1. Introduction
The discovery of quasicrystals in melt-spun Al–Mn ribbons by Shechtman et al. in 1982 [
1] triggered a revolution in crystallography and changed our understanding of the structure of solids. Just a few years later, the existence of a thermodynamically stable quasicrystal was reported in the Al–Cu–Fe system by Tsai et al. [
2]. A stable quasicrystal can have several interesting properties, such as a high hardness, a low coefficient of friction, a low surface energy and a high oxidation and corrosion resistance [
3,
4]. These properties are appropriate for technological applications such as low-stick surfaces or mechanical devices with reduced friction [
5]. Therefore, it is necessary to assess the surface energy of a quasicrystal material that comes into contact with an antagonist such as water or a lubricant in low-stick applications [
6] or a solid such as hard steel in mechanical applications [
7]. Among the methods used for surface-energy investigations, the sessile droplet technique is convenient for laboratory experiments. The measurements yield a contact angle
between the droplet of the test liquid and the surface of interest, neglecting the equilibrium pressure (
) of the adsorbed vapour from the liquid. The Young equation defines the angle
at equilibrium between the solid, the liquid, and its vapour:
where
,
and
are the solid–vapour, liquid–vapour, and solid–liquid interfacial tensions [
8].
At thermodynamic equilibrium, the solid–vapour interfacial tension
is usually taken as the solid surface energy
. Similarly, the interfacial tension
is the liquid surface energy
. The authentic surface energy is determined as the surface excess free energy per unit area of a particular crystal facet and is a physical property of the material. The state of the solid surface can be quantified through the surface energy [
9]. It determines the equilibrium shape of the crystal and the adhesion energy at the interfaces with other materials. It also plays an essential role in roughening, faceting, crystal growth and in the nucleation kinetics of precipitates [
10]. The surface energy can be computed [
9] for periodic crystals, for example, some elementary metals:
= 1200 mJ/m
2,
= 2200 mJ/m
2, and
= 1800 mJ/m
2. Unfortunately, computational methods are restricted to relatively simple compounds and do not apply to complex metallic alloys such as quasicrystals or their approximants with giant unit cells of up to thousands of atoms. Due to the absence of a periodic lattice for the surface energy of a quasicrystal, we have to rely on indirect techniques, for instance, friction experiments in a vacuum [
11] or thin-film growth [
12]. The typical surface energy
of a quasicrystal is in the range 600–800 mJ/m
2 for aluminium-based alloys [
13], i.e., significantly less than the surface energy of the individual constituents [
9]. Theory suggests a reduction in the surface energy of quasicrystals as a consequence of the icosahedral symmetry and the formation of a deep pseudo-gap [
13] or the excitation of phason modes [
14]. In addition, freshly polished and dried samples have contact angles with water in the range of 100–120°, much larger than the contact angle observed for aluminium, the main component of the alloy. This implies a sensitivity to the density of itinerant electrons in the bulk of the quasicrystal, beneath its superficial native oxide. Below 10–12 nm of oxide thickness, it has been shown to vary in inverse proportion to the thickness of the oxide [
13], suggesting a coupling between the electronic distribution on the liquid molecules (e.g., the electric dipole of the water molecule) on the one hand and the topology of the quasicrystal’s Fermi surface on the other [
15]. The same trend was observed with water deposited on Al-based quasicrystalline coatings [
16,
17,
18,
19] and films [
20], with quasicrystal-reinforced composites [
21], and even with liquid metals on Al–Co approximants [
22].
