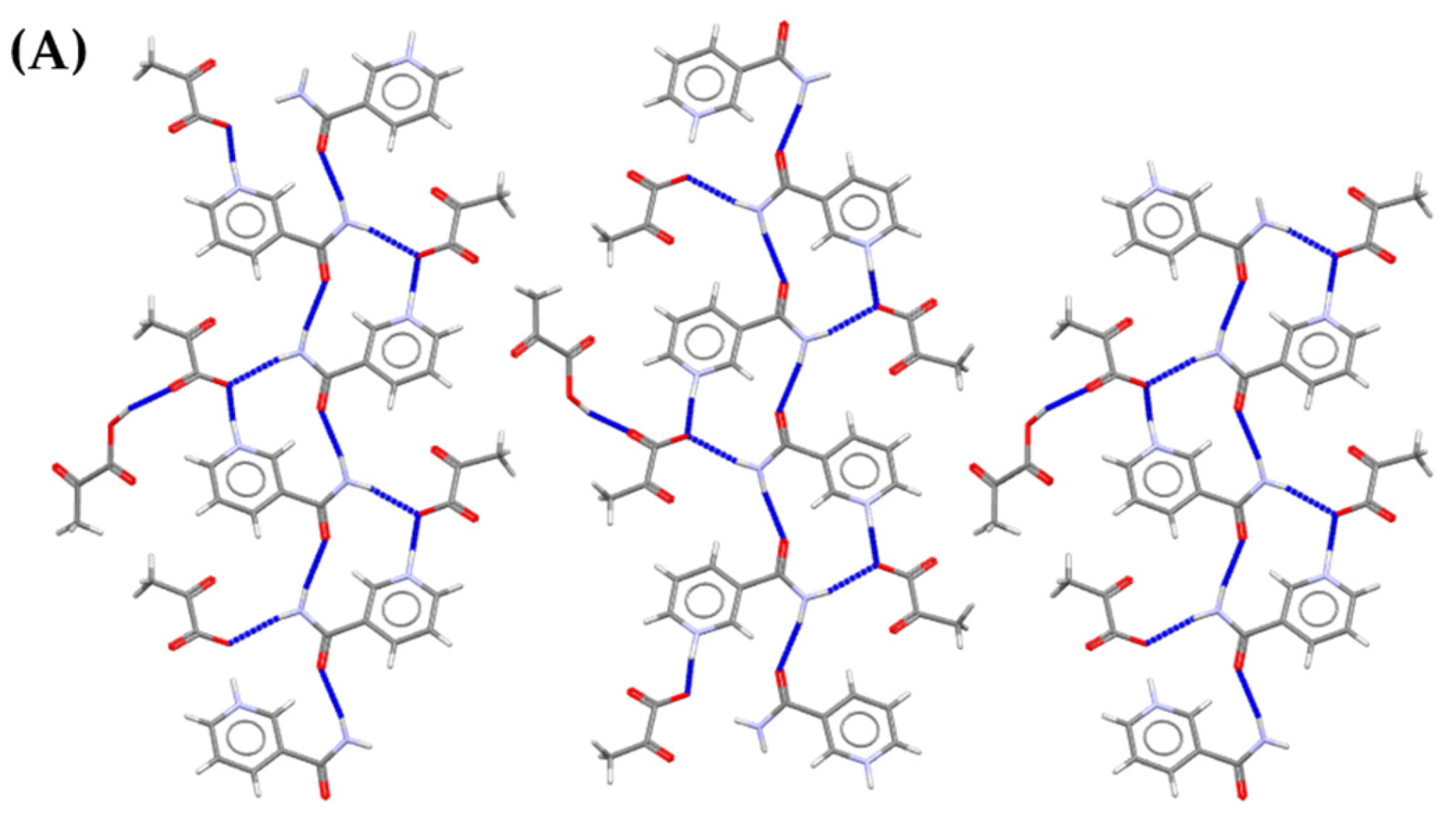
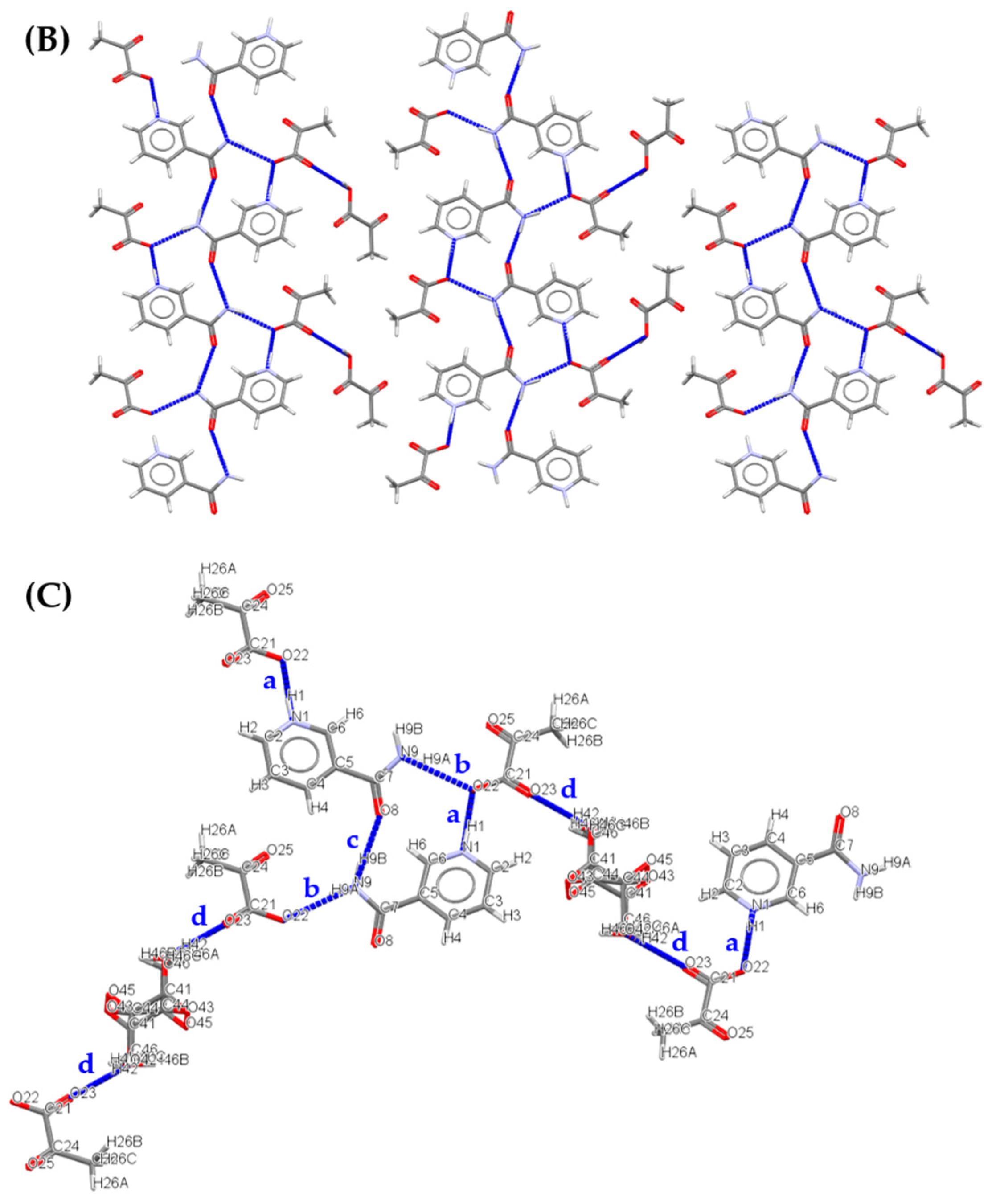

| Solid forms | 1:1 Pyruvic Acid- 4-Nitrobenzamide | 1:1 Pyruvic Acid- Carbamazepine | 1:1 Pyruvic Acid- Isonicotinamide | 2:3 Pyruvic Acid- Nicotinamide |
|---|---|---|---|---|
| Abbrev. | PANB | PACBZ | PAINAM | PANAM |
| Structural formula | C7H6N2O3, C3H4O3 | C15H12N2O, C3H4O3 | C6H7N2O, C3H3O3 | 2(C6H7N2O),2(C3H3O3), C3H4O3 |
| FW | 254.19 | 324.33 | 210.19 | 210.19 |
| Crystal system | Primitive Monoclinic | Primitive Monoclinic | Primitive Monoclinic | Primitive Monoclinic |
| Space group | P21/n | P21/n | P21/n | P21/n |
| a, b and c (Å) | 5.3463(5), 19.6423(14), 10.8234(7) | 5.3912(6),16.6752(15), 18.2383(18) | 3.8349(6), 33.160(5), 7.6010(11) | 3.82010(12), 33.2921(9), 9.5234(2) |
| α, β and γ (°) | 90, 90.316(7), 90 | 90, 97.324(10), 90 | 90, 97.015(13), 90 | 90, 98.606(3), 90 |
| Cell vol. (Å3) | 1136.59 | 1626.23 | 959.348 | 1197.54 |
| Z | 4 | 4 | 4 | 2 |
| Interatomic Distances (Å) | Angles (°) | ||||||
|---|---|---|---|---|---|---|---|
| Descriptors | Donors | H⋯ | Acceptors | D-H | H⋯A | D⋯A | D-H⋯A |
| a | N11 | H11A | O22 | 0.86 | 2.14 | 2.982(3) | 164 |