These quasicrystals, however, are always covered by a native oxide layer when exposed to the atmosphere. The amorphous alumina layer formed after a very brief time is a few nanometres thick [
23]. Annealing above room temperature, typically at 500 °C, leads to the significant growth of the oxide layer and causes the partition of the chemical species below the layer due to the migration of Al atoms to feed the growth of the oxide [
24]. These studies were performed with laboratory samples made of the pure QC phase. Technological applications require the preparation of the material under industrial conditions, using raw materials with an acceptable economic cost, containing impurities, and following an industrial protocol that does not necessarily provide a perfect quasicrystalline material [
23]. For instance, commercially available precursor quasicrystalline powders can be applied to prepare surface coatings via plasma spraying. These coatings need to be additionally heat treated after the deposition to eliminate undesirable crystalline phases that endanger the corrosion resistance of the coating and provoke the formation of the QC phases [
25]. The main property affecting this behaviour is the surface energy. However, as the material is covered by an oxide layer, we probe not the surface energy of the metallic material but rather the surface energy of the material containing the oxide layer. To avoid confusion between these two entities, we coin a particular word to label the surface energy probed on top of the oxide layer: “surfenergy”. The surfenergy is a few tens of mJ/m
2 and it should not be confused with the surface energy of the bare metallic materials found underneath the oxide, which is in the range of several hundred mJ/m
2 or more. In reality, the surfaces have physical (e.g., roughness) and chemical (e.g., functional groups) heterogeneities. The influences of various surface heterogeneities were studied by Wenzel [
26] and Good [
27] (for physical heterogeneity) and Cassie [
28] (for chemical heterogeneity). Oxidation behaviour has an influence on the physical properties of the interface, especially at elevated temperatures, and it is relevant for technological applications and determines the viability of valuable materials [
13,
23,
29].
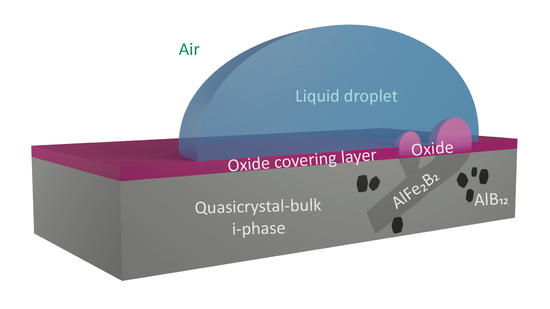
This article reviews the surface behaviour of a sample made from atomised powder via sintering at a temperature close to the peritectic reaction, leading to quasicrystal growth. We study the equilibrium shape of the droplets of two different liquids, which are placed in contact with the surface of a quasicrystalline material based on Al, Cu, Fe and B. The goal is to investigate the oxidation behaviour of the quasicrystalline sample in the air and to determine to what extent the constituent species take part in the formation of the oxide layer.
2. Experimental Details and Analytical Methods
2.1. Sample Preparation
An atomised powder with the nominal composition Al
59Cu
25Fe
13B
3 (at.%) was purchased from the Saint-Gobain company. It forms a stable icosahedral quasicrystal of the Mackay type and an FCC lattice in six dimensions [
23]. The powder was produced in a large-scale atomisation tower for industrial purposes. The addition of 3 at.% boron was intended to decrease the alloy’s melting point [
30]. This powder was mechanically sieved in the range of 20–70 μm and sintered under an argon flow using a uniaxial sintering furnace operated at 930 °C and a pressure of 100 MPa [
31]. After sintering, a dense orthogonal 11 × 15 × 2 mm
3 polygrained quasicrystalline body was obtained and cut (hereafter called “the sample”) for further studies. The largest surface of this sample was water polished to its final surface roughness by applying a finishing 4000 grit abrasive paper. No grinding paste was used during the polishing procedure to avoid trapping any residues in the sample’s open pores. The ageing experiment was performed under ambient conditions with an atmospheric humidity of around 40%. During the ageing experiments, the sample was kept in a dust-free box. In the subsequent experiments, the sample was annealed at 500 °C in the air using a resistance box furnace for time periods varying between a few minutes and several hours. The duration of the cooling stage was identical for all the annealing times, with around 5 h needed to reach a temperature of 20 °C. Before every subsequent ageing and annealing period, the specimen’s surface was re-polished and dried in a flow of hot air.
2.2. Scanning Electron Microscopy (SEM)
A JEOL JSM-7600F (JEOL, Tokyo, Japan) field-emission-gun scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy (EDXS) was used to characterise the microstructure and elemental composition of the prepared sample. The quantitative EDXS analyses were performed using the Oxford Instruments INCA Microanalysis Suite with an X-Max 20 SDD detector. The sample used for SEM/EDXS microstructural characterisation was prepared using standard metallographic procedures for aluminium alloys. The investigation was performed on a quasicrystalline sample to examine the phases and surface morphology. Images were taken from the central area of the sample in the SEI and BSE modes of operation to reveal phases with different compositions. The sample was characterised after polishing, ageing at different times in air and after annealing at 500 °C for different times in air.
2.3. X-ray Powder Diffraction (PXRD)
The X-ray powder diffraction (PXRD) data were collected with a Malvern Panalytical Empyrean X-ray (Malvern Panalytical, Almelo, the Netherlands) diffractometer using a monochromated X-ray beam produced by a Cu-target tube (λKα
1 = 0.15406 nm and λKα
2 = 0.154439 nm). The measurements were obtained in Bragg–Brentano geometry by applying a divergence slit of 0.04 rad, in the range of 10° < 2
< 90°, using a step size of 0.0131° and with a counting time of 1 s per step. The PXRD data were analysed using the HighScore Plus XRD Analysis Software database PDF-4+ 2022 and on the basis of the literature relating to quasicrystals [
23].
2.4. X-ray Photoelectron Spectroscopy (XPS)
The XPS analysis was carried out on a PHI-TFA XPS spectrometer produced by Physical Electronics Inc (Chanhassen, Minnesota, MN, USA). and equipped with an Al monochromatic source. The vacuum in the XPS spectrometer was 1 × 10−9 mbar and the analysed area was 0.4 mm in diameter, which did not allow us to spatially resolve between the micro-size crystalline phases present on the surface. The XPS analysis depth profile was between 3 and 5 nm. The XPS depth profiles were obtained by sputtering the sample surface with Ar ions having an energy of 4 keV over an area of 3 × 3 mm2. The sputtering rate was calibrated on Ni/Cr layers of known thickness to be 2.0 nm/min.
2.5. Atomic Force Microscopy (AFM)
The morphology of the QC surface was investigated using a VEECO Dimension 3100 atomic force microscope (AFM, Veeco Instruments Inc., Plainview, NY, USA). Three-dimensional (x–y–z) topographic images of the sample surface were obtained, and the surface roughness were extracted with a sub-nanometre resolution on a height scale (z scale) [
32]. The images were scanned using the contact mode with a silicon nitride AFM tip at a scan rate of 1 Hz within an image area of 30 × 30 µm
2. The measurements were performed at room temperature in air.
2.6. Focused Ion Beam (FIB) System and Transmission Electron Microscopy (TEM)
For the atomic-scale structural analysis of the oxide layer’s thickness, a thin, electron-transparent lamella was prepared in the cross-sectional view using focused ion beam–scanning electron microscopy (FIB–SEM, HeliosNanoLab NL650, Thermo Fisher Scientific Inc., Waltham, MA, USA). Prior to the lamella’s preparation, a 30 nm thick carbon layer was deposited on the sample to protect the surface layer during the FIB lamella’s preparation. The experimental, high-angle annular dark-field–scanning transmission electron microscopy (HAAD–FSTEM) images were recorded to assess the oxide layer and other bulk phases. The probe Cs-aberration-corrected TEM (JEM-ARM 200CF; JEOL, Tokyo, Japan) was operated at 200 keV, with a spatial resolution of 0.08 nm in STEM mode. The elemental distribution was assessed via standard-less energy-dispersive X-ray spectroscopy in STEM mode (STEM–EDX) using a JEOL Centurio 100 mm2 EDXS detector and a Gatan GIF Quantum ER spectrometer (Gatan, Pleasanton, CA, USA). The convergence and collection semi-angles for the probe and the HAADF detector were set to 24 mrad and 68–280 mrad, respectively.
2.7. Contact-Angle Measurements
The contact-angle measurements were performed using a Theta Lite-Biolin Scientific instrument (Biolin Scientific, Göteborg, Sweden) to determine the wettability of the flat surface of the sample. The measurements of the contact angle were performed at room temperature in the air with a relative humidity of 40–50% using purified Milli-Q water as a polar liquid and diiodomethane (CH2I2) as a non-polar liquid. The maximum volume of the applied droplets was 10 µL. For an individual experiment, 10 contact-angle measurements of each liquid were carried out, yielding the average value and the standard deviation. After every single set of experiments, the sample was re-polished to avoid contamination of the surface by the previous droplets of diiodomethane. The duration of every experiment using both liquids was approximately 20 min.