| b | N11 | H11B | O26 | 0.86 | 2.31 | 3.138(3) | 161 |
| c | O23 | H23 | O12 | 0.82 | 1.78 | 2.589(3) | 168 |
| a’ | N11 | H11A | O22 | 0.86 | 2.50 | 2.912(3) | 110 |
| Interatomic Distances (Å) | Angles (°) | ||||||
|---|---|---|---|---|---|---|---|
| Descriptors | Donors | H⋯ | Acceptors | D-H | H⋯A | D⋯A | D-H⋯A |
| a | N18 | H18A | O22B | 0.86 | 2.27 | 3.019(17) | 145 |
| b | N18 | H18B | O25B | 0.86 | 2.32 | 3.101(19) | 152 |
| c | O23 | H23B | O17 | 0.82 | 1.71 | 2.515(11) | 165 |
| a’ | N18 | H18A | O22B | 0.86 | 2.54 | 2.990(19) | 114 |
| Interatomic Distances (Å) | Angles (°) | ||||||
|---|---|---|---|---|---|---|---|
| Descriptors | Donors | H⋯ | Acceptors | D-H | H⋯A | D⋯A | D-H⋯A |
| a | N13 | H13 | O3 | 0.86 | 1.72 | 2.566(5) | 169 |
| c | N19 | H19B | O2 | 0.86 | 2.07 | 2.894(5) | 159 |
| > b > b | N19 | H19A | O18 | 0.86 | 2.07 | 2.929(5) | 179 |
| Interatomic Distances (Å) | Angles (°) | ||||||
|---|---|---|---|---|---|---|---|
| Descriptors | Donors | H⋯ | Acceptors | D-H | H⋯A | D⋯A | D-H⋯A |
| a | N18 | H18A | O22B | 0.86 | 2.27 | 3.019(17) | 145 |
| b | N18 | H18B | O25B | 0.86 | 2.32 | 3.101(19) | 152 |