2.8. Calculation of the Surfenergy
The surfenergy
appearing in Equation (1) as the solid–vapour interfacial tension
can be calculated from the contact-angle
measurement as graphically illustrated in
Figure 1a. In contrast, the liquid–vapour interfacial tension
and the solid–liquid contribution
need to be determined from Equation (1). Therefore, it is necessary to perform the measurement of the contact angle with two different liquids. In our case, with water and diiodomethane. The quantities
and
can be decomposed into their dispersive (
d) and polar (
p) components:
The values of the latter for the applied liquids are listed in
Table 1.
To calculate the solid–vapour interfacial tension
(or, equivalently, the surfenergy
), we used the OWRK (Owens, Wendt, Rabel and Kaelble) method [
8]. It is based on the assumption that the solid–liquid tension
can be expressed in terms of the geometrical mean of the dispersive and polar components:
After considering Equations (2) and (3) and inserting Equation (4) into Equation (1) for the two liquids, as denoted by the superscripts 1 and 2, we can derive the following expressions:
For the solid–liquid system interacting solely through the dispersion forces and from Equation (4), when an apolar liquid is used, we obtain a linear relation:
which yields the dispersive component
as the square of the respective line slope.
This approach was formulated by Fowkes [
34] in 1950 and confirmed later by other authors, e.g., Fox and Zisman [
35] and Dubois [
23], on different materials and with different experimental liquids.
4. Discussion
The surface oxidation is responsible for the samples’ properties, as proven by the XPS analysis, which revealed a diverse chemistry of various carbon-based hydrogen and dangling-oxide bonds on the top-most layer of the aged QC surface. In particular, the revealed oxidation states of the elements deduced from the surface XPS spectra were in accordance with previous studies of the comparable QC systems of Al–Fe–Cu–Cr [
29,
38], Al–Fe–Cu [
39] and Al–Fe–Cu–B [
40]. The measured XPS depth profile revealed an enrichment of the oxide and the aluminium, accompanied by a depletion of the iron and copper in the top surface layer. From this, we concluded that the Al-oxide layer serves as a passivation agent, hindering any further oxidation in the interior of the QC bulk, as previously reported [
39]. The electron-diffraction analysis confirmed the presence of the i-phase with a two-fold symmetry pattern, which is in agreement with the literature [
41], coexisting with an orthorhombic, needle-like AlFe
2B
2 phase and a AlB
12 phase with a globular morphology. The same was observed by Brien et al. [
30] for a sintered Al–Fe–Cu–B alloy. The TEM cross-sectional analysis of the i-phase revealed a smooth, 10 nm thick, amorphous, oxide Al-rich layer after the longest ageing time. After annealing at 500 °C for 1 h, the initially amorphous oxide layer on top of the i-phase was converted into an aluminium-oxide-rich nanocrystalline film with an average thickness of 20 nm. The needle-like AlFe
2B
2 phase grew in a more complex oxide assembly. The corresponding oxide thickness after annealing for 1 h at 500 °C increased to 50–60 nm, as confirmed by the AFM measurements. The transformation due to annealing was accompanied by a dramatic change in the topography of the surface, which was quantified by a 44% greater roughness in comparison to that of the aged sample. The general composition of the oxide film on the matrix phase is aluminium (33 at.%) and oxygen (66 at.%). The secondary AlFe
2B
2 phase consists of iron (27 at.%), oxygen (65 at.%) and a very small amount of aluminium (6 at.%), while the B content is unknown. As confirmed by the EDXS measurements, the matrix i-phase Al
62Cu
25Fe
13 and its surface oxide did not exhibit any detectable morphology changes. In contrast, the oxidation of the secondary AlFe
2B
2 phase implies morphological changes after ageing and even more drastically after annealing. This complex oxide grows in different forms, either in terms of sharp needles or dome-like objects, depending on the annealing time.
The amount of boron trapped in the quasicrystalline sample is an additional technological optimisation parameter, where the surface roughness and the resulting surfenergy can be, to some extent, controlled by the amount of B-doping without significantly altering the dominant matrix QC phase. The oxidation behaviour for both cases, during ageing and annealing, exposes different trends for the surface oxidation, which is directly related to the underlying crystal phase. However, the average oxide film thickness remains significantly lower for the aged samples when compared to their annealed counterparts. It can be assumed that the surface energy is also influenced by the subsurface chemistry and not just the formation of the oxide layer and the resulting surface morphology.
The surface roughness and the oxide population influence the surfenergy. The tendency of the QC-treated surfaces to exhibit hydrophobic behaviour can be, in the simplest terms, explained by their increased surface roughness in the nm range. However, it is known that hydrophobic behaviour can be controlled by the surface roughness, as previously shown in reference [
26]. As a rule of thumb, the level of hydrophobicity grows with the roughness, which is affected by the shape and size of the oxide forms. On the other hand, there is a relation between the oxides and the two components of the surfenergy.
The changes in roughness are mostly associated with nanocrystalline oxides above the AlFe2B2 phase at various time intervals. Namely, for shorter annealing times, the oxide phase is mainly in the form of sharp needles forming above the surface. In contrast, for the longest annealing times, the formation of various oxides becomes largely delocalised in a uniform, dome-shaped oxide layer, populating the sample’s surface.
The presence of needles and domes is reflected in an enhanced surface roughness and energy. The latter phenomenon can be explained at the atomistic level by means of the charge accumulation at the tips [
42]. A high charge density due to a specific morphology, in our case in the oxides above the secondary phase, is associated with an increased surface energy in comparison with a flat sample, which is in agreement with previous reports (Dubois [
23]).
The experimental setup makes it possible to assess the surfenergy using its two main constituents, i.e., the dispersive and polar surfenergy components. To better understand the behaviour of the surfenergy components, a droplet experiment was performed on different materials (see
Table 2). The results revealed a significant difference between our material and the pure aluminium, the main component of the quasicrystalline sample. The dispersive components match to within 8%, because it is less sensitive to the structure, morphology and chemical composition. However, there is a significant difference in the polar components, which is ascribed to the influence of the processing techniques. As highlighted in the following text, in strong contrast to these reference materials, the quasicrystalline sample does exhibit a much lower polar component under suitable preparation conditions. We assigned these differences to coexistence with the majority i-phase of a small amount of a needle-like AlFe
2B
2 phase that promotes the formation of specific oxides on the sample surface.
The surface morphology has direct and indirect influences on the behaviour of the liquid droplets. The indirect influence is related to the distribution of electric charges and, consequently, to the energy of the surface, whereas the shape of the droplet is directly affected by the surface landscape.
Conceptually, both polar and dispersive components can contribute to the overall surfenergy. However, as shown in this study, an oxide film thickness of a minimum of 5–10 nm completely masks the QC contribution of the dispersive component, which can be attributed solely to the amorphous oxide layer. Therefore, the dispersive component remains time independent, regardless of the oxide-layer thickness. The only noticeable drop in the dispersive component occurs at an early stage of ageing, when the oxide-layer thickness is sufficiently low (below 5 nm). It is worth mentioning that the droplet experiment for the surfenergy was performed about 5 min after polishing, whereas the XPS measurements were carried out at least 15 min later, preventing us from obtaining an insight into the surface chemistry before complete oxidation. Extremely low values of the dispersive contribution to the QC surface energy, close to the data for polymers (Teflon) in previous studies [
13], were obtained by applying a high vacuum and so preventing surface oxidation. Such experiments are of limited technological importance for atmospheric/ambient conditions. Therefore, it is very desirable to prevent excessive oxide-layer growth and to retain the surface influence of the QC electronic states. This can be achieved by means of tuning the sample-processing parameters. The goal is to find a balance between the polar and the dispersive components, since the former is decreased with the ageing/annealing times. The rapid decay of the polar component can be explained by the liquid-molecule reorientation and the surface-charge redistribution due to the electrostatic forces, leading to energy minimisation. This is reflected in the relaxation of the respective atomic positions and the lowering of the surfenergy. The restriction is maintaining the pseudo gap in the electronic structure, which is characteristic of QC materials and apparently responsible for desirable frictional and wetting properties [
43].
Since the majority of the oxide layer is formed above the B-containing phase, it might be important to control the content of boron. The amount of boron trapped in the quasicrystalline sample opens an additional technological optimisation parameter, directly influencing the roughness without changing the matrix phase. Utilising the investigated material for future technologies should be focused on the processing optimisation and the boron content related to the interplay between the polar and the dispersive components of the surfenergy.