| c | O23 | H23B | O17 | 0.82 | 1.71 | 2.515(11) | 165 |
| a’ | N18 | H18A | O22B | 0.86 | 2.54 | 2.990(19) | 114 |
| Cocrystal/Salt Former | Ratio | Formed Solid | M.P./Degradation Range (°C) |
|---|---|---|---|
| 4-Nitrobenzamide | 1:1 | Cocrystal | 90–100 |
| Carbamazepine | 1:1 | Cocrystal | 100 |
| Isonicotinamide | 1:1 | Salt | 120–130 |
| Nicotinamide | 2:3 | Salt cocrystal | 73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caro Garrido, C.; Robeyns, K.; Debecker, D.P.; Luis, P.; Leyssens, T. From Liquid to Solid: Cocrystallization as an Engineering Tool for the Solidification of Pyruvic Acid. Crystals 2023, 13, 808. https://doi.org/10.3390/cryst13050808
Caro Garrido C, Robeyns K, Debecker DP, Luis P, Leyssens T. From Liquid to Solid: Cocrystallization as an Engineering Tool for the Solidification of Pyruvic Acid. Crystals. 2023; 13(5):808. https://doi.org/10.3390/cryst13050808
Chicago/Turabian StyleCaro Garrido, Camila, Koen Robeyns, Damien P. Debecker, Patricia Luis, and Tom Leyssens. 2023. "From Liquid to Solid: Cocrystallization as an Engineering Tool for the Solidification of Pyruvic Acid" Crystals 13, no. 5: 808. https://doi.org/10.3390/cryst13050808









